Epidemiology and Clinical Burden of Meningococcal Disease in France: Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Research Question
2.3. Searching Strategy
2.4. Categorization Strategy
2.5. Data Extraction
3. Results
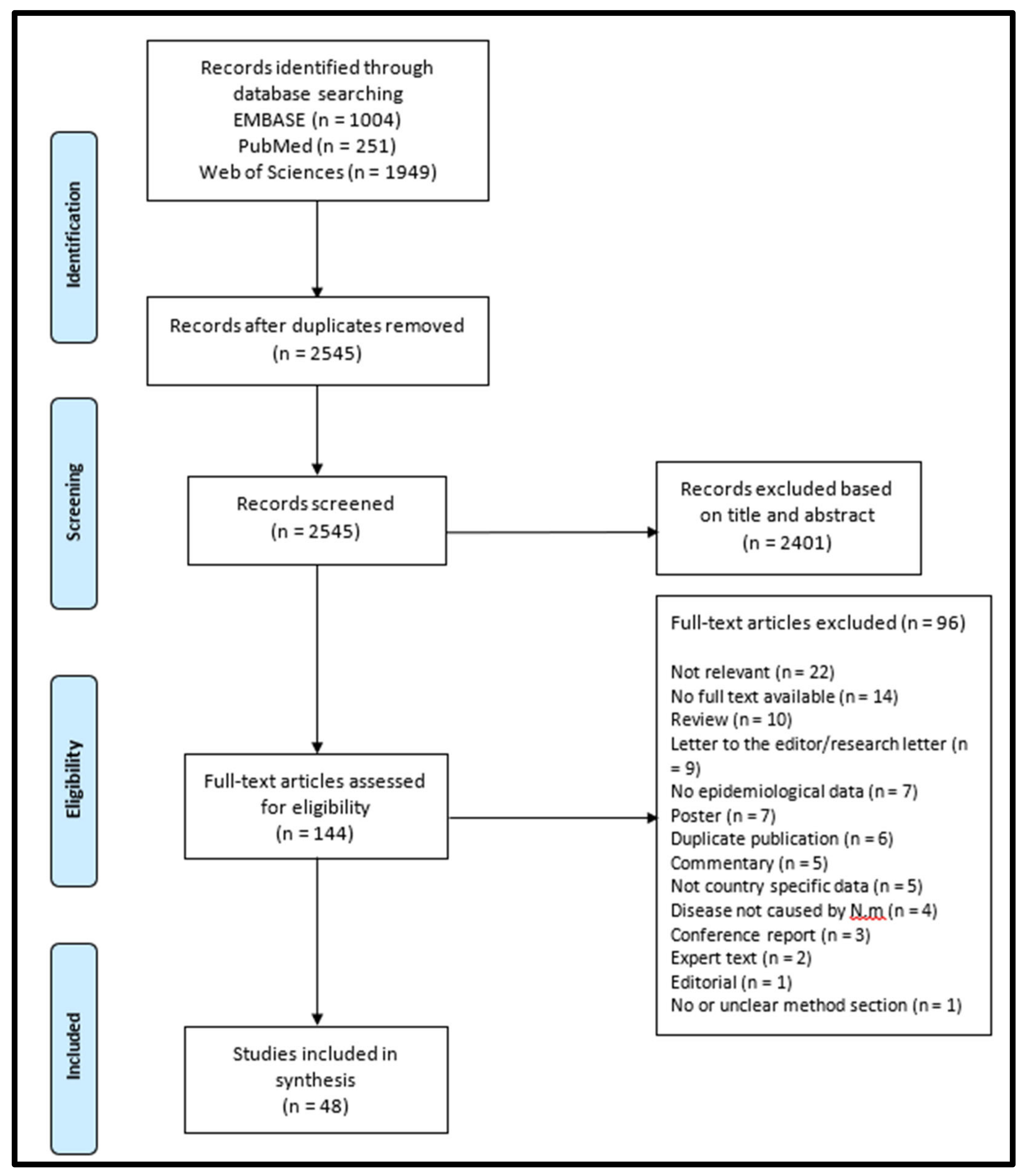
3.1. Study Selection Process
3.2. Characteristics of Publications Included
3.2.1. Data sources and Scope of Included Studies
3.2.2. Outcomes of Included Studies
3.2.3. Quality of Included Studies
3.3. Results of Included Studies
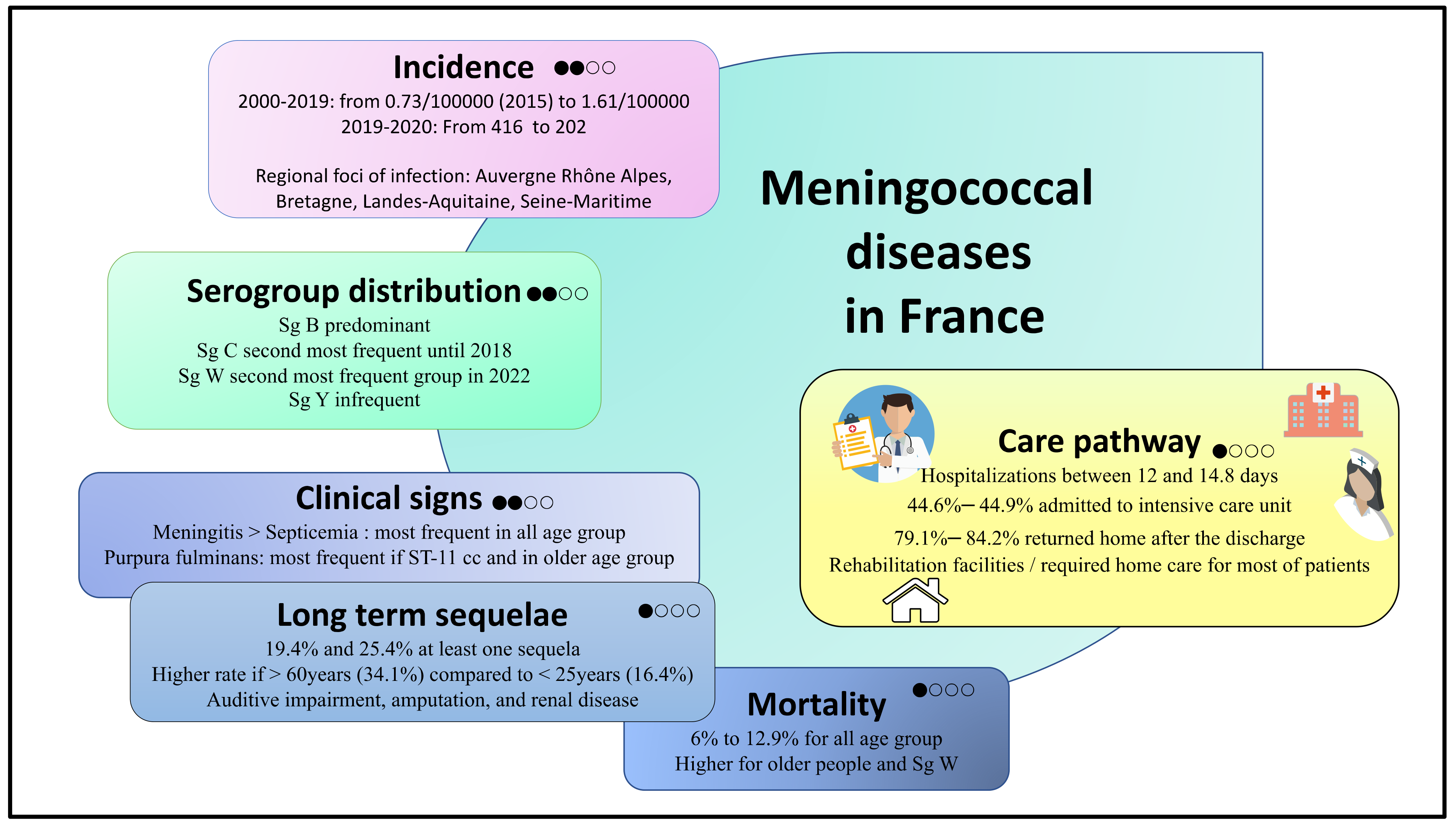
3.3.1. Epidemiology of IMD in France
3.3.2. The Clinical Burden of IMD in France
3.3.3. Care pathways of Patients with IMD in France
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollingshead, S.; Tang, C.M. An Overview of Neisseria meningitidis. Methods Mol. Biol. 2019, 1969, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.R.; Campbell, H.; Bettinger, J.A.; Harrison, L.H.; Marshall, H.S.; Martinon-Torres, F.; Safadi, M.A.; Shao, Z.; Zhu, B.; von Gottberg, A.; et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 2020, 81, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Alarcon, P.; Carlos, J.; Caugant, D.A.; Christensen, H.; Debbag, R.; De Wals, P.; Echaniz-Aviles, G.; Findlow, J.; Head, C.; et al. The Global Meningococcal Initiative: Global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert. Rev. Vaccines 2017, 16, 313–328. [Google Scholar] [CrossRef]
- ECDC. Disease data from ECDC Surveillance Atlas for Meningococcal Disease. Available online: https://www.ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas (accessed on 15 August 2022).
- Whittaker, R.; Dias, J.G.; Ramliden, M.; Kodmon, C.; Economopoulou, A.; Beer, N.; Pastore Celentano, L.; disease, E.n.m.f.i.m. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine 2017, 35, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Ninis, N.; Perera, R.; Mayon-White, R.; Phillips, C.; Bailey, L.; Harnden, A.; Mant, D.; Levin, M. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006, 367, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Martinon-Torres, F. Deciphering the Burden of Meningococcal Disease: Conventional and Under-recognized Elements. J. Adolesc. Health 2016, 59, S12–S20. [Google Scholar] [CrossRef]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef]
- Shen, J.; Begum, N.; Ruiz-Garcia, Y.; Martinon-Torres, F.; Bekkat-Berkani, R.; Meszaros, K. Range of invasive meningococcal disease sequelae and health economic application—A systematic and clinical review. BMC Public Health 2022, 22, 1078. [Google Scholar] [CrossRef]
- Olbrich, K.J.; Muller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic Review of Invasive Meningococcal Disease: Sequelae and Quality of Life Impact on Patients and Their Caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef]
- Greenwood, B.; Sow, S.; Preziosi, M.P. Defeating meningitis by 2030—An ambitious target. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1099–1101. [Google Scholar] [CrossRef]
- INSTRUCTION N° DGS/SP/2018/163 du 27 Juillet 2018 Relative à la Prophylaxie des Infections Invasives à Méningocoque Santé. Available online: https://www.legifrance.gouv.fr/circulaire/id/43909 (accessed on 15 August 2022).
- Sucharew, H.; Macaluso, M. Progress Notes: Methods for Research Evidence Synthesis: The Scoping Review Approach. J. Hosp. Med. 2019, 14, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Deghmane, A.E.; Parent du Chatelet, I.; Szatanik, M.; Hong, E.; Ruckly, C.; Giorgini, D.; Levy-Bruhl, D.; Alonso, J.M.; Taha, M.K. Emergence of new virulent Neisseria meningitidis serogroup C sequence type 11 isolates in France. J. Infect. Dis. 2010, 202, 247–250. [Google Scholar] [CrossRef]
- Parent du Chatelet, I.; Deghmane, A.E.; Antona, D.; Hong, E.; Fonteneau, L.; Taha, M.K.; Levy-Bruhl, D. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006-2015. J. Infect. 2017, 74, 564–574. [Google Scholar] [CrossRef]
- Deghmane, A.E.; Taha, M.K. Changes in Invasive Neisseria meningitidis and Haemophilus influenzae Infections in France during the COVID-19 Pandemic. Microorganisms 2022, 10, 907. [Google Scholar] [CrossRef]
- Thabuis, A.; Tararbit, K.; Taha, M.K.; Dejour-Salamanca, D.; Ronin, V.; Parent du Chatelet, I.; Spaccaferri, G. Community outbreak of serogroup B invasive meningococcal disease in Beaujolais, France, February to June 2016: From alert to targeted vaccination. Eurosurveillance 2018, 23, 1700590. [Google Scholar] [CrossRef]
- Pivette, M.; Taha, M.K.; Barret, A.S.; Polard, E.; Hautier, M.B.; Dufour, J.B.; Faisant, M.; King, L.A.; Antona, D.; Levy-Bruhl, D.; et al. Targeted vaccination campaigns of teenagers after two clusters of B invasive meningococcal disease in Brittany, France, 2017. BMC Public Health 2020, 20, 1382. [Google Scholar] [CrossRef]
- Delisle, E.; Larrieu, S.; Simoes, J.; Laylle, N.; De Pommerol, M.; Taha, M.K.; Termignon, J.L.; Parent du Chatelet, I. Community outbreak of group B meningococcal disease in southwest France--December 2008 to September 2009. Eurosurveillance 2010, 15, 19665. [Google Scholar] [CrossRef]
- Caron, F.; du Chatelet, I.P.; Leroy, J.P.; Ruckly, C.; Blanchard, M.; Bohic, N.; Massy, N.; Morer, I.; Floret, D.; Delbos, V.; et al. From tailor-made to ready-to-wear meningococcal B vaccines: Longitudinal study of a clonal meningococcal B outbreak. Lancet Infect. Dis. 2011, 11, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Parent du Chatelet, I.; Taha, M.K.; Sesboue, C.; Rouaud, P.; Perrocheau, A.; Levy-Bruhl, D. [Increased incidence of invasive meningococcal disease in Seine-Maritime. The evolving epidemiology due to the B:14:P1.7,16 strain]. Arch. Pediatr. 2007, 14, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Zarantonelli, M.L.; Lancellotti, M.; Deghmane, A.E.; Giorgini, D.; Hong, E.; Ruckly, C.; Alonso, J.M.; Taha, M.K. Hyperinvasive genotypes of Neisseria meningitidis in France. Clin. Microbiol. Infect. 2008, 14, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Levy-Bruhl, D.; Fonteneau, L.; Vaux, S.; Barret, A.S.; Antona, D.; Bonmarin, I.; Che, D.; Quelet, S.; Coignard, B. Assessment of the impact of the extension of vaccination mandates on vaccine coverage after 1 year, France, 2019. Eurosurveillance 2019, 24, 1900301. [Google Scholar] [CrossRef]
- Taha, M.K.; Deghmane, A.E. Impact of COVID-19 pandemic and the lockdown on invasive meningococcal disease. BMC Res. Notes 2020, 13, 399. [Google Scholar] [CrossRef]
- Taha, M.K.; Giorgini, D.; Ducos-Galand, M.; Alonso, J.M. Continuing diversification of Neisseria meningitidis W135 as a primary cause of meningococcal disease after emergence of the serogroup in 2000. J. Clin. Microbiol. 2004, 42, 4158–4163. [Google Scholar] [CrossRef]
- Hong, E.; Barret, A.S.; Terrade, A.; Denizon, M.; Antona, D.; Aouiti-Trabelsi, M.; Deghmane, A.E.; Parent du Chatelet, I.; Levy-Bruhl, D.; Taha, M.K. Clonal replacement and expansion among invasive meningococcal isolates of serogroup W in France. J. Infect. 2018, 76, 149–158. [Google Scholar] [CrossRef]
- Rosain, J.; Hong, E.; Fieschi, C.; Martins, P.V.; El Sissy, C.; Deghmane, A.E.; Ouachee, M.; Thomas, C.; Launay, D.; de Pontual, L.; et al. Strains Responsible for Invasive Meningococcal Disease in Patients With Terminal Complement Pathway Deficiencies. J. Infect. Dis. 2017, 215, 1331–1338. [Google Scholar] [CrossRef]
- Faye, A.; Mariani-Kurkjian, P.; Taha, M.K.; Louzeau, C.; Bingen, E.; Bourrillon, A. Clinical aspects and outcome of meningococcal disease due to Neisseria meningitidis of serogroup W135 in 5 children. Arch. Pediatr. 2005, 12, 291–294. [Google Scholar] [CrossRef]
- Dubos, F.; Marechal, I.; Tilmont, B.; Courouble, C.; Leclerc, F.; Martinot, A. Incidence of invasive meningococcal diseases in children in Northern France: Usefulness and limits of the discharge code database for correcting compulsory notification data. Arch. Pediatr. 2009, 16, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Gaschignard, J.; Levy, C.; Deghmane, A.E.; Dubos, F.; Muszlak, M.; Cohen, R.; Bingen, E.; Faye, A.; Taha, M.K. Invasive serogroup w meningococcal disease in children: A national survey from 2001 to 2008 in France. Pediatr. Infect. Dis. J. 2013, 32, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Floret, D. Pediatric deaths due to community-acquired bacterial infections. A French survey in Pediatric Intensive Care Units. Arch. Pédiatrie 2001, 8, 705–711. [Google Scholar] [CrossRef]
- Lorton, F.; Chalumeau, M.; Martinot, A.; Assathiany, R.; Roue, J.M.; Bourgoin, P.; Chantreuil, J.; Boussicault, G.; Gaillot, T.; Saulnier, J.P.; et al. Epidemiology of Community-Onset Severe Bacterial Infections in Children and Its Evolution: A Population-Based Study in France. Pediatr. Crit Care Med. 2020, 21, e325–e332. [Google Scholar] [CrossRef]
- Bilal, A.; Taha, M.K.; Caeymaex, L.; Cohen, R.; Levy, C.; Durrmeyer, X.; Groupe des Pediatres et microbiologistes de l’Observatoire National des, M.; Members of the National Reference Center for, M. Neonatal Meningococcal Meningitis in France from 2001 to 2013. Pediatr. Infect. Dis. J. 2016, 35, 1270–1272. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Taha, M.K.; Weil Olivier, C.; Quinet, B.; Lecuyer, A.; Alonso, J.M.; Aujard, Y.; Bingen, E.; Cohen, R.; Groupe des pédiatres et microbiologistes de l’Observatoire National des Méningites. Association of meningococcal phenotypes and genotypes with clinical characteristics and mortality of meningitis in children. Pediatr. Infect. Dis. J. 2010, 29, 618–623. [Google Scholar] [CrossRef]
- Levy, C.; Bingen, E.; Aujard, Y.; Boucherat, M.; Floret, D.; Gendrel, D.; Cohen, R.; Groupe des pediatres et microbiologistes de l’Observatoire National des, M. Surveillance network of bacterial meningitis in children, 7 years of survey in France. Arch Pediatr. 2008, 15 (Suppl. 3), S99–S104. [Google Scholar] [CrossRef]
- Duval, X.; Taha, M.K.; Lamaury, I.; Escaut, L.; Gueit, I.; Manchon, P.; Tubiana, S.; Hoen, B.; group, C.s. One-Year Sequelae and Quality of Life in Adults with Meningococcal Meningitis: Lessons from the COMBAT Multicentre Prospective Study. Adv. Ther. 2022, 39, 3031–3041. [Google Scholar] [CrossRef]
- Bassi, C.; Taha, M.K.; Merle, C.; Hong, E.; Levy-Bruhl, D.; Barret, A.S.; Mounchetrou Njoya, I. A cluster of invasive meningococcal disease (IMD) caused by Neisseria meningitidis serogroup W among university students, France, February to May 2017. Eurosurveillance 2017, 22, 30574. [Google Scholar] [CrossRef]
- Deghmane, A.E.; Haeghebaert, S.; Hong, E.; Jousset, A.; Barret, A.S.; Taha, M.K. Emergence of new genetic lineage, ST-9316, of Neisseria meningitidis group W in Hauts-de-France region, France 2013-2018. J. Infect. 2020, 80, 519–526. [Google Scholar] [CrossRef]
- Huang, L.; Fievez, S.; Goguillot, M.; Marie, L.; Benard, S.; Elkaim, A.; Tin Tin Htar, M. A database study of clinical and economic burden of invasive meningococcal disease in France. PLoS ONE 2022, 17, e0267786. [Google Scholar] [CrossRef] [PubMed]
- Perrocheau, A.; Taha, M.K.; Levy-Bruhl, D. Epidemiology of invasive meningococcal disease in France in 2003. Eurosurveillance 2005, 10, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Taha, M.K.; Weill Olivier, C.; Quinet, B.; Lecuyer, A.; Alonso, J.M.; Cohen, R.; Bingen, E.; Groupe des pédiatres et microbiologistes de l’Observatoire National des Méningites. Characteristics of meningococcal meningitis in children in France. Arch. Pediatr. 2008, 15 (Suppl. 3), S105-10. [Google Scholar] [CrossRef]
- Weil-Olivier, C.; Taha, M.K.; Bouee, S.; Emery, C.; Loncle-Provot, V.; Nachbaur, G.; Beck, E.; Pribil, C. Care pathways in invasive meningococcal disease: A retrospective analysis of the French national public health insurance database. Hum. Vaccin. Immunother. 2022, 18, 2021764. [Google Scholar] [CrossRef]
- Contou, D.; Sonneville, R.; Canoui-Poitrine, F.; Colin, G.; Coudroy, R.; Pene, F.; Tadie, J.M.; Cour, M.; Beduneau, G.; Marchalot, A.; et al. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: A French multicenter retrospective cohort study. Intensive Care Med. 2018, 44, 1502–1511. [Google Scholar] [CrossRef]
- Shen, J.; Bouee, S.; Aris, E.; Emery, C.; Beck, E.C. Long-Term Mortality and State Financial Support in Invasive Meningococcal Disease-Real-World Data Analysis Using the French National Claims Database (SNIIRAM). Infect. Dis. Ther. 2021, 11, 249–262. [Google Scholar] [CrossRef]
- Levy, C.; Taha, M.K.; Bingen, E.; Cohen, R. Méningites à méningocoques de l’enfant en France: Résultats de l’observatoire ACTIV/GPIP. Arch. Pédiatrie 2012, 19, S49–S54. [Google Scholar] [CrossRef]
- Levy, C.; Varon, E.; Taha, M.K.; Béchet, S.; Bonacorsi, S.; Cohen, R.; Bingen, E. Change in French bacterial meningitis in children resulting from vaccination. Arch. Pédiatrie 2014, 21, 736–744. [Google Scholar] [CrossRef]
- Bingen, E.; Levy, C.; de la Rocque, F.; Boucherat, M.; Varon, E.; Alonso, J.M.; Dabernat, H.; Reinert, P.; Aujard, Y.; Cohen, R.; et al. Bacterial meningitis in children: A French prospective study. Clin. Infect. Dis. 2005, 41, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.F.; Perrocheau, A.; Meffre, C.; Hahne, S.; Group, W.W. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg. Infect. Dis. 2002, 8, 761–767. [Google Scholar] [CrossRef]
- Lorton, F.; Chalumeau, M.; Assathiany, R.; Martinot, A.; Bucchia, M.; Roue, J.M.; Bourgoin, P.; Chantreuil, J.; Boussicault, G.; Gaillot, T.; et al. Vaccine-preventable severe morbidity and mortality caused by meningococcus and pneumococcus: A population-based study in France. Paediatr. Perinat. Epidemiol. 2018, 32, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Arlet, J.B.; de Lajudie, E.; Despujol, C.; Ranque, B.; Pouchot, J. Meningitis and loss of visual acuity. Presse. Med. 2010, 39, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Briand, C.; Levy, C.; Baumie, F.; Joao, L.; Bechet, S.; Carbonnelle, E.; Grimprel, E.; Cohen, R.; Gaudelus, J.; de Pontual, L. Outcomes of bacterial meningitis in children. Med. Mal. Infect. 2016, 46, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Weil-Olivier, C.; Taha, M.K.; Emery, C.; Bouee, S.; Beck, E.; Aris, E.; Loncle-Provot, V.; Nachbaur, G.; Pribil, C. Healthcare Resource Consumption and Cost of Invasive Meningococcal Disease in France: A Study of the National Health Insurance Database. Infect. Dis. Ther. 2021, 10, 1607–1623. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.K.; Gaudelus, J.; Deghmane, A.E.; Caron, F. Recent changes of invasive meningococcal disease in France: Arguments to revise the vaccination strategy in view of those of other countries. Hum. Vaccin. Immunother. 2020, 16, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Santé Publique France. Les Infections Invasives à Méningocoques en 2019. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-prevention-vaccinale/infections-invasives-a-meningocoque/donnees/#tabs (accessed on 15 August 2022).
- Knol, M.J.; Ruijs, W.L.; Antonise-Kamp, L.; de Melker, H.E.; van der Ende, A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Eurosurveillance 2018, 23, 18–00158. [Google Scholar] [CrossRef]
- Campbell, H.; Saliba, V.; Borrow, R.; Ramsay, M.; Ladhani, S.N. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Eurosurveillance 2015, 20, 21188. [Google Scholar] [CrossRef]
- Gruhn, S.; Witte, J.; Greiner, W.; Damm, O.; Dietzsch, M.; Kramer, R.; Knuf, M. Epidemiology and economic burden of meningococcal disease in Germany: A systematic review. Vaccine 2022, 40, 1932–1947. [Google Scholar] [CrossRef]
- Guiddir, T.; Gros, M.; Hong, E.; Terrade, A.; Denizon, M.; Deghmane, A.E.; Taha, M.K. Unusual Initial Abdominal Presentations of Invasive Meningococcal Disease. Clin. Infect. Dis. 2018, 67, 1220–1227. [Google Scholar] [CrossRef]
- Huang, L.; Heuer, O.D.; Janssen, S.; Hackl, D.; Schmedt, N. Clinical and economic burden of invasive meningococcal disease: Evidence from a large German claims database. PLoS ONE 2020, 15, e0228020. [Google Scholar] [CrossRef]
- Voss, S.S.; Nielsen, J.; Valentiner-Branth, P. Risk of sequelae after invasive meningococcal disease. BMC Infect. Dis. 2022, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E.; Li, Y.; Bita, A.; Moureau, A.; Nair, H.; Kyaw, M.H.; Meningococcal Surveillance, G.; Abad, R.; Bailey, F.; Garcia, I.F.; et al. Meningococcal serogroups and surveillance: A systematic review and survey. J. Glob. Health 2019, 9, 010409. [Google Scholar] [CrossRef] [PubMed]
- Guedes, S.; Bertrand-Gerentes, I.; Evans, K.; Coste, F.; Oster, P. Invasive meningococcal disease in older adults in North America and Europe: Is this the time for action? A review of the literature. BMC Public Health 2022, 22, 380. [Google Scholar] [CrossRef] [PubMed]
- Guedes, S.; Bricout, H.; Langevin, E.; Tong, S.; Bertrand-Gerentes, I. Epidemiology of invasive meningococcal disease and sequelae in the United Kingdom during the period 2008 to 2017—A secondary database analysis. BMC Public Health 2022, 22, 521. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.J.; Brouwer, M.C.; van de Beek, D. Neurological sequelae of bacterial meningitis. J. Infect. 2016, 73, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.G.; van Stel, H.F.; Schrijvers, A.J.; Rommes, J.H.; Bakker, J.; Spronk, P.E. Health-related quality of life in critically ill patients: How to score and what is the clinical impact? Curr. Opin. Crit. Care 2009, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Scanferla, E.; Gorwood, P.; Fasse, L. Familial experience of acute bacterial meningitis in children: A transversal qualitative study using interpretative phenomenological analysis. BMJ Open 2021, 11, e047465. [Google Scholar] [CrossRef]
- Borg, J.; Christie, D.; Coen, P.G.; Booy, R.; Viner, R.M. Outcomes of meningococcal disease in adolescence: Prospective, matched-cohort study. Pediatrics 2009, 123, e502–e509. [Google Scholar] [CrossRef]
- Antignac, A.; Ducos-Galand, M.; Guiyoule, A.; Pires, R.; Alonso, J.M.; Taha, M.K. Neisseria meningitidis strains isolated from invasive infections in France (1999–2002): Phenotypes and antibiotic susceptibility patterns. Clin. Infect. Dis. 2003, 37, 912–920. [Google Scholar] [CrossRef]
- Aubert, L.; Taha, M.; Boo, N.; Le Strat, Y.; Deghmane, A.E.; Sanna, A.; Barret, A.S.; Lévy-Bruhl, D.; Vandentorren, S.; Parent du Châtelet, I. Serogroup C invasive meningococcal disease among men who have sex with men and in gay-oriented social venues in the Paris region: July 2013 to December 2014. Eurosurveillance 2015, 20, 21016. [Google Scholar] [CrossRef]
- Barret, A.S.; Clinard, F.; Taha, M.K.; Girard, I.; Hong, E.; Tessier, S.; Zurbaran, M.; de Bort, C.; Antona, D.; Deghmane, A.E.; et al. Cluster of serogroup W invasive meningococcal disease in a university campus. Med. Mal. Infect. 2020, 50, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; de La Rocque, F.; Aujard, Y.; Bingen, E. National surveillance of bacterial meningitis in children. Arch. Pediatr. 2003, 10 (Suppl 1), 114s–115s. [Google Scholar] [CrossRef] [PubMed]
- Garnier, F.; Courouble, M.; Denis, F.; Ploy, M.C. Emergence of 2 Neisseria meningitidis serogroup C clones in a French county. Diagn. Microbiol. Infect. Dis. 2011, 69, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Grodet, C.; Dequin, P.F.; Watt, S.; Lanotte, P.; de Gialluly, C.; Taha, M.K.; Alonso, J.M.; Quentin, R.; Goudeau, A.; Mereghetti, L. Outbreak in France of Neisseria meningitidis B:15:P1.12 belonging to sequence type 1403. Clin. Microbiol. Infect. 2004, 10, 845–848. [Google Scholar] [CrossRef]
- Hong, E.; Terrade, A.; Muzzi, A.; De Paola, R.; Boccadifuoco, G.; La Gaetana, R.; Deghmane, A.-E.; Pizza, M.; Serino, L.; Taha, M.-K. Evolution of strain coverage by the multicomponent meningococcal serogroup B vaccine (4CMenB) in France. Hum. Vaccin. Immunother. 2021, 17, 5614–5622. [Google Scholar] [CrossRef]
- Levy-Bruhl, D.; Perrocheau, A.; Mora, M.; Taha, M.K.; Dromell-Chabrier, S.; Beytout, J.; Quatresous, I. Vaccination campaign following an increase in incidence of serogroup C meningococcal diseases in the department of Puy-de-Dome (France). Eurosurveillance 2002, 7, 74–76. [Google Scholar] [CrossRef]
- Sarlangue, J.; Levy, C.; Cohen, R.; Bingen, E.; Aujard, Y. Epidemiology of bacterial meningitis in children in France. Arch. Pediatr. 2006, 13, 569–571. [Google Scholar] [CrossRef]
| Proportion of IMD Diagnoses | References | |
|---|---|---|
| Meningitis | 43–79% (all 1) 61.1% (children 2) 56.5–63.1% (adult 3) | [19,43,44] [43] [40,43] |
| Septicemia | 12.2–25.1% (all 1) 22.2% (children 2) 28.2% (adult 3) | [19,43,44] [43] [43] |
| Septicemia and meningitis | 9.4–18% (all 1) 12.3% (children 2) 6.5% (adult 3) | [19,43,44] [43] [43] |
| Purpura fulminans | 25.6–57% (all 1) 9.1–29.7% (children 2) 36.9% (adult) | [19,44] [37,38,45] [40] |
| Unspecified or other type of IMD | 1.1–6.6% (all 1) 4.5% (children 2) 8.8% (adult 3) | [43,44] [43] [43] |
| Case Fatality Rate 1 | References | |
|---|---|---|
| Overall | 6.0–12.9% (all 2) | [19,43,48] |
| Serogroup | ||
| B | 7.8–8.8% (all 2) 5.3–8.2% (children 3) | [19,25,30] [39,49,50,51] |
| C | 12.3–13.2% (all 2) 8.4–9.9% (children 3) | [19,30] [39,49,50,51] |
| W | 11.9–22.1% (all 2) 6% (children 3) | [19,30,52] [34] |
| Y | 15.5–17.2% | [19,30] |
| Age group | ||
| <1 year | 5.1–9.9% (all 2) | [19,37,38,46] |
| 1–4 years | 5.1–8.9% (all 2) | [19,38,46] |
| 5–14 years | 4.1–5.9% (all 2) | [19,46] |
| 15–24 years | 7.7–10.3% (all 2) | [19,46] |
| 25–59 years | 9.3% (all 2) | [19] |
| >60 years | 20% (all 2) | [19] |
| Clinical presentation | ||
| Meningitis only | 5.6% (all 2) 21% (children 3) | [43] [35] |
| Septicemia only | 7.7% (all 2) | [43] |
| Both septicemia and meningitis | 3.9% (all 2) | [43] |
| Purpura fulminans | 36.6% (all 4) 36.9% (adults 4) 79% (children 3) | [44] [47] [35] |
| Hyperinvasive clonal complex 11 | ||
| Overall | 16% (all 2) | [26] |
| Sg C | 22% (all 2) | [18] |
| Sg W | 4.0–27.8% (all 2) | [30] |
| Rate | References | ||
|---|---|---|---|
| Age group | All ages | 19.4–25.4% | [43,46,53] |
| Children (<18 years) | 9.3–15.3% | [46] | |
| Adult (≥18 years) | 11.3–31.3% | [40,46] | |
| Clinical presentation | Meningitis | 19.9% | [43] |
| Septicemia | 19.3% | [43] | |
| Both septicemia and meningitis | 21.6% | [43] | |
| Sequelae | Rate | References |
|---|---|---|
| Physical | ||
| Amputations | 1.5–2.7% (all 1) 1.4–11.6% (adults 2) | [43,46] [46,47] |
| Skin necrosis/scarring | 2.3–2.7% (all 1) | [43,46] |
| Renal disease | 1.3–1.9% (all 1) | [21,43,46] |
| Persistent gonaglias * | [54] | |
| Mechanical arthralgias * | [54] | |
| Persistent inflammatory syndrome * | [32] | |
| Sensorial | ||
| Hearing impairment | 2.8–4.8% (all 1) 15.5% (adults 2) | [43,46] [40] |
| Severe visual impairment/blindness | 1.7% (all 1) | [46] |
| Neurological | ||
| Motor disorders | 3.5% (all 1) | [46] |
| Epilepsy | 5.8% (all 1) | [46] |
| Migraine/Headache | 32.9% (adults 2) | [40] |
| Severe neurological disorder | 5.5% (all 1) | [46] |
| Speech or communication problems | 1.7% (all 1) | [46] |
| Cognitive | ||
| Intellectual disability/ cognitive impairment | 1.7% (all 1) 10% (adults 2) | [43] [40] |
| Behavior or psychological | ||
| Sleep disorders | 42.9% (adults 2) | [40] |
| Depressive symptoms | 2.5% (all 1) 34.3% (adults 2) | [46] [40] |
| Anxiety | 5.5% (all 1) | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloche, A.; Dussart, C.; Bedouch, P.; Carrouel, F.; Mick, G. Epidemiology and Clinical Burden of Meningococcal Disease in France: Scoping Review. J. Clin. Med. 2023, 12, 849. https://doi.org/10.3390/jcm12030849
Baloche A, Dussart C, Bedouch P, Carrouel F, Mick G. Epidemiology and Clinical Burden of Meningococcal Disease in France: Scoping Review. Journal of Clinical Medicine. 2023; 12(3):849. https://doi.org/10.3390/jcm12030849
Chicago/Turabian StyleBaloche, Alexiane, Claude Dussart, Pierrick Bedouch, Florence Carrouel, and Gérard Mick. 2023. "Epidemiology and Clinical Burden of Meningococcal Disease in France: Scoping Review" Journal of Clinical Medicine 12, no. 3: 849. https://doi.org/10.3390/jcm12030849
APA StyleBaloche, A., Dussart, C., Bedouch, P., Carrouel, F., & Mick, G. (2023). Epidemiology and Clinical Burden of Meningococcal Disease in France: Scoping Review. Journal of Clinical Medicine, 12(3), 849. https://doi.org/10.3390/jcm12030849








