Abstract
Intravenous sedative drugs are commonly administered during regional anesthesia. However, reducing the excessive use of sedatives while providing adequate sedation is important from the clinical perspective, since the use of sedatives can cause considerable complications. We hypothesized that the application of earmuffs and eye masks would help reduce the sedative dose required to maintain proper sedation by blocking external stimuli. Patients who underwent orthopedic surgery under spinal anesthesia were randomly allocated to the control (no intervention) or intervention group (wearing earmuffs and eye masks). Intravenous sedation was administered using target-controlled infusion of propofol. The target concentration was controlled to maintain a Modified Observer’s Assessment of Alertness and Sedation score of 3 or 4. The primary outcome was the intraoperative propofol requirement. We also investigated the incidence of apnea, and patient satisfaction. Propofol requirement was significantly lower in the intervention group than that in the control group (2.3 (2.0–2.7) vs. 3.1 (2.7–3.4) mg·kg−1·h−1; p < 0.001). Intraoperative apnea occurred less frequently (p = 0.038) and patient satisfaction was higher (p = 0.002) in the intervention group compared to the control group. This study demonstrated that the use of earmuffs and eye masks during sedation was associated with lower propofol requirement and improved sedation quality.
1. Introduction
Intravenous sedative drugs are commonly administered during regional anesthesia to reduce patients’ anxiety and increase their comfort during surgery [1,2]. Perioperative anxiety is not restricted to simple psychological stress, but can also lead to various complications, such as increased postoperative pain and analgesic requirements and prolonged hospitalization [3,4,5].
However, sedatives should be administered cautiously because of their considerable side effects. Intravenous sedatives can cause respiratory depression and hypotension during surgery, necessitating close monitoring and prompt treatment of patients to avoid medical emergencies [6,7,8]. Thus, non-pharmacological interventions that could help reduce the dose of sedatives as well as the patient’s anxiety are important clinically.
Environmental factors should be considered when maintaining appropriate sedation during surgery [9,10,11]. Bright lighting and surgical noise in the operating room can disturb the patient’s ability to relax and interfere with proper sedation. According to previous studies, earplugs and eye masks, which were employed to reduce external noise and light stimulation, improved the patient’s quality of sleep and decreased the incidence of postoperative disorientation [9,12]. Studies have reported that playing music to patients through headphones could reduce anxiety and sedative requirements during spinal anesthesia [1,13]. However, it has not been explored whether blocking external noise and light stimulation, without any music, might help to sedate patients intraoperatively.
We hypothesized that the application of earmuffs and eye masks during spinal anesthesia would block external stimuli, help patients relax, and reduce the sedative dose required to maintain proper sedation. This study entailed a randomized controlled trial to investigate the effect of the use of earmuffs and eye masks in patients undergoing orthopedic surgeries under spinal anesthesia on the sedative dose requirements for appropriate sedation and sedation quality.
2. Materials and Methods
This prospective randomized control trial was approved by the Institutional Review Boards of Seoul National University Bundang Hospital (study number: B2102-664-309) and was registered at the University Hospital Medical Information Network Clinical Trials Registry (registration number: UMIN000043496). This study adhered to the CONSORT guidelines. Written informed consent was obtained from all patients before surgery. Patients were enrolled between 25 March 2021 and 4 August 2022 at Seoul National University Bundang Hospital.
2.1. Patients
This study enrolled adult patients aged 19–64 years, with American Society of Anesthesiologists physical status I or II, who were scheduled to undergo elective orthopedic surgeries under spinal anesthesia. Patients who underwent treatment under general or epidural anesthesia and those with a history of heart failure, respiratory failure, sleep apnea, or propofol allergy were excluded. Patients who did not want intravenous sedation, earmuffs, or eye masks during surgery were excluded. We also excluded patients undergoing procedures requiring the lateral decubitus position during surgery, owing to the impossibility of applying earmuffs in these cases. Patients who required intensive care unit admission after surgery were also excluded. The participants were randomly assigned to the control group or intervention group using a computer-generated random code (Random Allocation Software Version 1.0; University of Medical Sciences, Isfahan, Iran) in an allocation ratio of 1:1. Randomization was performed by an independent anesthesiologist who was only involved in patient assignment and measurement of the sedation score.
2.2. Spinal Anesthesia and Intervention
After the patient entered the operating room, standard monitoring was initiated using electrocardiography, noninvasive blood pressure, peripheral oxygen saturation, and bispectral index measurement (BIS; Medtronic, Dublin, Ireland).
Spinal anesthesia was induced with an intrathecal injection of an optimal dose of 0.5% hyperbaric bupivacaine with fentanyl 10–20 μg through a 26-G Quincke needle into the L3–4 or L4–5 interspace. After the appropriate level of sensory blockade and hemodynamic stability were confirmed, earmuffs (Optime 105, 3M, Saint Paul, MI, USA) and eye masks (Dreamweaver-Contoured Sleep Mask, MACK’S, Michigan, IN, USA) were placed over the ears and eyes, respectively, of the patients in the intervention group. Earmuffs and eye masks were not used in the control group. The eye masks were discarded after single use and the earplugs were cleaned with an alcohol-containing cleaner.
Intravenous sedation was provided after intervention. Target-controlled infusion (TCI) of propofol was performed using an Orchestra infusion pump system (Fresenius vial, Berzins, France); the initial target effect-site concentration was set to 1.2 μg·mL−1. During sedation, the Modified Observer’s Assessment of Alertness and Sedation (MOAA/S) was assessed every 10 min by an independent anesthesiologist who was responsible for patient allocation. For patients in the intervention group, the earmuffs were lifted slightly from the left ear for sedation assessment. The definition of the MOAA/S score was as follows: (5) responds readily to name spoken in normal tone, (4) lethargic response to name spoken in normal tone, (3) responds only after the name is called loudly and/or repeatedly, (2) responds only after mild prodding or shaking, and (1) does not respond to mild prodding or shaking. The target sedation score was 3 or 4, and the effect-site concentration was adjusted according to the MOAA/S score every 10 min as follows: if the MOAA/S was 5, the target concentration was increased by 0.2 μg·mL−1; if it was 3 or 4, the concentration was maintained as the same; if the MOAA/S score was 2 or less, the target effect-site concentration was decreased by 0.2 μg·mL−1. During surgery, 10–20 µg of phenylephrine was administered intravenously when the mean blood pressure (MBP) decreased by more than 20% from baseline or fell below 60 mmHg. Atropine 0.5 mg was administered when the heart rate fell below 40 beats/min.
2.3. Study Outcomes
The primary outcome was the total intraoperative requirement of propofol, which was defined as the total dose of propofol · patient’s body weight−1 · administration time−1 (mg·kg−1·h−1). The MBP, heart rate, BIS score, and target effect-site concentration of propofol were recorded every 10 min during surgery. The time taken to reach a BIS value of 80 or less from the start of propofol administration was also measured and termed as the induction time. The incidence of apnea due to sedative administration and inotropic requirements during surgery were also recorded. An independent blinded researcher asked patients to estimate their satisfaction with intraoperative sedation using a numerical rating scale (score: 0, very dissatisfied, to 10, very satisfied) 24 h after surgery. The incidence of postoperative nausea and vomiting during the first 24 h was also investigated.
2.4. Statistical Analysis
Continuous data were expressed as median (interquartile range) and categorical variables were presented as numbers (percentages). Continuous outcomes between the control and intervention groups were compared using the Mann–Whitney U test. The chi-squared or Fisher exact test was used to analyze the categorical outcomes. SPSS version 21.0 (SPSS Inc., IBM, Chicago, IL, USA) was used for all statistical analyses. Statistical significance was defined as a two-sided p-value of <0.05.
2.5. Sample Size Calculation
In a pilot study of 20 patients (10 in each group) undergoing orthopedic surgery under spinal anesthesia, the mean (standard deviation) propofol requirement was 3.3 (0.8) and 2.8 (0.7) mg·kg−1·h−1 for the control and intervention groups, respectively. A power analysis was performed with G*Power 3.1.2 (Heinrich-Heine University, Düsseldorf, Germany) on the basis of the results of the pilot study. Our calculation showed that a sample size of 40 patients per group would ensure a statistical power of 80%, after accounting for a risk of 0.05 for type I errors in two-tailed analysis, and a 10% dropout rate.
3. Results
A total of 3 of the 83 eligible patients declined to participate in the study, and 80 patients were randomly allocated to the control (40 patients) or intervention group (40 patients). The anesthetic method was changed to general anesthesia for one patient in each group. Two patients in the control group and one patient in the intervention group declined to participate in the study before spinal anesthesia. Therefore, 75 patients completed the study (37 in the control group and 38 in the intervention group; Figure 1).
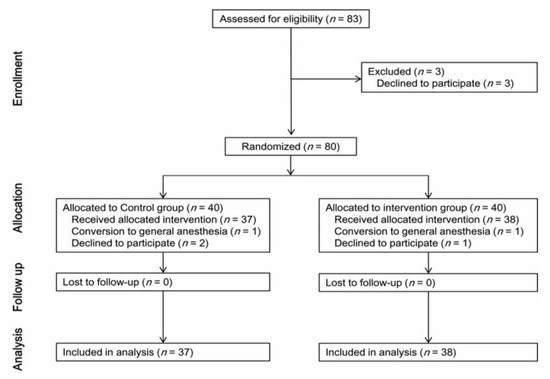
Figure 1.
Flow chart of patient selection.
The patient characteristics did not differ between the two groups (Table 1). Surgical data, including surgery/sedation time, spinal anesthetics, and type of surgery, did not differ between the groups (Table 2).

Table 1.
Baseline characteristics before and after propensity score matching.

Table 2.
Operational data.
The intraoperative propofol requirement for controlled sedation was significantly lower in the intervention group than that in the control group (2.3 (2.0–2.7) vs. 3.1 (2.7–3.4) mg·kg−1·h−1; p < 0.001; Table 3). The mean effect-site concentration of propofol was also lower in the intervention group than that in the control group during the surgery (0.9 (0.8–1.1) vs. 1.2 (1.1–1.4) mcg·mL−1; p < 0.001). The mean BIS did not significantly differ between the groups (p = 0.478). The induction time was shorter in the intervention group than that in the control group (190.0 (120.0–321.0) vs. 280.0 (180.0–452.0) s, p = 0.006). The incidence of intraoperative apnea was significantly lower in the intervention group (3 vs. 11, p = 0.038). The incidence of postoperative nausea and vomiting was comparable between the two groups (p = 0.190). Patient satisfaction with intraoperative sedation was significantly higher in the intervention group than that in the control group (10.0 (9.0–10.0) vs. 8.0 (8.0–10.0), p = 0.002).

Table 3.
Anesthesia and complication data.
The basal MBP and heart rate were comparable between the control and intervention groups (p = 0.379 and 0.461, respectively; Table 4). However, the intraoperative MBP was significantly higher in the intervention group (77.3 (72.9–96.1) vs. 75.1 (69.5–78.9) mmHg; p = 0.036) than that in the non-intervention group. The mean heart rate did not differ significantly between the two groups during surgery (p = 0.546), and neither did the intraoperative inotropic requirement (p = 0.563).

Table 4.
Intraoperative propofol dose and mean propofol target concentration among surgeries with high noise or low noise level.
The subgroup analysis of propofol use was also performed for cases with high noise and low noise levels (Table 4). During orthopedic surgery with high noise level as well as low noise, propofol dose was significantly lower in the intervention group compared with that in the control group (p < 0.001 for both levels). Likewise, mean propofol target concentration during surgery was also lower in the intervention group than that in the control group for both noise levels (p = 0.023 and 0.001 for high noise level surgery and low noise, respectively).
4. Discussion
This randomized controlled study confirmed that the application of earmuffs and eye masks to block external stimuli significantly decreased the propofol dose required for controlled sedation during orthopedic surgery under spinal anesthesia. The intervention group also showed a lower mean effect-site concentration of propofol, shorter induction time, lower frequency of apnea, and greater patient satisfaction compared to the control group. This was the first clinical trial to investigate the effect of earmuffs and eye masks on the sedative dose and sedation quality during spinal anesthesia.
Excessive propofol use for sedation during spinal anesthesia could be detrimental for the patient. Deep sedation using propofol infusion is reportedly associated with greater postoperative delirium and even mortality [14,15,16]. Therefore, the administration of appropriate sedative doses to maintain proper sedation are vital. In this study, the effect-site concentration of propofol was lower in the intervention group than that in the control group, albeit the BIS values were similar in both groups; thus, blocking external stimuli seemed to aid in the relaxation and sedation of patients intraoperatively. Moreover, the induction time was also significantly shorter in the intervention group, even though the initial target effect-site concentration of propofol was the same in both groups. A previous study reported that blocking noise by packing the ears significantly reduced the BIS score during propofol-induced sedation in noisy environments, which is consistent with our results [10].
The intervention in our protocol reduced the incidence of intraoperative apnea, which may be attributed to the significantly decreased propofol dose in the intervention group. Intraoperative apnea is one of the most common and critical complications of propofol administration [17,18,19]. It is fundamentally critical that anesthesiologists maintain spontaneous ventilation during intravenous sedation, and avoid hypercapnia or hypoxemia, because sedative agents inevitably depress respiration [20]. Even respiratory arrest cases due to intravenous sedation have been reported [21]; the role of anesthesiologists to direct intravenous sedation outside the operating room as well as intraoperatively is becoming increasingly important [22,23].
There was also a significant difference in the intraoperative MBP between the two groups, however, the hemodynamic difference did not seem clinically significant. Since the basal MBP was comparable between the two groups before the initiation of sedation, the difference of MBP may be attributed to the difference in the propofol dose between them. Along with respiratory depression, hypotension is one of well-known side effects of propofol infusion [24,25]. However, the inotropic requirement or intraoperative heart rate did not differ between the groups.
In this study, both the BIS and MOAA/S were assessed during TCI of propofol. TCI has been utilized for propofol sedation in patients with spinal anesthesia at our institution, owing to its superiority over the manually controlled method with respect to hemodynamic stability and convenience of management [1,26]. MOAA/S is a more sensitive sedation monitoring method than BIS in patients under spinal anesthesia [27]. Therefore, in our protocol, the target concentration of TCI propofol was regulated according to the MOAA/S score every 10 min. However, BIS is also known to have a good correlation with the sedation depth during propofol anesthesia [28,29,30]; we defined the induction time based on BIS and compared the BIS profile between the two groups.
A previous study found that (listening to) music significantly decreased the induction time and propofol dose required for sedation during regional anesthesia [1]. However, this approach would require greater attention to detail and preparation, such as determining the patient’s music preference, volume level, and arrangement of audio devices, compared to our intervention. Earmuffs and eye masks can be applied simply and easily in various clinical settings. Furthermore, noise blockade reportedly reduces BIS more effectively than listening to music in noisy environments [10].
Research on the effects of earplugs and eye masks during sleep has shown that they significantly improve sleep quality [9,12]. However, controlled clinical data on the effects of active blockade of external stimuli on sedation quality are insufficient. In our study, patient satisfaction with intraoperative sedation was also significantly higher in the intervention group than that in the control group. Some patients in the control group complained of noise or inappropriate conversations among the medical staff, which could be experienced by patients when intraoperative sedation was insufficient, especially at the beginning of propofol administration. Earmuffs and eye masks can also interrupt these unnecessary stimuli.
To check the effects of earmuffs and eye masks at different intraoperative noise levels, we performed the subgroup analysis of propofol use in cases with high noise and low noise levels. The analysis confirmed that, during surgery with high noise level as well as low noise, propofol dose and propofol target concentration were significantly lower in the intervention group than those in the control group. It seems that earmuffs effectively blocked external noise stimuli, regardless of the level of noise during surgery, causing a clinically significant improvement in sedation quality.
This study has some limitations. First, the attending anesthesiologists and patients were not blinded to the allocated intervention. However, each procedure or assessment was performed precisely, according to the study protocol. Patient allocation, MOAA/S monitoring, and propofol concentration adjustment were performed by an independent anesthesiologist who did not participate in other anesthetic procedures. Furthermore, postoperative outcomes were measured by one blinded researcher. Second, we utilized a numerical rating scale to estimate patient satisfaction. Other instruments such as the Evaluation du Vécu de l’Anesthésie LocoRégionale survey might be more robust [31,32,33]. However, we needed to focus on intraoperative sedation rather than on the entire anesthesia process; a blinded researcher tried to help patients to freely self-assess their satisfaction about the quality of sedation. Third, we enrolled patients who agreed to wearing earmuffs and eye masks, which could contain a selection bias, as only patients who were expecting positive effects were included in the protocol. However, the number of patients who refused the intervention was relatively small compared to the total number of screened patients. Moreover, it should be a clinically important finding that the intervention in patients who did not have an objection to wearing earmuffs and eye masks significantly reduced propofol dose and improve sedation quality. Fourth, the generalizability of our results is limited because this study was conducted at a single medical center. Multicenter data are required to confirm the generalizability of these results.
In conclusion, this prospective study demonstrated that the application of earmuffs and eye masks during elective orthopedic surgery with propofol sedation under spinal anesthesia was helpful in reducing propofol requirement, which also reduced the pharmacological side effects and enhanced patient satisfaction. Further studies will be needed to confirm the effects of the intervention on other sedatives and in different clinical environments.
Author Contributions
Conceptualization, S.I.B. and S.-H.D.; methodology, S.H.C. and S.-H.D.; investigation and data curation, J.-W.P. and S.-H.D.; formal analysis, S.I.B., S.H.C. and S.-H.D.; writing—original draft preparation, J.-W.P., S.I.B. and J.R.; writing—review and editing, S.H.C. and S.-H.D.; visualization, J.R.; supervision, J.-W.P. and S.-H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (study number: B2102-664-309) and was registered at the University Hospital Medical Information Network Clinical Trials Registry (registration number: UMIN000043496).
Informed Consent Statement
Written informed consent was obtained from all patients involved in this study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.W.; Fan, Y.; Manyande, A.; Tian, Y.K.; Yin, P. Effects of music on target-controlled infusion of propofol requirements during combined spinal-epidural anaesthesia. Anaesthesia 2005, 60, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Ki, M.J.; Kim, D.; Oh, Y.J.; Lee, J. The effect of an eye mask on midazolam requirement for sedation during spinal anesthesia: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.; Mushtaq, A.; Mukhtar, S.; Khan, Z. Preoperative anxiety before elective surgery. Neurosciences 2007, 12, 145–148. [Google Scholar] [PubMed]
- Nigussie, S.; Belachew, T.; Wolancho, W. Predictors of preoperative anxiety among surgical patients in Jimma University Specialized Teaching Hospital, South Western Ethiopia. BMC Surg. 2014, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, K.H.; Lee, C.S.; Lee, H.S.; Moon, S.Y.; Hwang, S.I.; Song, J.H. The effect-site concentration of propofol producing respiratory depression during spinal anesthesia. Korean J. Anesthesiol. 2011, 61, 122–126. [Google Scholar] [CrossRef]
- Conrad, B.; Larsen, R.; Rathgeber, J.; Lange, H.; Stuber, H.; Crozier, T. Propofol infusion for sedation in regional anesthesia. A comparison with midazolam. Anasth Intensiv. Notf. 1990, 25, 186–192. [Google Scholar] [CrossRef]
- Frank, L.R.; Strote, J.; Hauff, S.R.; Bigelow, S.K.; Fay, K. Propofol by infusion protocol for ED procedural sedation. Am. J. Emerg. Med. 2006, 24, 599–602. [Google Scholar] [CrossRef]
- Hu, R.F.; Jiang, X.Y.; Zeng, Y.M.; Chen, X.Y.; Zhang, Y.H. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit. Care 2010, 14, R66. [Google Scholar] [CrossRef]
- Kang, J.G.; Lee, J.J.; Kim, D.M.; Kim, J.A.; Kim, C.S.; Hahm, T.S.; Lee, B.D. Blocking noise but not music lowers bispectral index scores during sedation in noisy operating rooms. J. Clin. Anesth. 2008, 20, 12–16. [Google Scholar] [CrossRef]
- Al-Samsam, R.H.; Cullen, P. Sleep and adverse environmental factors in sedated mechanically ventilated pediatric intensive care patients. Pediatr. Crit. Care Med. 2005, 6, 562–567. [Google Scholar] [CrossRef]
- Le Guen, M.; Nicolas-Robin, A.; Lebard, C.; Arnulf, I.; Langeron, O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: A randomized study. Br. J. Anaesth. 2014, 112, 89–95. [Google Scholar] [CrossRef]
- Lepage, C.; Drolet, P.; Girard, M.; Grenier, Y.; DeGagne, R. Music decreases sedative requirements during spinal anesthesia. Anesth Analg. 2001, 93, 912–916. [Google Scholar] [CrossRef]
- Brown, C.H.T.; Azman, A.S.; Gottschalk, A.; Mears, S.C.; Sieber, F.E. Sedation depth during spinal anesthesia and survival in elderly patients undergoing hip fracture repair. Anesth Analg. 2014, 118, 977–980. [Google Scholar] [CrossRef]
- Sieber, F.E.; Zakriya, K.J.; Gottschalk, A.; Blute, M.R.; Lee, H.B.; Rosenberg, P.B.; Mears, S.C. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin. Proc. 2010, 85, 18–26. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Lee, S.Y.; Kim, T.Y.; Kim, D.K.; Lee, K.M.; Woo, N.S.; Chang, Y.J.; Lee, M.A. Spectral entropy for assessing the depth of propofol sedation. Korean J. Anesthesiol. 2012, 62, 234–239. [Google Scholar] [CrossRef]
- Aikawa, M.; Uesato, M.; Urahama, R.; Hayano, K.; Kunii, R.; Kawasaki, Y.; Isono, S.; Matsubara, H. Predictor of respiratory disturbances during gastric endoscopic submucosal dissection under deep sedation. World J. Gastrointest. Endosc. 2020, 12, 378–387. [Google Scholar] [CrossRef]
- Calderwood, A.H.; Chapman, F.J.; Cohen, J.; Cohen, L.B.; Collins, J.; Day, L.W.; Early, D.S. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest. Endosc. 2014, 79, 363–372. [Google Scholar] [CrossRef]
- Sasaki, T.; Tanabe, S.; Azuma, M.; Sato, A.; Naruke, A.; Ishido, K.; Katada, C.; Higuchi, K.; Koizumi, W. Propofol sedation with bispectral index monitoring is useful for endoscopic submucosal dissection: A randomized prospective phase II clinical trial. Endoscopy 2012, 44, 584–589. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, E.Y.; Hwang, J.W.; Do, S.H.; Na, H.S. Comparison of upper airway patency in patients with mild obstructive sleep apnea during dexmedetomidine or propofol sedation: A prospective, randomized, controlled trial. BMC Anesthesiol. 2018, 18, 120. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, S.; Choi, Y.J.; Kwon, H.J.; Jung, K.; Kim, S.E.; Park, M.I.; Moon, W.; Park, S.J. Efficacy and Safety of Etomidate in Comparison with Propofol or Midazolam as Sedative for Upper Gastrointestinal Endoscopy. Clin. Endosc. 2020, 53, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.; Roth, N.; Motamedi, N.; Lee, T.; Leonor, P.; Salem, M.; Gibbs, D.; Vargo, J. Anesthetist-Directed Sedation Favors Success of Advanced Endoscopic Procedures. Am. J. Gastroenterol. 2017, 112, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Goudra, B.G.; Singh, P.M.; Gouda, G.; Borle, A.; Gouda, D.; Dravida, A.; Chandrashakhara, V. Safety of Non-anesthesia Provider-Administered Propofol (NAAP) Sedation in Advanced Gastrointestinal Endoscopic Procedures: Comparative Meta-Analysis of Pooled Results. Dig. Dis. Sci. 2015, 60, 2612–2627. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.R.; Absalom, A.R.; Barends, C.R.M.; Jones, J.B. Hypotension during propofol sedation for colonoscopy: A retrospective exploratory analysis and meta-analysis. Br. J. Anaesth. 2022, 128, 610–622. [Google Scholar] [CrossRef]
- Mannion, S. Sedation, spinal anesthesia and older patients. J. Postgrad. Med. 2007, 53, 155. [Google Scholar] [CrossRef]
- Passot, S.; Servin, F.; Allary, R.; Pascal, J.; Prades, J.M.; Auboyer, C.; Molliex, S. Target-controlled versus manually-controlled infusion of propofol for direct laryngoscopy and bronchoscopy. Anesth Analg. 2002, 94, 1212–1216, table of contents. [Google Scholar] [CrossRef]
- Pollock, J.E.; Neal, J.M.; Liu, S.S.; Burkhead, D.; Polissar, N. Sedation during spinal anesthesia. Anesthesiology 2000, 93, 728–734. [Google Scholar] [CrossRef]
- Struys, M.M.R.F.; Vereecke, H.; Moerman, A.; Jensen, E.W.; Verhaeghen, D.; De Neve, N.; Dumortier, F.J.E.; Mortier, E.P. Ability of the Bispectral Index, Autoregressive Modelling with Exogenous Input-derived Auditory Evoked Potentials, and Predicted Propofol Concentrations to Measure Patient Responsiveness during Anesthesia with Propofol and Remifentanil. Anesthesiology 2003, 99, 802–812. [Google Scholar] [CrossRef]
- Liu, J.; Singh, H.; White, P.F. Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesth Analg. 1997, 84, 185–189. [Google Scholar] [CrossRef]
- Singh, H. Bispectral index (BIS) monitoring during propofol-induced sedation and anaesthesia. Eur. J. Anaesthesiol. 1999, 16, 31–36. [Google Scholar] [CrossRef]
- Maurice-Szamburski, A.; Bruder, N.; Loundou, A.; Capdevila, X.; Auquier, P. Development and validation of a perioperative satisfaction questionnaire in regional anesthesia. Anesthesiology 2013, 118, 78–87. [Google Scholar] [CrossRef]
- Wu, C.L.; Naqibuddin, M.; Fleisher, L.A. Measurement of patient satisfaction as an outcome of regional anesthesia and analgesia: A systematic review. Reg. Anesth. Pain Med. 2001, 26, 196–208. [Google Scholar] [CrossRef]
- Wu, C.L.; Fleisher, L.A. Outcomes research in regional anesthesia and analgesia. Anesth Analg. 2000, 91, 1232–1242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).