Construction and Verification of the Molecular Subtype and a Novel Prognostic Signature Based on Inflammatory Response-Related Genes in Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Inflammatory Response-Related Genes Collection
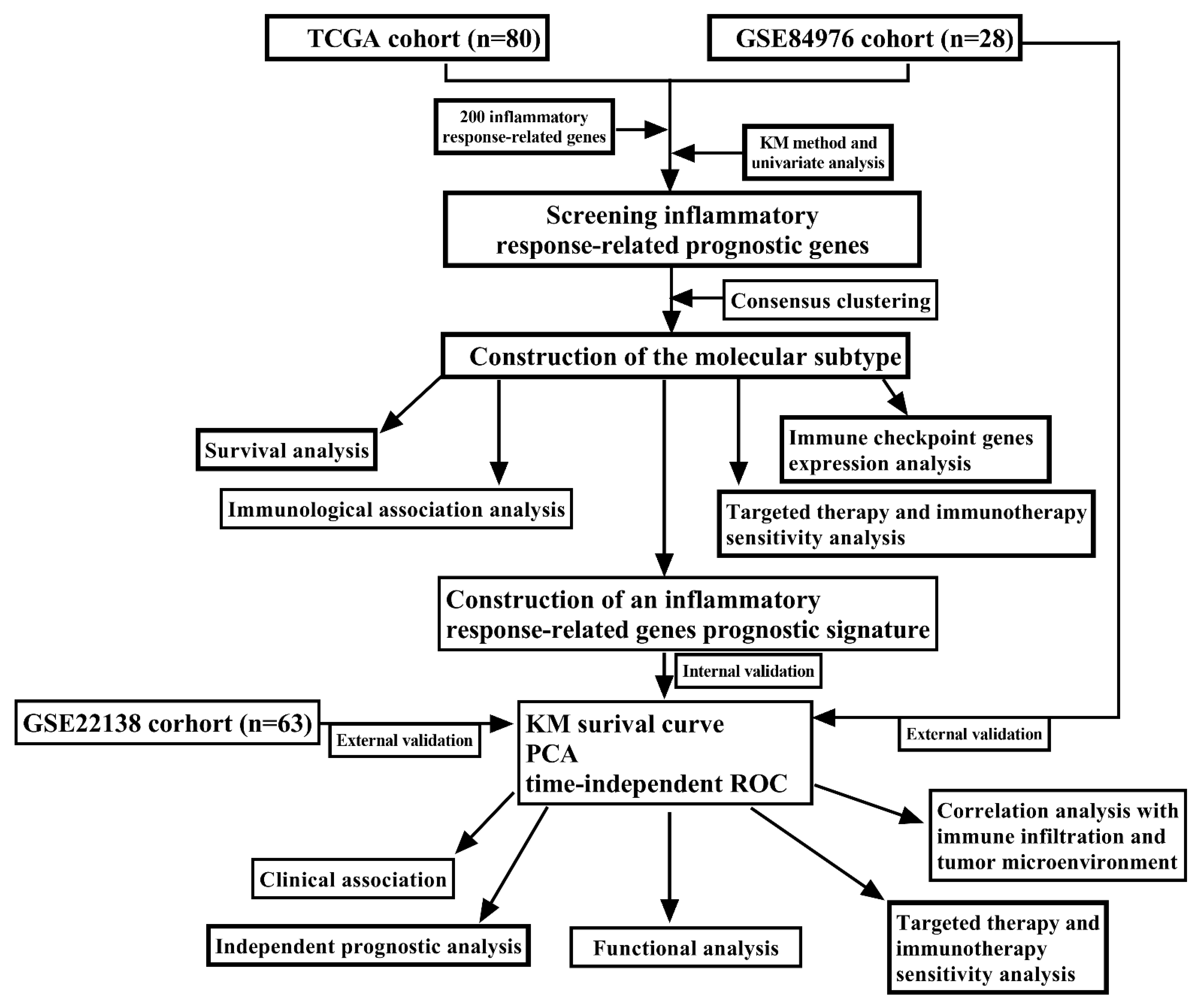
2.2. Molecular Typing Based on Inflammatory Response-Related Genes
2.3. Comparison of Immune Signature, Immune Cell Infiltration Level and Immune-Inflammation Microenvironment between the Molecular Subtypes
2.4. Sensitivity and Efficacy Analysis of Immunotherapy, Targeted Therapy and Chemotherapy between the Molecular Subtypes
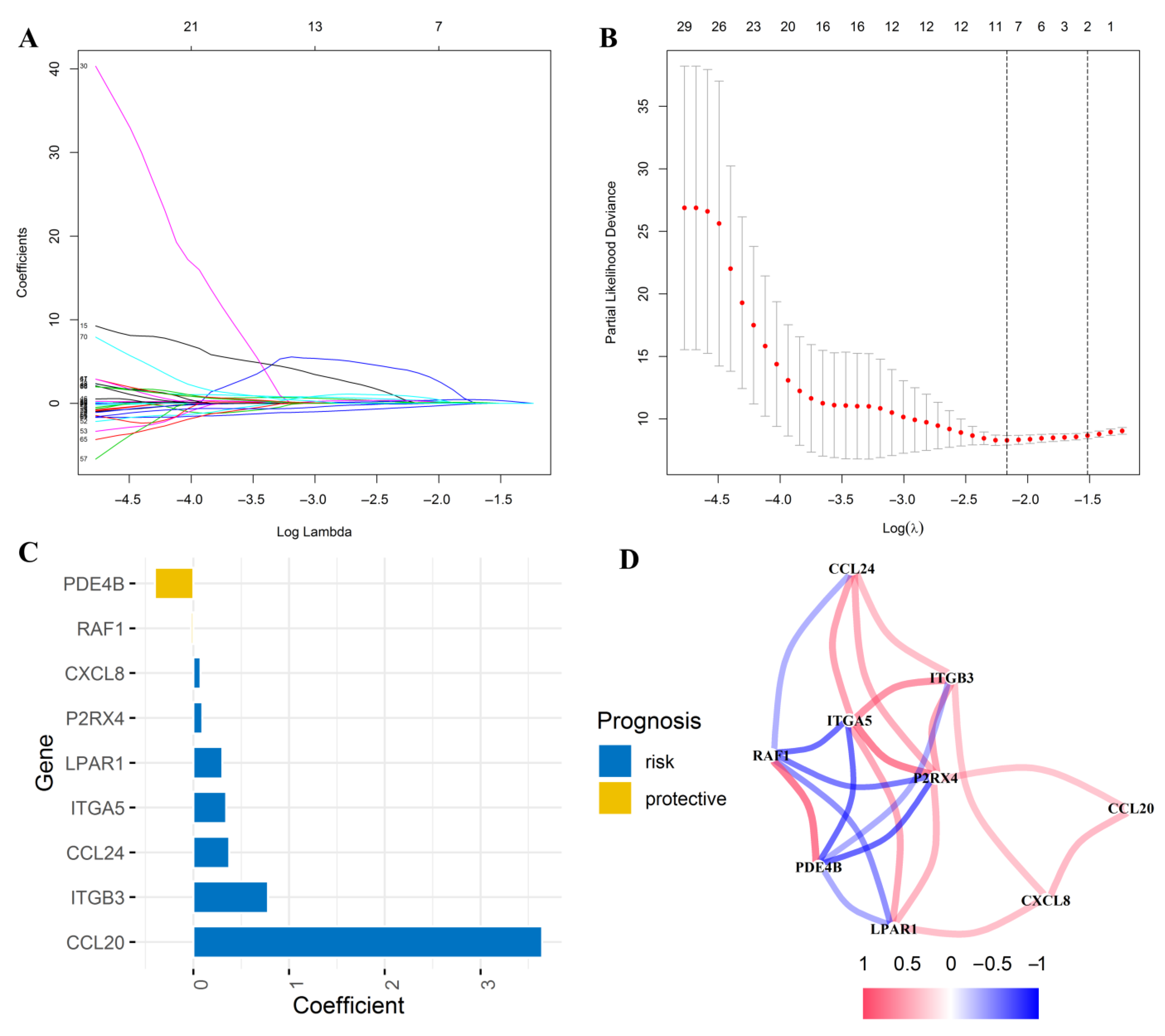
2.5. Construction of an Inflammatory Response-Related Genes Risk Signature in the TCGA Cohort
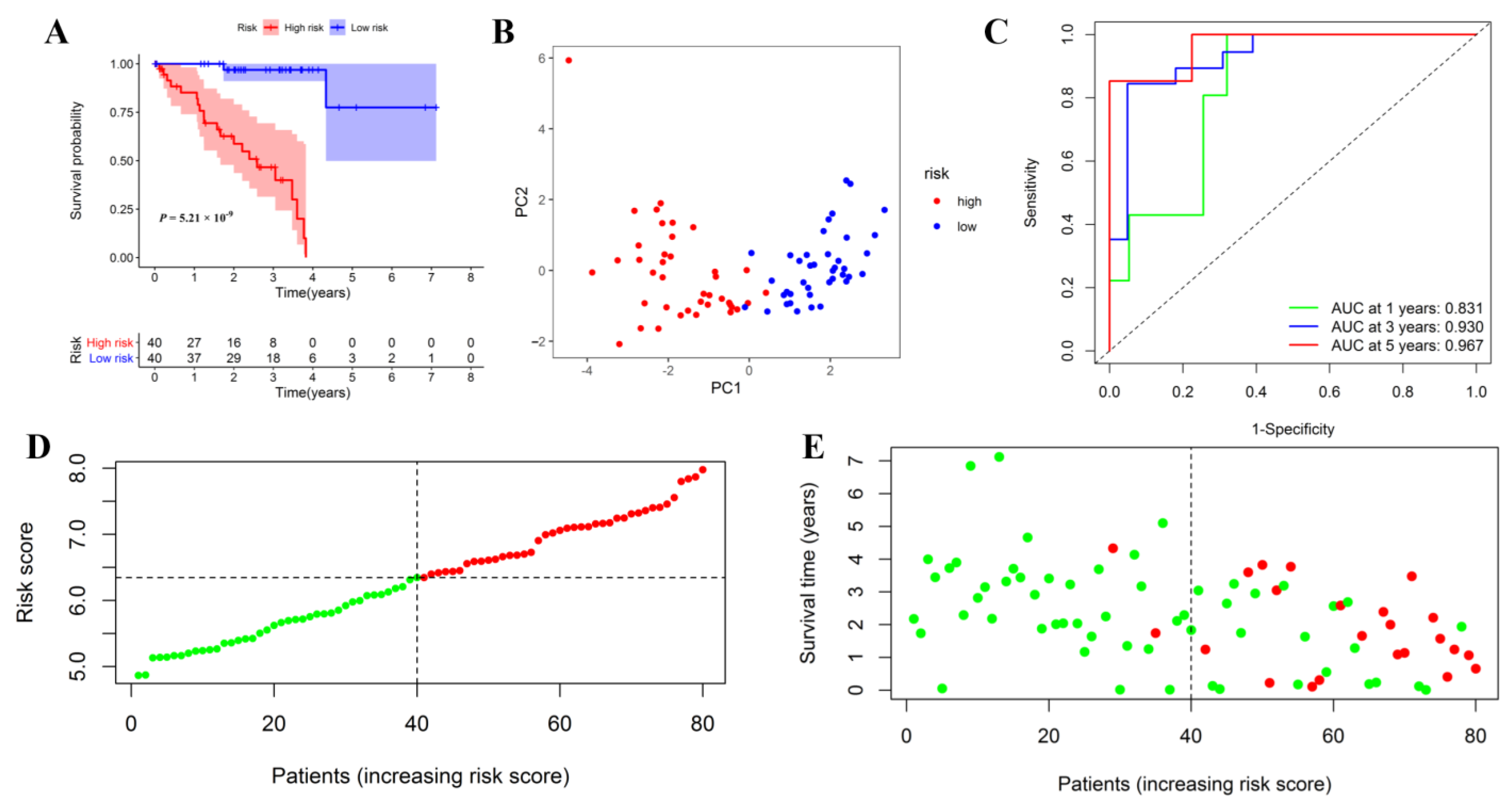
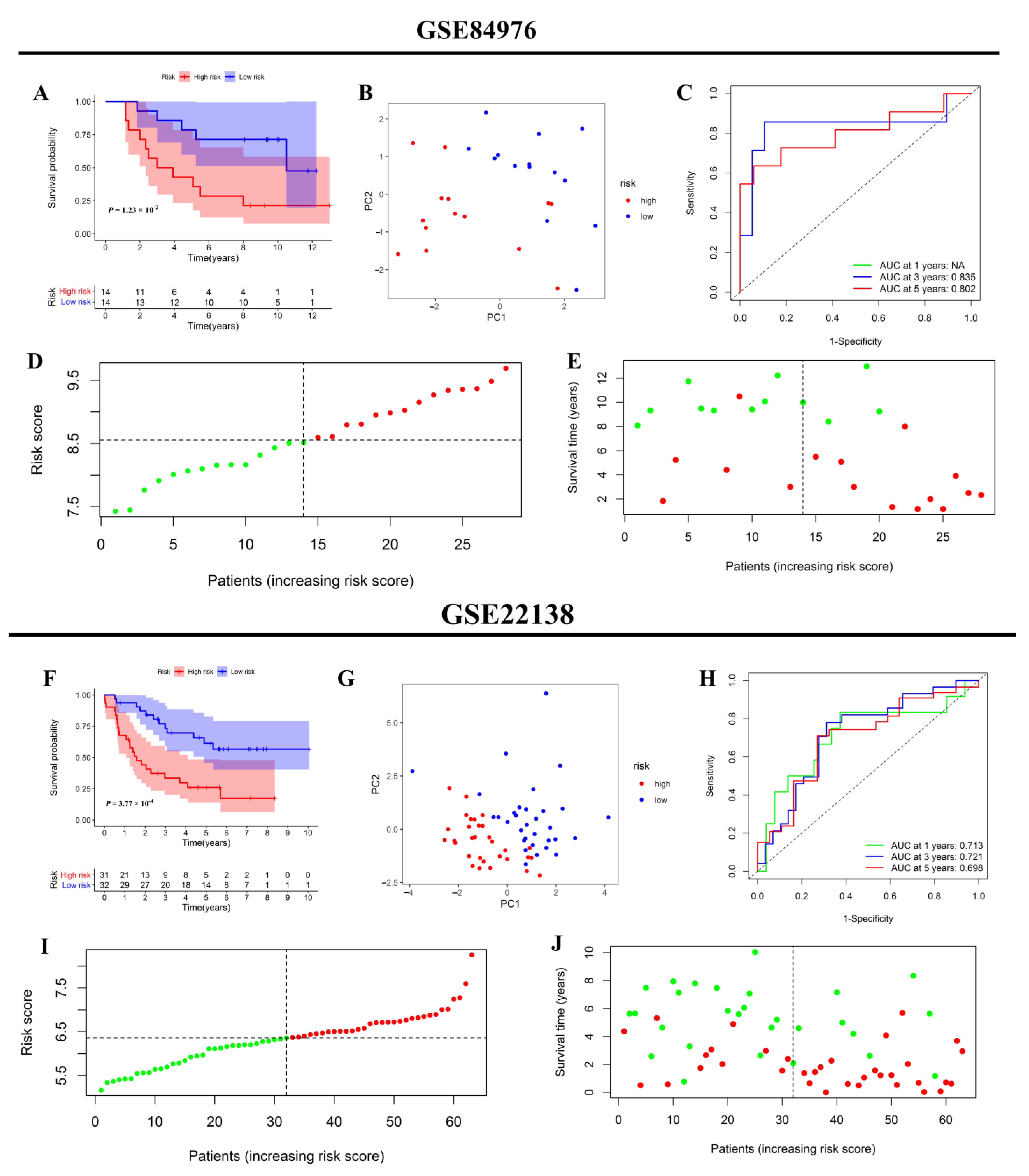
2.6. Internal and External Validation of the Inflammatory Response-Related Genes Prognostic Signature
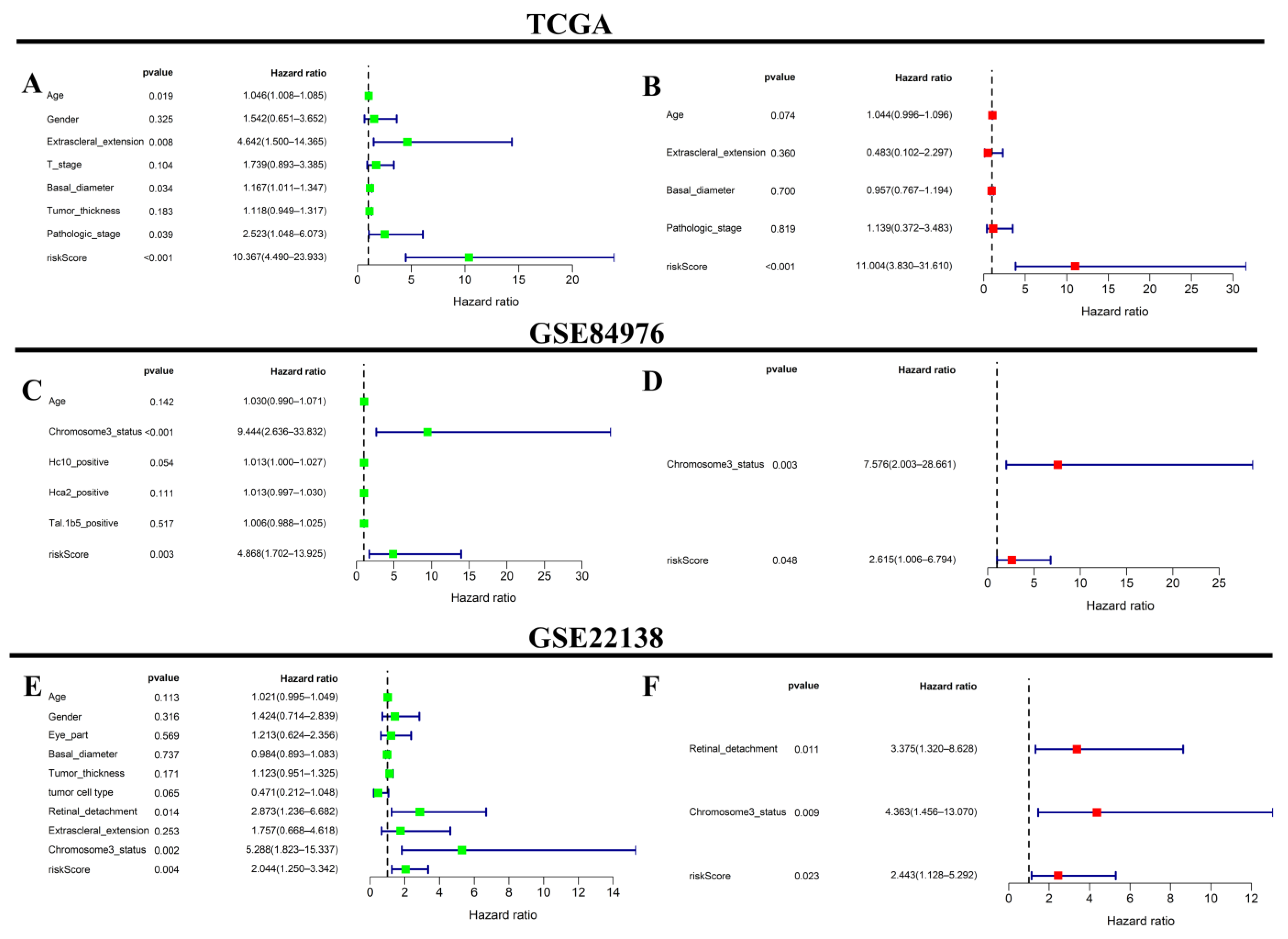
2.7. Independent Prognostic Analysis of the Prognostic Signature
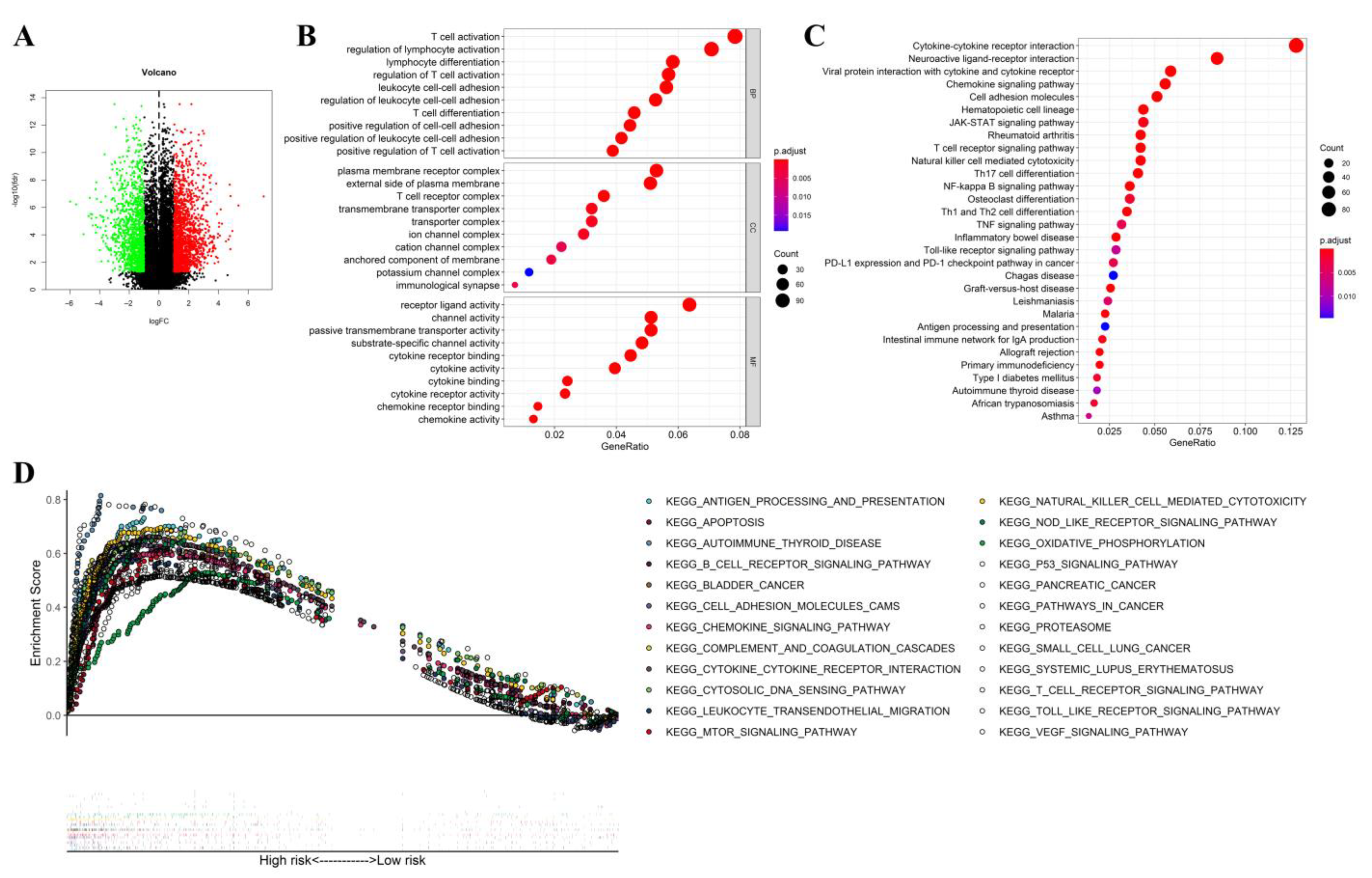
2.8. Functional Analysis
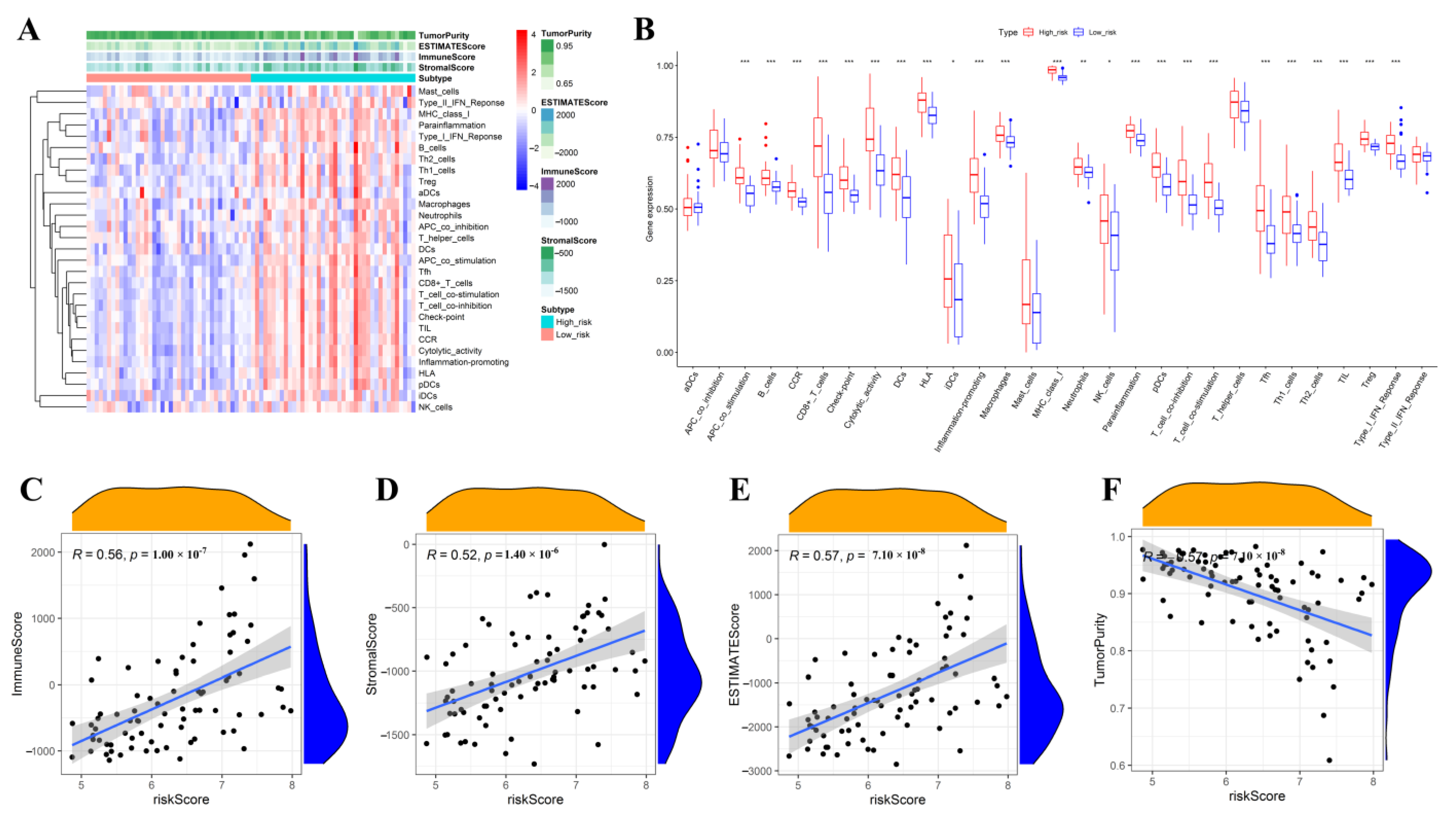
2.9. Correlations Analysis of the Riskscore with Immune Signature, Immune Cell Infiltration Level, and Tumor Microenvironment-Related Scores
2.10. Statistical Analysis
3. Results
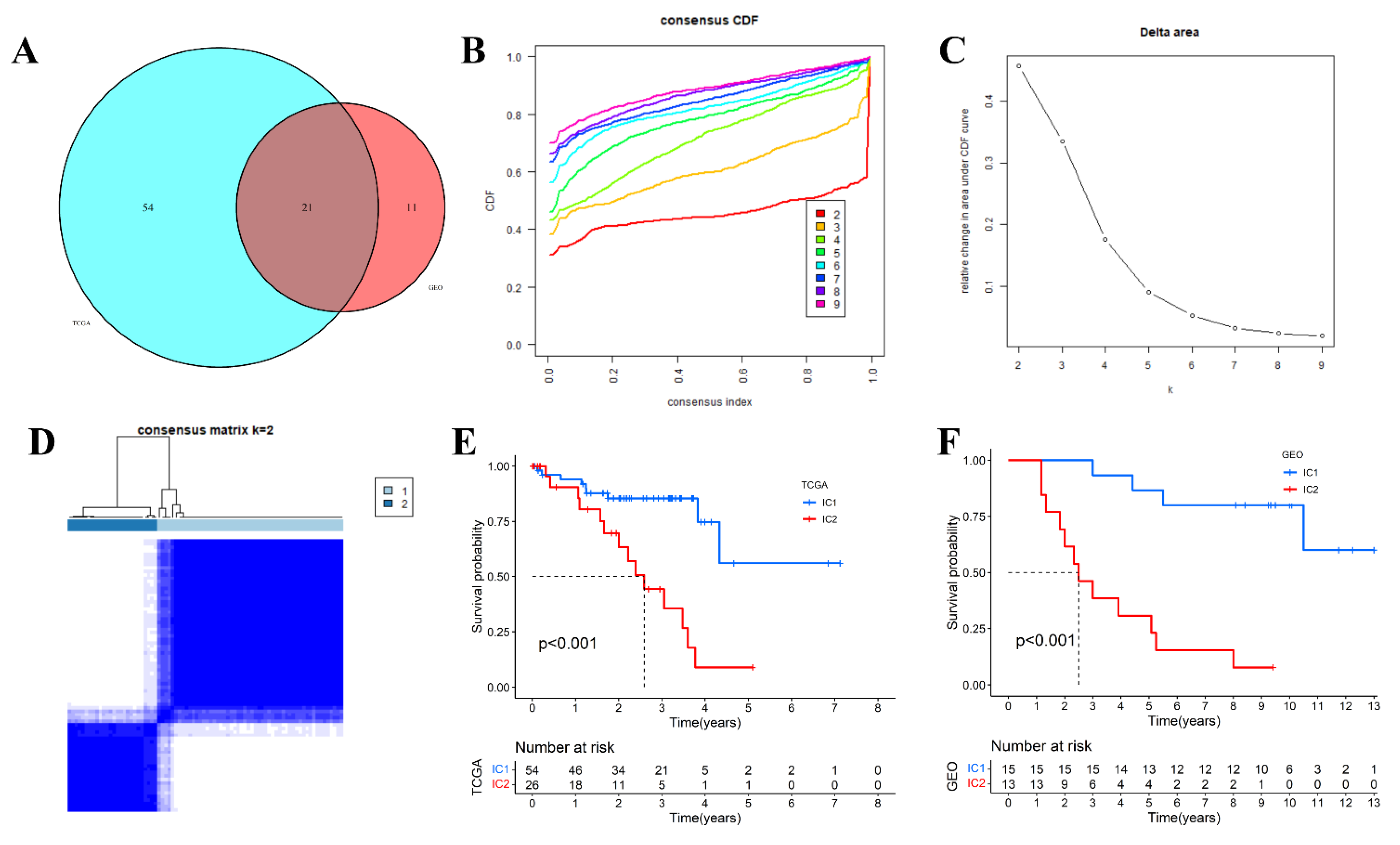
3.1. Molecular Typing Based on Inflammatory Response-Related Genes
3.2. Comparative Analysis of Immune Signature, Immune Cell Infiltration Level and Immune-Inflammation Microenvironment in Different Molecular Subtypes
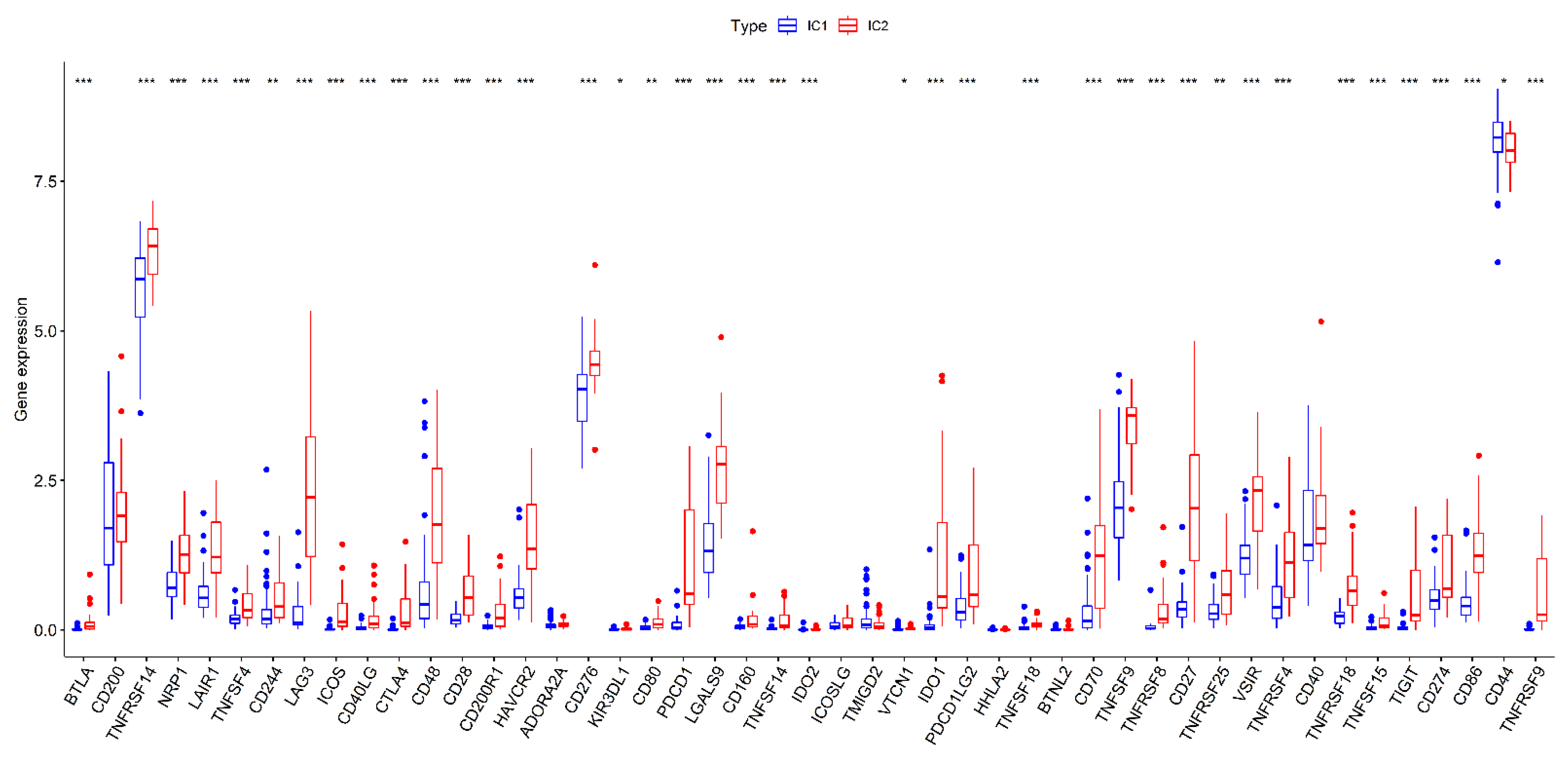
3.3. Comparison of Immune Checkpoint Genes Expression in Different Subtypes
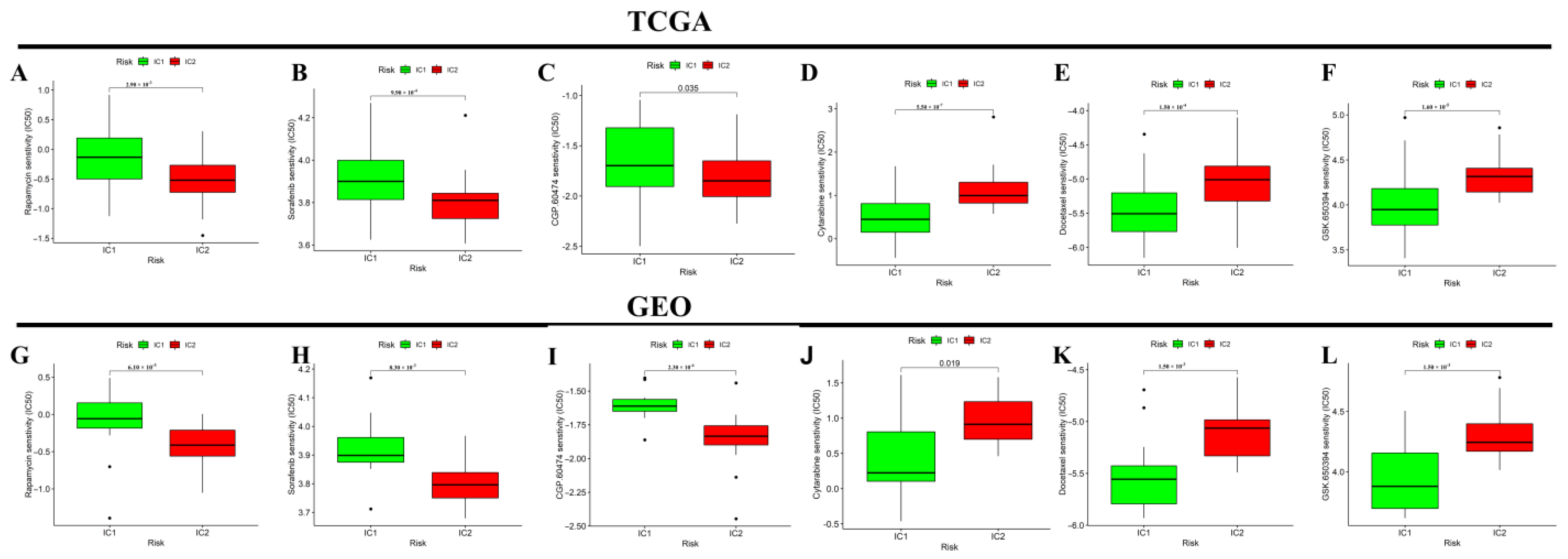
3.4. Differential Analysis of Targeted Therapy and Chemotherapy Sensitivity between Different Molecular Subtypes
3.5. Construction of an Inflammatory Response-Related Genes Prognostic Signature in the TCGA Cohort
3.6. Internal and External Validation of the Prognostic Signature
3.7. Correlation Analysis and Independent Prognostic Analysis between the Riskscore and Clinicopathological Features
3.8. Functional Analysis
3.9. Comparative Analysis of Immune Activation and Immune-Inflammation Microenvironment between the High- and Low-Risk Subgroups
3.10. Sensitivity and Efficacy Prediction of Immunotherapy and Targeted Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLaughlin, C.C.; Wu, X.-C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973–2013). Ocul. Oncol. Pathol. 2017, 4, 145–151. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal Melanoma: Trends in Incidence, Treatment, and Survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.; Shields, J. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef]
- Bolling, J.P.; Dagan, R.; Rutenberg, M.; Mamalui-Hunter, M.; Buskirk, S.J.; Heckman, M.G.; Hochwald, A.P.; Slopsema, R. Treatment of Uveal Melanoma with Radioactive Iodine 125 Implant Compared with Proton Beam Radiotherapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 6, 27–36. [Google Scholar] [CrossRef]
- Wei, A.Z.; Maniar, A.B.; Carvajal, R.D. New targeted and epigenetic therapeutic strategies for the treatment of uveal melanoma. Cancer Gene Ther. 2022, 29, 1819–1826. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, W.; Mao, Y. Recent Advances and Challenges in Uveal Melanoma Immunotherapy. Cancers 2022, 14, 3094. [Google Scholar] [CrossRef]
- Castet, F.; Garcia-Mulero, S.; Sanz-Pamplona, R.; Cuellar, A.; Casanovas, O.; Caminal, J.M.; Piulats, J.M. Uveal Melanoma, Angiogenesis and Immunotherapy, Is There Any Hope? Cancers 2019, 11, 834. [Google Scholar] [CrossRef]
- Rothermel, L.D.; Sarnaik, A.A.; Khushalani, N.I.; Sondak, V.K. Current Immunotherapy Practices in Melanoma. Surg. Oncol. Clin. North Am. 2019, 28, 403–418. [Google Scholar] [CrossRef]
- Martinez-Perez, D.; Viñal, D.; Solares, I.; Espinosa, E.; Feliu, J. Gp-100 as a Novel Therapeutic Target in Uveal Melanoma. Cancers 2021, 13, 5968. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Prim. 2020, 6, 24. [Google Scholar] [CrossRef]
- Zhang, F.; Deng, Y.; Wang, D.; Wang, S. Construction and validation of a pyroptosis-related gene signature associated with the tumor microenvironment in uveal melanoma. Sci. Rep. 2022, 12, 1640. [Google Scholar] [CrossRef]
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliç, E. Uveal melanoma: Towards a molecular understanding. Prog. Retin. Eye Res. 2019, 75, 100800. [Google Scholar] [CrossRef]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58, 10. [Google Scholar] [CrossRef]
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory breast cancer biology: The tumour microenvironment is key. Nat. Rev. Cancer 2018, 18, 485–499. [Google Scholar] [CrossRef]
- Amara, S.; Tiriveedhi, V. Inflammatory role of high salt level in tumor microenvironment (Review). Int. J. Oncol. 2017, 50, 1477–1481. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Pas de Deux: Control of Anti-tumor Immunity by Cancer-Associated Inflammation. Immunity 2019, 51, 15–26. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, F.; Yu, X.; Huo, C.-L.; Sun, Z.-G.; Wang, S. Prognostic Value of Preoperative Systemic Inflammatory Biomarkers In Patients With Gallbladder Cancer And The Establishment Of A Nomogram. Cancer Manag. Res. 2019, 11, 9025–9035. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Y.; Yu, X.; Zhang, X.-W.; Huo, C.-L.; Sun, Z.-G.; Chang, H. Prognostic significance of preoperative systemic inflammatory biomarkers in patients with hepatocellular carcinoma after microwave ablation and establishment of a nomogram. Sci. Rep. 2021, 11, 13814. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, J.; Zhu, X.; Liang, J. Cytokines concentrations in aqueous humor of eyes with uveal melanoma. Medicine 2019, 98, e14030. [Google Scholar] [CrossRef]
- Midena, E.; Parrozzani, R.; Midena, G.; Trainiti, S.; Marchione, G.; Cosmo, E.; Londei, D.; Frizziero, L. In vivo intraocular biomarkers. Medicine 2020, 99, e22091. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Trevino, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Breazzano, M.P.; Milam, R.W.; Batson, S.A.; Johnson, D.B.; Daniels, A.B. Immunotherapy for Uveal Melanoma. Int. Ophthalmol. Clin. 2017, 57, 29–39. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.; Jager, M.J. Uveal Melanoma: The Inflammatory Microenvironment. J. Innate Immun. 2012, 4, 454–462. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.G.; Jager, M.J. Inflammation in uveal melanoma. Eye 2012, 27, 217–223. [Google Scholar] [CrossRef]
- Krishna, Y.; McCarthy, C.; Kalirai, H.; Coupland, S.E. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum. Pathol. 2017, 66, 159–166. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Triozzi, P.L.; Schoenfield, L.; Plesec, T.; Saunthararajah, Y.; Tubbs, R.R.; Singh, A.D. Molecular profiling of primary uveal melanomas with tumor-infiltrating lymphocytes. Oncoimmunology 2014, 8, e947169. [Google Scholar] [CrossRef]
- Jin, L.; Liu, W.-R.; Tian, M.-X.; Jiang, X.-F.; Wang, H.; Zhou, P.-Y.; Ding, Z.-B.; Peng, Y.-F.; Dai, Z.; Qiu, S.-J.; et al. CCL24 contributes to HCC malignancy via RhoB- VEGFA-VEGFR2 angiogenesis pathway and indicates poor prognosis. Oncotarget 2016, 8, 5135–5148. [Google Scholar] [CrossRef]
- Lim, S.-J. CCL24 Signaling in the Tumor Microenvironment. Single Mol. Single Cell Seq. 2021, 1302, 91–98. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2016, 387, 61–68. [Google Scholar] [CrossRef]
- Kuninty, P.R.; Bansal, R.; De Geus, S.W.L.; Mardhian, D.F.; Schnittert, J.; van Baarlen, J.; Storm, G.; Bijlsma, M.F.; van Laarhoven, H.W.; Metselaar, J.M.; et al. ITGA5 inhibition in pancreatic stellate cells attenuates desmoplasia and potentiates efficacy of chemotherapy in pancreatic cancer. Sci. Adv. 2019, 5, eaax2770. [Google Scholar] [CrossRef]
- Zhou, C.; Shen, Y.; Wei, Z.; Shen, Z.; Tang, M.; Shen, Y.; Deng, H. ITGA5 is an independent prognostic biomarker and potential therapeutic target for laryngeal squamous cell carcinoma. J. Clin. Lab. Anal. 2022, 36, e24228. [Google Scholar] [CrossRef]
- Fuentes, P.; Sesé, M.; Guijarro, P.J.; Emperador, M.; Sánchez-Redondo, S.; Peinado, H.; Hümmer, S.; Cajal, S.R.Y. ITGB3-mediated uptake of small extracellular vesicles facilitates intercellular communication in breast cancer cells. Nat. Commun. 2020, 11, 4261. [Google Scholar] [CrossRef]
- Ma, X.; Feng, J.; Lu, M.; Tang, W.; Han, J.; Luo, X.; Zhao, Q.; Yang, L. microRNA-501-5p promotes cell proliferation and migration in gastric cancer by downregulating LPAR1. J. Cell. Biochem. 2019, 121, 1911–1922. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, D.; Yang, S.; Zhang, X.; Wang, J.; Liu, Y.; Sun, Y.; Lu, Y.; Yang, K. LPAR1, Correlated with Immune Infiltrates, Is a Potential Prognostic Biomarker in Prostate Cancer. Front. Oncol. 2020, 10, 846. [Google Scholar] [CrossRef]
- Zhao, P.; Yun, Q.; Li, A.; Li, R.; Yan, Y.; Wang, Y.; Sun, H.; Damirin, A. LPA3 is a precise therapeutic target and potential biomarker for ovarian cancer. Med. Oncol. 2022, 39, 17. [Google Scholar] [CrossRef]
- Alharbi, A.F.; Parrington, J. The role of genetic polymorphisms in endolysosomal ion channels TPC2 and P2RX4 in cancer pathogenesis, prognosis, and diagnosis: A genetic association in the UK Biobank. NPJ Genom. Med. 2021, 6, 58. [Google Scholar] [CrossRef]
- Kashiwagi, E.; Shiota, M.; Yokomizo, A.; Itsumi, M.; Inokuchi, J.; Uchiumi, T.; Naito, S. Downregulation of phosphodiesterase 4B (PDE4B) activates protein kinase A and contributes to the progression of prostate cancer. Prostate 2011, 72, 741–751. [Google Scholar] [CrossRef]
- Kim, D.U.; Kwak, B.; Kim, S.-W. Phosphodiesterase 4B is an effective therapeutic target in colorectal cancer. Biochem. Biophys. Res. Commun. 2018, 508, 825–831. [Google Scholar] [CrossRef]
- Ehrenreiter, K.; Kern, F.; Velamoor, V.; Meissl, K.; Galabova-Kovacs, G.; Sibilia, M.; Baccarini, M. Raf-1 Addiction in Ras-Induced Skin Carcinogenesis. Cancer Cell 2009, 16, 149–160. [Google Scholar] [CrossRef]
- Jeric, I.; Maurer, G.; Cavallo, A.L.; Raguz, J.; Desideri, E.; Tarkowski, B.; Parrini, M.; Fischer, I.; Zatloukal, K.; Baccarini, M. A cell-autonomous tumour suppressor role of RAF1 in hepatocarcinogenesis. Nat. Commun. 2016, 7, 13781. [Google Scholar] [CrossRef]


| Parameters | Group | Riskscore | ||
|---|---|---|---|---|
| N | Mean | p Value | ||
| Age (years) | <60 | 36 | 6.158 | 0.128 |
| ≥60 | 44 | 6.441 | ||
| Gender | Male | 45 | 6.329 | 0.853 |
| Female | 35 | 6.294 | ||
| Basal diameter | Low | 39 | 6.101 | 0.034 |
| High | 40 | 6.490 | ||
| Tumor thickness | Low | 42 | 6.360 | 0.604 |
| High | 38 | 6.263 | ||
| T stage | II | 14 | 5.896 | 0.019 |
| III | 32 | 6.268 | ||
| IV | 34 | 6.528 | ||
| Pathologic stage | II | 39 | 6.111 | 0.024 |
| III | 37 | 6.448 | ||
| IV | 4 | 7.049 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Deng, Y.; Wang, D.; Wang, S. Construction and Verification of the Molecular Subtype and a Novel Prognostic Signature Based on Inflammatory Response-Related Genes in Uveal Melanoma. J. Clin. Med. 2023, 12, 861. https://doi.org/10.3390/jcm12030861
Zhang F, Deng Y, Wang D, Wang S. Construction and Verification of the Molecular Subtype and a Novel Prognostic Signature Based on Inflammatory Response-Related Genes in Uveal Melanoma. Journal of Clinical Medicine. 2023; 12(3):861. https://doi.org/10.3390/jcm12030861
Chicago/Turabian StyleZhang, Feng, Yan Deng, Dong Wang, and Shuai Wang. 2023. "Construction and Verification of the Molecular Subtype and a Novel Prognostic Signature Based on Inflammatory Response-Related Genes in Uveal Melanoma" Journal of Clinical Medicine 12, no. 3: 861. https://doi.org/10.3390/jcm12030861
APA StyleZhang, F., Deng, Y., Wang, D., & Wang, S. (2023). Construction and Verification of the Molecular Subtype and a Novel Prognostic Signature Based on Inflammatory Response-Related Genes in Uveal Melanoma. Journal of Clinical Medicine, 12(3), 861. https://doi.org/10.3390/jcm12030861





