Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs and the Risk of Vascular Dementia in Patients with Spondyloarthritis: A Database Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. The Data Source
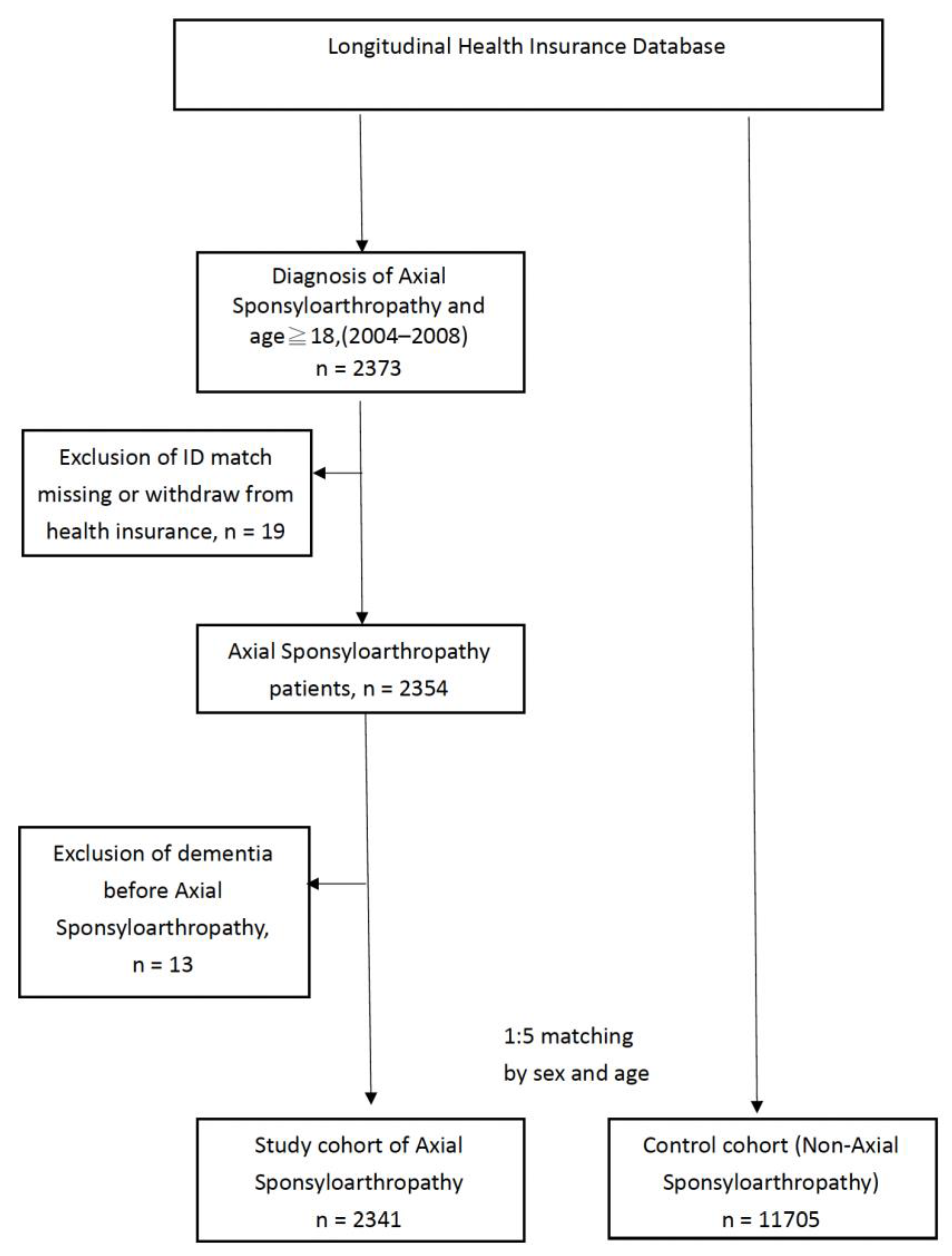
2.2. Axial SpA Study Group Selection and Exclusion Process
2.3. Non-axSpA Control Cohort
2.4. Outcome and Relevant Comorbidities
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, E.; Herzberg, I.; Laiho, K.; Barnardo, M.C.; Pointon, J.J.; Kauppi, M.; Kaarela, K.; Tuomilehto-Wolf, E.; Tuomilehto, J.; Wordsworth, B.P.; et al. Finnish HLA studies confirm the increased risk conferred by HLA-B27 homozygosity in ankylosing spondylitis. Ann. Rheum. Dis. 2006, 65, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Haibel, H.; Baraliakos, X.; Listing, J.; Marker-Hermann, E.; Zeidler, H.; Braun, J.; Sieper, J. The early disease stage in axial spondylarthritis: Results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009, 60, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res. 2016, 68, 1320–1331. [Google Scholar] [CrossRef]
- van Tubergen, A. The changing clinical picture and epidemiology of spondyloarthritis. Nat. Rev. Rheumatol. 2015, 11, 110–118. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Michaud, K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 2009, 11, 229. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Zhu, J.; Du, X.; Huang, F. Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: A case-control study. Arthritis Res. Ther. 2012, 14, R215. [Google Scholar] [CrossRef]
- Batmaz, I.; Sariyildiz, M.A.; Dilek, B.; Bez, Y.; Karakoc, M.; Cevik, R. Sleep quality and associated factors in ankylosing spondylitis: Relationship with disease parameters, psychological status and quality of life. Rheumatol. Int. 2013, 33, 1039–1045. [Google Scholar] [CrossRef]
- Jang, H.D.; Park, J.S.; Kim, D.W.; Han, K.; Shin, B.J.; Lee, J.C.; Choi, S.W.; Suh, S.W.; Yang, J.H.; Park, S.Y.; et al. Relationship between dementia and ankylosing spondylitis: A nationwide, population-based, retrospective longitudinal cohort study. PLoS ONE 2019, 14, e0210335. [Google Scholar] [CrossRef]
- Fleming, K.C.; Adams, A.C.; Petersen, R.C. Dementia: Diagnosis and evaluation. Mayo Clin. Proc. 1995, 70, 1093–1107. [Google Scholar] [CrossRef]
- Haapasalo, A.; Pikkarainen, M.; Soininen, H. Alzheimer’s disease: A report from the 7th Kuopio Alzheimer symposium. Neurodegener. Dis. Manag. 2015, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Lovestone, S. The dementias. Lancet 2002, 360, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Fabrigoule, C.; Amieva, H.; Helmer, C.; Dartigues, J.F. The disablement process: A conceptual framework for dementia-associated disability. Dement. Geriatr. Cogn. Disord. 2002, 13, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Schram, M.T.; Euser, S.M.; de Craen, A.J.; Witteman, J.C.; Frolich, M.; Hofman, A.; Jolles, J.; Breteler, M.M.; Westendorp, R.G. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007, 55, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lin, K.P.; Chen, Y.C. Risk factors for dementia. J. Formos. Med. Assoc. 2009, 108, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease—A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Basu, A.; Krady, J.K.; Levison, S.W. Interleukin-1: A master regulator of neuroinflammation. J. Neurosci. Res. 2004, 78, 151–156. [Google Scholar] [CrossRef]
- Chang, R.; Yee, K.L.; Sumbria, R.K. Tumor necrosis factor alpha Inhibition for Alzheimer’s Disease. J. Central Nerv. Syst. Dis. 2017, 9, 1179573517709278. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kiyota, T.; Horiba, M.; Buescher, J.L.; Walsh, S.M.; Gendelman, H.E.; Ikezu, T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am. J. Pathol. 2007, 170, 680–692. [Google Scholar] [CrossRef]
- Sun, X.; Steffens, D.C.; Au, R.; Folstein, M.; Summergrad, P.; Yee, J.; Rosenberg, I.; Mwamburi, D.M.; Qiu, W.Q. Amyloid-associated depression: A prodromal depression of Alzheimer disease? Arch. Gen. Psychiatry 2008, 65, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Boss, B.; Teichmann, J.; Klor, H.U.; Neeck, G. Serum amyloid A—An indicator of inflammation in ankylosing spondylitis. Rheumatol. Int. 2000, 19, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Park, M.C.; Park, Y.B.; Lee, S.K. Serum amyloid a as a useful indicator of disease activity in patients with ankylosing spondylitis. Yonsei Med. J. 2007, 48, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Baysal, O.; Durmus, B.; Ersoy, Y.; Altay, Z.; Senel, K.; Nas, K.; Ugur, M.; Kaya, A.; Gur, A.; Erdal, A.; et al. Relationship between psychological status and disease activity and quality of life in ankylosing spondylitis. Rheumatol. Int. 2011, 31, 795–800. [Google Scholar] [CrossRef]
- Jedrziewski, M.K.; Lee, V.M.; Trojanowski, J.Q. Physical activity and cognitive health. Alzheimer’s Dement. 2007, 3, 98–108. [Google Scholar] [CrossRef]
- Lee, D.H.; Choi, Y.J.; Han, I.B.; Hong, J.B.; Do Han, K.; Choi, J.M.; Sohn, S. Association of ischemic stroke with ankylosing spondylitis: A nationwide longitudinal cohort study. Acta Neurochir. 2018, 160, 949–955. [Google Scholar] [CrossRef]
- Lin, Y.R.; Chou, L.C.; Chen, H.C.; Liou, T.H.; Huang, S.W.; Lin, H.W. Increased Risk of Dementia in Patients with Systemic Lupus Erythematosus: A Nationwide Population-Based Cohort Study. Arthritis Care Res. 2016, 68, 1774–1779. [Google Scholar] [CrossRef]
- Judge, A.; Garriga, C.; Arden, N.K.; Lovestone, S.; Prieto-Alhambra, D.; Cooper, C.; Edwards, C.J. Protective effect of antirheumatic drugs on dementia in rheumatoid arthritis patients. Alzheimer’s Dement. 2017, 3, 612–621. [Google Scholar] [CrossRef]
- Newby, D.; Prieto-Alhambra, D.; Duarte-Salles, T.; Ansell, D.; Pedersen, L.; van der Lei, J.; Mosseveld, M.; Rijnbeek, P.; James, G.; Alexander, M.; et al. Methotrexate and relative risk of dementia amongst patients with rheumatoid arthritis: A multi-national multi-database case-control study. Alzheimer’s Res. 2020, 12, 38. [Google Scholar] [CrossRef]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Box, S.A.; Pullar, T. Sulphasalazine in the treatment of rheumatoid arthritis. Br. J. Rheumatol. 1997, 36, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Morabito, L.; Montesinos, M.C.; Schreibman, D.M.; Balter, L.; Thompson, L.F.; Resta, R.; Carlin, G.; Huie, M.A.; Cronstein, B.N. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J. Clin. Investig. 1998, 101, 295–300. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, R.; Kaelber, D.C.; Gurney, M.E. Tumor Necrosis Factor (TNF) blocking agents are associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis. PLoS ONE 2020, 15, e0229819. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Steroid modulation of hippocampal plasticity: Switching between cognitive and emotional memories. Front. Cell. Neurosci. 2012, 6, 12. [Google Scholar] [CrossRef]
- Brown, E.; Woolston, D.J.; Frol, A.; Bobadilla, L.; A Khan, D.; Hanczyc, M.; Rush, A.; Fleckenstein, J.; Babcock, E.; Cullum, C. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol. Psychiatry 2004, 55, 538–545. [Google Scholar] [CrossRef]

| Baseline Variable | Patients with axSpA (n = 2341) | Patients without axSpA (n = 11,705) | p Value | ||
|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||
| Characteristics | |||||
| Age (years) | 1.00 | ||||
| 18–30 | 632 | 27.0 | 3160 | 27.0 | |
| 31–40 | 563 | 24.0 | 2815 | 24.0 | |
| 41–50 | 483 | 20.6 | 2415 | 20.6 | |
| 51–60 | 322 | 13.8 | 1610 | 13.8 | |
| 61–70 | 195 | 8.3 | 975 | 8.3 | |
| >70 | 146 | 6.2 | 730 | 6.2 | |
| Sex | 1.00 | ||||
| Male | 1535 | 65.6 | 7675 | 65.6 | |
| Female | 806 | 34.4 | 4030 | 34.4 | |
| Urbanization | <0.001 | ||||
| Level 1 | 675 | 28.8 | 3723 | 31.8 | |
| Level 2 | 665 | 28.4 | 3297 | 28.2 | |
| Level 3 | 370 | 15.8 | 1973 | 16.9 | |
| Level 4 | 365 | 15.6 | 1478 | 12.6 | |
| Level 5 | 266 | 11.4 | 1234 | 10.5 | |
| Monthly income | <0.001 | ||||
| 0 (Financially dependent) | 363 | 15.5 | 2496 | 21.3 | |
| 1–20,000 TWD | 511 | 21.8 | 4840 | 41.3 | |
| 20,001–40,000 TWD | 1005 | 42.9 | 2476 | 21.2 | |
| ≥40,001 TWD | 462 | 19.7 | 1893 | 16.2 | |
| Comorbid medical disorders | |||||
| DM | 0.440 | ||||
| Yes | 185 | 7.9 | 871 | 7.4 | |
| No | 2156 | 92.1 | 10,834 | 92.6 | |
| Hypertension | 0.045 | ||||
| Yes | 425 | 18.2 | 1927 | 16.5 | |
| No | 1916 | 81.8 | 9778 | 83.5 | |
| Coronary heart disease | <0.001 | ||||
| Yes | 191 | 8.2 | 722 | 6.2 | |
| No | 2150 | 91.8 | 10,983 | 93.8 | |
| Parkinson’s disease | 1.00 | ||||
| Yes | 9 | 0.4 | 44 | 0.4 | |
| No | 2332 | 99.6 | 11,661 | 99.6 | |
| Hyperlipidemia | 0.001 | ||||
| Yes | 301 | 12.9 | 1235 | 10.6 | |
| No | 2040 | 87.1 | 10,470 | 89.4 | |
| Stroke | |||||
| Yes | 91 | 3.9 | 406 | 3.5 | |
| No | 2250 | 96.1 | 11,299 | 96.5 | |
| Autoimmune disease (RA, SLE) | <0.001 | ||||
| Yes | 97 | 4.1 | 90 | 0.8 | |
| No | 2244 | 95.9 | 11,615 | 99.2 | |
| Thyroid disease | <0.001 | ||||
| Yes | 150 | 6.4 | 457 | 3.9 | |
| No | 2191 | 93.6 | 11,248 | 96.1 | |
| Gout | <0.001 | ||||
| Yes | 319 | 13.6 | 1242 | 10.6 | |
| No | 2022 | 86.4 | 10,463 | 89.4 | |
| COPD | <0.001 | ||||
| Yes | 507 | 21.7 | 2009 | 17.2 | |
| No | 1834 | 78.3 | 9696 | 82.8 | |
| Medication therapy | |||||
| csDMARDs | <0.001 | ||||
| Yes | 1026 | 43.8 | 83 | 0.7 | |
| No | 1315 | 56.2 | 11,622 | 99.3 | |
| PREDNISOLONE | <0.001 | ||||
| Yes | 1031 | 44.0 | 3451 | 29.5 | |
| No | 1310 | 56.0 | 8254 | 70.5 | |
| METHYLPREDNISOLONE | <0.001 | ||||
| Yes | 264 | 11.3 | 890 | 7.6 | |
| No | 2077 | 88.7 | 10,815 | 92.4 | |
| Presence of Dementia | Patients without axSpA | Patients with axSpA |
|---|---|---|
| Follow-up period | ||
| Yes/Total | 157/11,705 | 40/2341 |
| Person-years | 67,341 | 11,961 |
| Incidence per 100,000 person-years | 233 | 334 |
| Crude hazard ratio (95% CI) | 1.00 | 1.53 *** (1.08–2.17) |
| a Model 1 Adjusted hazard ratio (95% CI) | 1.00 | 1.73 ** (1.22–2.46) |
| b Model 2 Adjusted hazard ratio (95% CI) | 1.00 | 1.55 * (1.08–2.23) |
| c Model 3 Adjusted hazard ratio (95% CI) | 1.00 | 1.95 ** (1.31–2.91) |
| Presence of Overall Dementia | Patients without axSpA | Patients with axSpA | |
|---|---|---|---|
| Without csDMARDs | With csDMARDs | ||
| Crude HR (95% CI) | 1.00 | 1.93 ** (1.29–2.89) | 1.03 (0.57–1.85) |
| Adjusted HR (95% CI) | 1.00 | 1.98 ** (1.32–2.99) | 1.01 (0.55–1.85) |
| Presence of AD | |||
| Crude HR (95% CI) | 1.00 | 1.19 (0.27–5.17) | 0.74 (0.10–5.60) |
| Adjusted HR (95% CI) | 1.00 | 1.23 (0.28–5.39) | 0.77 (0.10–5.89) |
| Presence of vascular dementia | |||
| Crude HR (95% CI) | 1.00 | 2.03 ** (1.33–3.09) | 1.06 (0.57–1.97) |
| Adjusted HR (95% CI) | 1.00 | 2.09 ** (1.36–3.20) | 1.05 (0.56–1.97) |
| Presence of Overall Dementia Medication Therapy | Hazard Ratio | (95% CI) | p-Value |
|---|---|---|---|
| csDMARDs | 0.45 | 0.22–0.89 | 0.023 |
| Prednisolone | 2.53 | 1.29–4.96 | 0.007 |
| Methylprednisolone | 2.40 | 1.15–4.97 | 0.019 |
| Adjusted hazard ratio | |||
| csDMARDs | 0.45 | 0.22–0.90 | 0.024 |
| Prednisolone | 2.53 | 1.29–4.99 | 0.006 |
| Methylprednisolone | 2.31 | 1.11–4.80 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Huang, S.-W.; Chen, C.-K.; Hong, J.-P.; Chen, Y.-W.; Lin, H.-W. Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs and the Risk of Vascular Dementia in Patients with Spondyloarthritis: A Database Cohort Study. J. Clin. Med. 2023, 12, 950. https://doi.org/10.3390/jcm12030950
Lee Y-H, Huang S-W, Chen C-K, Hong J-P, Chen Y-W, Lin H-W. Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs and the Risk of Vascular Dementia in Patients with Spondyloarthritis: A Database Cohort Study. Journal of Clinical Medicine. 2023; 12(3):950. https://doi.org/10.3390/jcm12030950
Chicago/Turabian StyleLee, Yu-Hao, Shih-Wei Huang, Chih-Kuang Chen, Jia-Pei Hong, Yi-Wen Chen, and Hui-Wen Lin. 2023. "Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs and the Risk of Vascular Dementia in Patients with Spondyloarthritis: A Database Cohort Study" Journal of Clinical Medicine 12, no. 3: 950. https://doi.org/10.3390/jcm12030950
APA StyleLee, Y.-H., Huang, S.-W., Chen, C.-K., Hong, J.-P., Chen, Y.-W., & Lin, H.-W. (2023). Conventional Synthetic Disease-Modifying Anti-Rheumatic Drugs and the Risk of Vascular Dementia in Patients with Spondyloarthritis: A Database Cohort Study. Journal of Clinical Medicine, 12(3), 950. https://doi.org/10.3390/jcm12030950






