Circulating Inflammatory Cytokine Associated with Poor Prognosis in Moyamoya Disease: A Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Subjects
2.2. Clinical Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
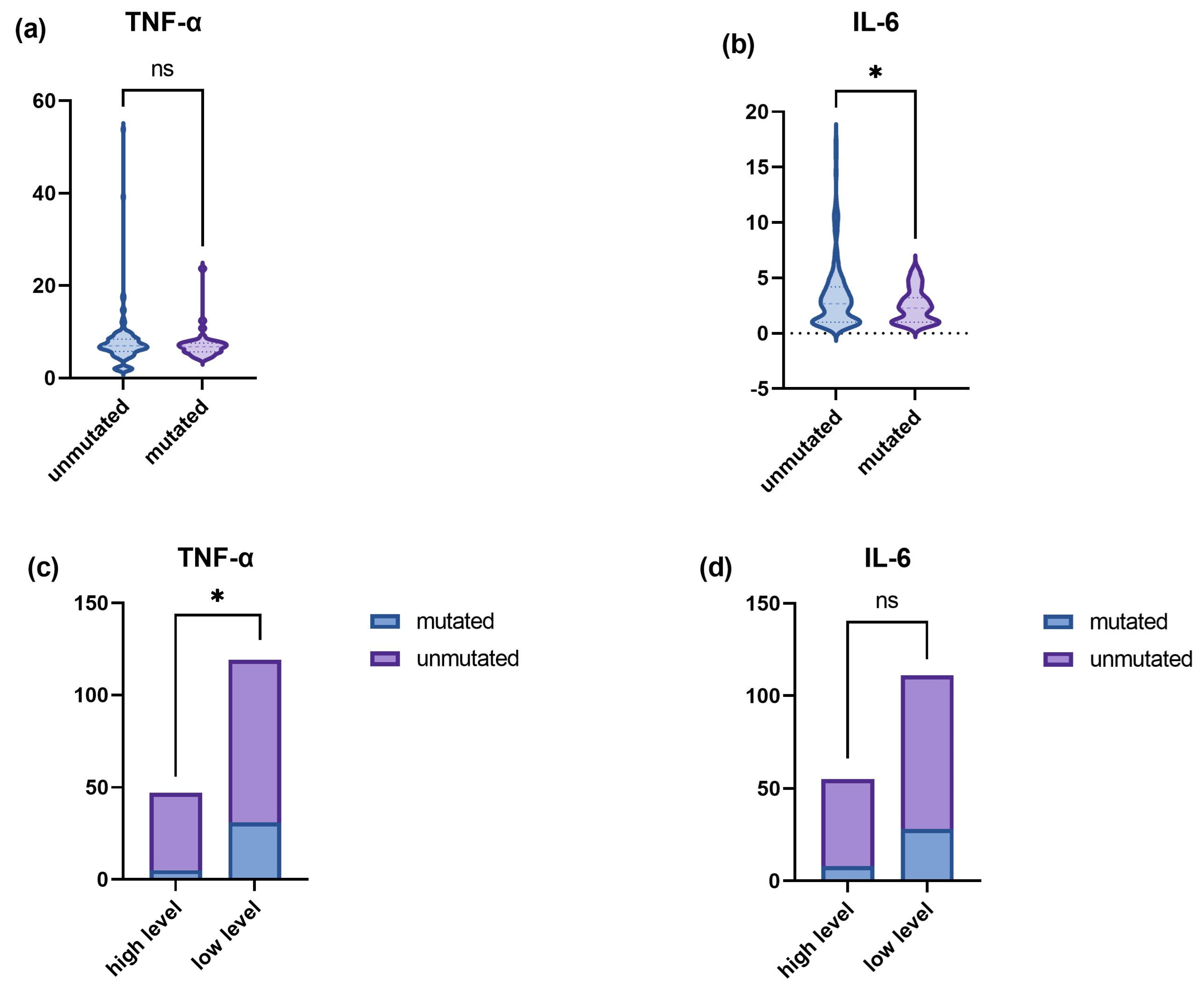
3.2. Higher TNF-α and IL-6 Levels in the p.R4810K Variant of RNF213
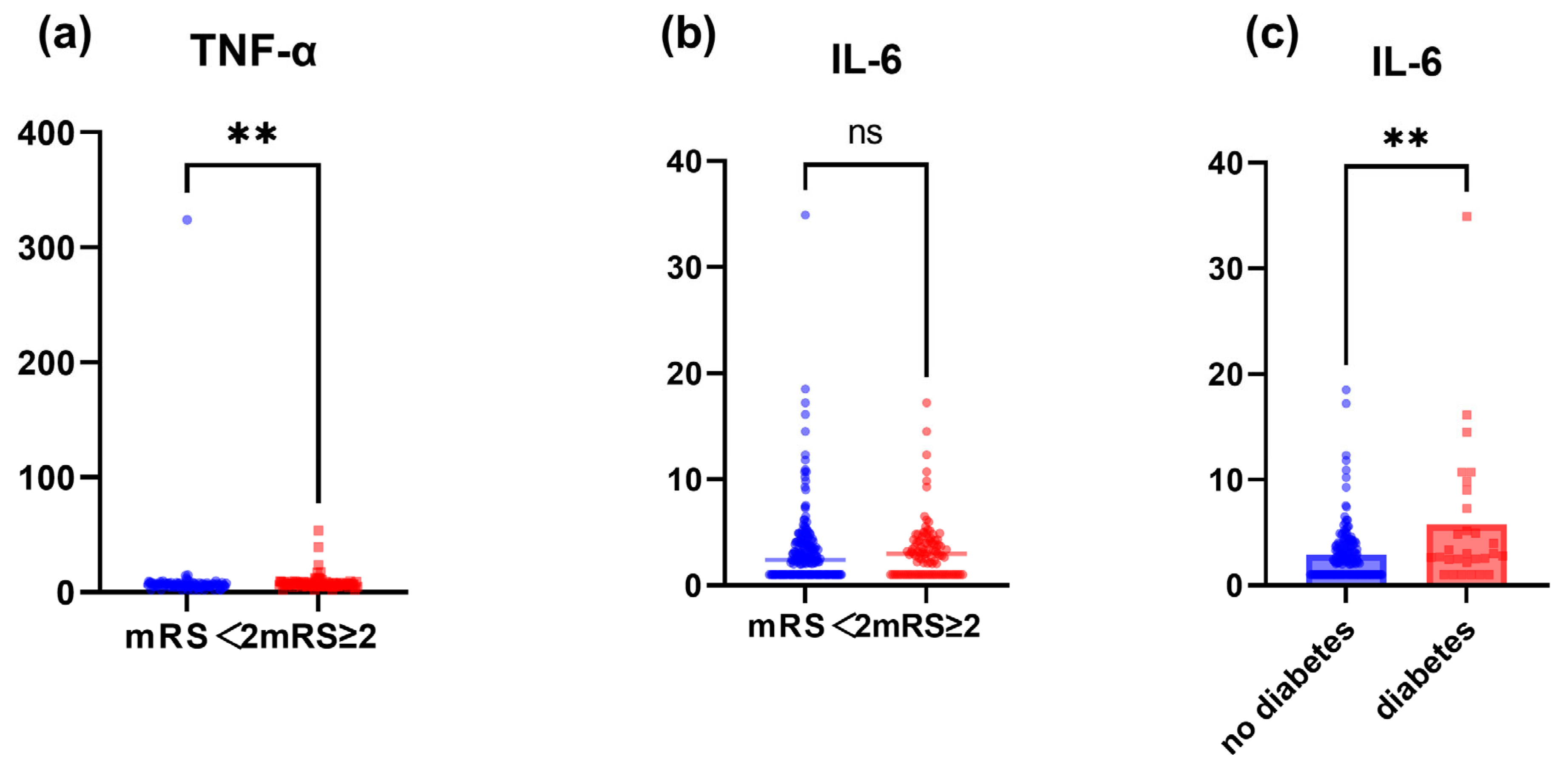
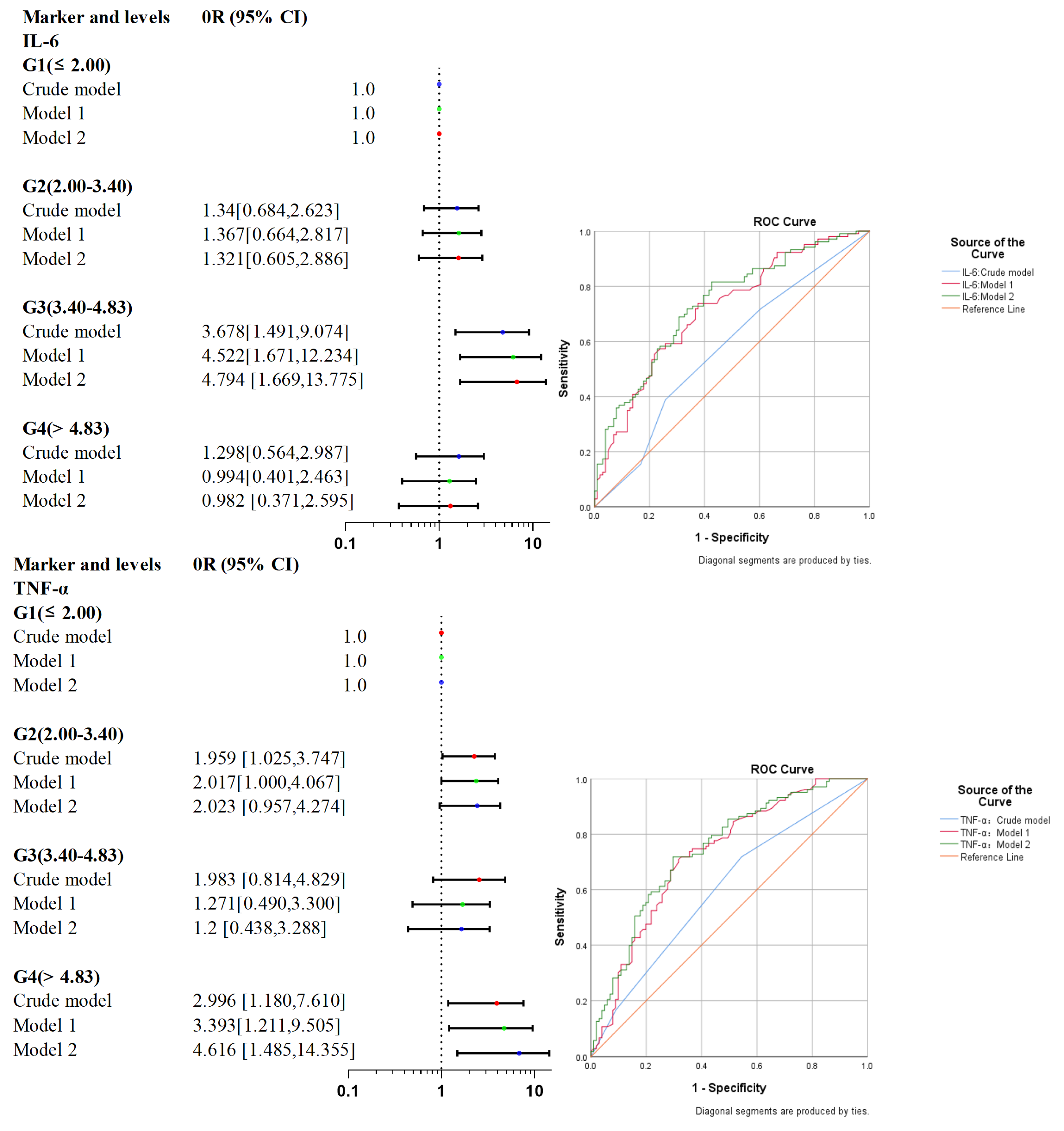
3.3. IL-6 and TNF-α Are Associated with Poor Functional Prognosis after Surgery for Adult MMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Guo, Z.N.; Shi, M.; Yang, Y.; Rao, M. Etiology and pathogenesis of Moyamoya Disease: An update on disease prevalence. Int. J. Stroke 2017, 12, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.G.; Arnaout, O.M.; Rahme, R.J.; Aoun, S.G.; Batjer, H.H.; Bendok, B.R. Moyamoya disease: A review of histopathology, biochemistry, and genetics. Neurosurg. Focus 2011, 30, E20. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Huang, Z.; Zhou, Q.; Yang, X.; Gu, H.; Li, Z.; Shi, Y.; Gan, L.; Wang, H.; et al. Epidemiology of Moyamoya disease in China: A nationwide hospital-based study. Lancet Reg Health West Pac. 2022, 18, 100331. [Google Scholar] [CrossRef]
- Birkeland, P.; Tharmabalan, V.; Lauritsen, J.; Ganesan, V.; Bjarkam, C.R.; von Weitzel-Mudersbach, P. Moyamoya disease in a European setting: A Danish population-based study. Eur. J. Neurol. 2020, 27, 2446–2452. [Google Scholar] [CrossRef]
- Ghaffari-Rafi, A.; Ghaffari-Rafi, S.; Leon-Rojas, J. Socioeconomic and demographic disparities of moyamoya disease in the United States. Clin. Neurol. Neurosurg. 2020, 192, 105719. [Google Scholar] [CrossRef]
- Mejia-Munne, J.C.; Ellis, J.A.; Feldstein, N.A.; Meyers, P.M.; Connolly, E.S. Moyamoya and Inflammation. World Neurosurg. 2017, 100, 575–578. [Google Scholar] [CrossRef]
- Kronenburg, A.; Braun, K.P.J.; van der Zwan, A.; Klijn, C.J.M. Recent advances in moyamoya disease: Pathophysiology and treatment. Curr. Neurol. Neurosci. Rep. 2014, 4, 423. [Google Scholar] [CrossRef]
- Mikami, T.; Suzuki, H.; Komatsu, K.; Mikuni, N. Influence of Inflammatory Disease on the Pathophysiology of Moyamoya Disease and Quasi-moyamoya Disease. Neurol. Med. Chir. 2019, 59, 361–370. [Google Scholar] [CrossRef]
- Weng, L.; Cao, X.; Han, L.; Zhao, H.; Qiu, S.; Yan, Y.; Wang, X.; Chen, X.; Zheng, W.; Xu, X.; et al. Association of increased Treg and Th17 with pathogenesis of moyamoya disease. Sci. Rep. 2017, 7, 3071. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Sonobe, S.; Nishijima, Y.; Niizuma, K.; Sakata, H.; Kure, S.; Tominaga, T. Genetics and Biomarkers of Moyamoya Disease: Significance of RNF213 as a Susceptibility Gene. J. Stroke 2014, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ahel, J.; Lehner, A.; Vogel, A.; Schleiffer, A.; Meinhart, A.; Haselbach, D.; Clausen, T. Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism. Elife 2020, 9, e56185. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Sakai, Y.; Inoue, H.; Akamine, S.; Ishizaki, Y.; Matsushita, Y.; Sanefuji, M.; Torisu, H.; Ihara, K.; Sardiello, M.; et al. Moyamoya disease susceptibility gene RNF213 links inflammatory and angiogenic signals in endothelial cells. Sci. Rep. 2015, 5, srep13191. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Thirumurugan, K. New insights into TNFalpha/PTP1B and PPARgamma pathway through RNF213-a link between inflammation, obesity, insulin resistance, and Moyamoya disease. Gene 2021, 771, 145340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.; Pan, Y.; Wang, M.; Meng, X.; Li, H.; Wang, Y.; Zhao, X.; Qin, H.; Liu, L.; et al. Interleukin-6 and YKL-40 predicted recurrent stroke after ischemic stroke or TIA: Analysis of 6 inflammation biomarkers in a prospective cohort study. J. Neuroinflamm. 2022, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.J.; Chow, D.; Brevnova, E.E.; Garcia, K.C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science 2003, 300, 2101–2104. [Google Scholar] [CrossRef]
- Hitomi, T.; Habu, T.; Kobayashi, H.; Okuda, H.; Harada, K.H.; Osafune, K.; Taura, D.; Sone, M.; Asaka, I.; Ameku, T.; et al. Downregulation of Securin by the variant RNF213 R4810K (rs112735431, G>A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from moyamoya patients. Biochem. Biophys. Res. Commun. 2013, 438, 13–19. [Google Scholar] [CrossRef]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef]
- Zimmermann, M.; Aguilera, F.B.; Castellucci, M.; Rossato, M.; Costa, S.; Lunardi, C.; Ostuni, R.; Girolomoni, G.; Natoli, G.; Bazzoni, F.; et al. Chromatin remodelling and autocrine TNFα are required for optimal interleukin-6 expression in activated human neutrophils. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Yamashita, M.; Oka, K.; Tanaka, K. Cervico-cephalic arterial thrombi and thromboemboli in moyamoya disease—Possible correlation with progressive intimal thickening in the intracranial major arteries. Stroke 1984, 15, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.A.-O.; Rinaldo, L.; Lanzino, G.; Klaas, J.P. High prevalence of pro-thrombotic conditions in adult patients with moyamoya disease and moyamoya syndrome: A single center study. Acta Neurochir. 2020, 162, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Luo, M.Y.; Liang, N.; Gong, S.X.; Chen, W.; Huang, W.Q.; Tian, Y.; Wang, A.-P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021, 12, 745061. [Google Scholar] [CrossRef]
- Jenny, N.S.; Callas, P.W.; Judd, S.E.; McClure, L.A.; Kissela, B.; Zakai, N.A.; Cushman, M. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology 2019, 92, e2375–e2384. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Klimiec, E.; Weglarczyk, K.; Pera, J.; Slowik, A.; Siedlar, M.; Dziedzic, T. Reduced ex vivo release of pro-inflammatory cytokines and elevated plasma interleukin-6 are inflammatory signatures of post-stroke delirium. J. Neuroinflammation 2018, 15, 111. [Google Scholar] [CrossRef]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Da Silva, C.C.; Buisson, M.; Amaz, C.; Derex, L.; Ong, E.; Berthezene, Y.; Eker, O.F.; et al. Association of Interleukin-6 Levels and Futile Reperfusion After Mechanical Thrombectomy. Neurology 2020, 96, e752–e757. [Google Scholar] [CrossRef]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Crola Da Silva, C.; Buisson, M.; Amaz, C.; Derex, L.; Ong, E.; Berthezene, Y.; Dufay, N.; et al. A lower admission level of interleukin-6 is associated with first-pass effect in ischemic stroke patients. BMJ 2022, 14, 248–251. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, Z.-S.; Liu, W.-W.; Bao, X.-Y.; Li, D.-S.; Han, C.; Xian, P.; Zhao, F.; Wang, H.; Wang, H.; et al. Surgical outcomes following encephaloduroarteriosynangiosis in adult moyamoya disease associated with Type 2 diabetes. J. Neurosurg. 2016, 125, 308–314. [Google Scholar] [CrossRef]
- Clausen, B.H.; Wirenfeldt, M.; Høgedal, S.S.; Frich, L.H.; Nielsen, H.H.; Schrøder, H.D.; Østergaard, K.; Finsen, B.; Kristensen, B.W.; Lambertsen, K.L. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. J. Neurosurg. 2016, 125, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dziewulska, D.; Mossakowski, M.J. Cellular expression of tumor necrosis factor a and its receptors in human ischemic stroke. Clin. Neuropathol. 2003, 22, 35–40. [Google Scholar] [PubMed]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1677–1698. [Google Scholar] [CrossRef]
- Cui, G.; Wang, H.; Li, R.; Zhang, L.; Li, Z.; Wang, Y.; Hui, R.; Ding, H.; Wang, D.W. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J. Neuroinflamm. 2012, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tezuka, T.; Kim, M.; Choi, J.; Oichi, Y.; Kobayashi, H.; Harada, K.H.; Mizushima, T.; Taketani, S.; Koizumi, A.; et al. Moyamoya disease patient mutations in the RING domain of RNF213 reduce its ubiquitin ligase activity and enhance NFκB activation and apoptosis in an AAA+ domain-dependent manner. Biochem. Biophys. Res. Commun. 2020, 525, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.K.; Shapson-Coe, A.; Govender, R.; van Toorn, R.; Ndondo, A.; Wieselthaler, N.; Eley, B.; Mubaiwa, L.; Wilmshurst, J.M. Moyamoya Syndrome in South African Children With HIV-1 Infection. J. Child Neurol. 2016, 31, 1010–1017. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, X.; Shi, Y.; Yuan, F.; Hu, Z. Systemic sclerosis associated with moyamoya syndrome: A case report and literature review. Immunobiology 2020, 225, 121882. [Google Scholar] [CrossRef]
- Kamada, F.; Aoki, Y.; Narisawa, A.; Abe, Y.; Komatsuzaki, S.; Kikuchi, A.; Kanno, J.; Niihori, T.; Ono, M.; Ishii, N.; et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2011, 56, 34–40. [Google Scholar] [CrossRef]
- Imani, S.Z.H.; Hojati, Z.; Khalilian, S.; Dehghanian, F.; Kheirollahi, M.; Khorrami, M.; Shaygannejad, V.; Mirmosayyeb, O. Expression and clinical significance of IL7R, NFATc2, and RNF213 in familial and sporadic multiple sclerosis. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Lin, Y.A.-O.; Huang, H.; Hwang, W.Z. Moyamoya disease with Sjogren disease and autoimmune thyroiditis presenting with left intracranial hemorrhage after messenger RNA-1273 vaccination: A case report. Medicine 2022, 101, e28756. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Liu, Y.; Zhou, L.X.; Sun, H.; He, M.; You, C. Prevalence of autoimmune disease in moyamoya disease patients in Western Chinese population. J. Neurol. Sci. 2015, 351, 184–186. [Google Scholar] [CrossRef]
- Mineharu, Y.; Miyamoto, S. RNF213 and GUCY1A3 in Moyamoya Disease: Key Regulators of Metabolism, Inflammation, and Vascular Stability. Front. Neurol. 2021, 12, 687088. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Jin, F.; Zhang, H.; Yang, M.; Cui, C.; Wang, C.; Jiang, P. Association of Brain-Gut Peptides with Inflammatory Cytokines in Moyamoya Disease. Mediat. Inflamm. 2020, 2020, 5847478. [Google Scholar] [CrossRef] [PubMed]

| Variables | Value | ALL Patients (n = 204) | mRS < 2 (n = 101) | mRS ≥ 2 (n = 103) | p-Value |
|---|---|---|---|---|---|
| Age, median[IQR] | - | 44.000 [34.000, 50.000] | 38.000 [31.000, 47.000] | 47.000 [40.000, 52.000] | <0.001 |
| Gender/male, n (%) | - | 81 (39.706) | 37 (36.634) | 44 (42.718) | 0.375 |
| Clinical manifestation, n (%) | - | ||||
| ischemic | 143 (70.098) | 77 (76.238) | 66 (64.078) | 0.058 | |
| hemorrhagic | 61 (29.902) | 24 (23.762) | 37 (35.922) | ||
| RNF213 mutation, n (%) | |||||
| Unmutated | 130/166 (78.313) | 57/75 (76.000) | 73/91 (80.220) | 0.511 | |
| mutated | 36/166 (21.687) | 18/75 (24.000) | 18/91 (19.780) | ||
| Unilateral or bilateral, n (%) | |||||
| unilateral | 25 (12.255) | 17 (16.832) | 8 (7.767) | 0.048 | |
| bilateral | 179 (87.745) | 84 (83.168) | 95 (92.233) | ||
| Image Stage, n (%) | |||||
| early | 127 (62.255) | 65 (64.356) | 62 (60.194) | 0.54 | |
| late | 77 (37.745) | 36 (35.644) | 41 (39.806) | ||
| suzuki stage, n (%) | |||||
| 1 | 1 (0.490) | 1 (0.990) | 0 (0.000) | <0.001 | |
| 2 | 56 (27.451) | 32 (31.683) | 24 (23.301) | ||
| 3 | 70 (34.314) | 32 (31.683) | 38 (36.893) | ||
| 4 | 32 (15.686) | 13 (12.871) | 19 (18.447) | ||
| 5 | 29 (14.216) | 14 (13.861) | 15 (14.563) | ||
| 6 | 16 (7.843) | 9 (8.911) | 7 (6.796) | ||
| Hyperlipidemia, n (%) | 30 (14.706) | 12 (11.881) | 18 (17.476) | 0.259 | |
| Hypertension, n (%) | 68 (33.333) | 23 (22.772) | 45 (43.689) | 0.002 | |
| Diabetes, n (%) | 29 (14.216) | 11 (10.891) | 18 (17.476) | 0.178 | |
| BMI, median[IQR] | 24.977 [22.491, 28.040] | 25.352 [22.583, 28.040] | 24.768 [22.266, 28.028] | 0.549 | |
| WBC, median[IQR] | 6.810 [5.620, 8.300] | 6.940 [5.690, 8.510] | 6.780 [5.520, 8.140] | 0.456 | |
| RBC, mean (±SD) | 4.605 ± 0.529 | 4.573 ± 0.574 | 4.637 ± 0.480 | 0.394 | |
| HGB, mean (±SD) | 140.000 [129.000, 155.000] | 137.000 [129.000, 154.000] | 142.000 [130.000, 155.000] | 0.639 | |
| PLT, median[IQR] | 237.000 [204.000, 278.000] | 245.000 [204.000, 279.000] | 236.000 [204.000, 276.000] | 0.827 | |
| PCT, median[IQR] | 0.230 [0.200, 0.260] | 0.230 [0.190, 0.260] | 0.230 [0.200, 0.270] | 0.876 | |
| Hcy, median[IQR] | 10.560 [8.600, 13.100] | 10.330 [8.160, 12.940] | 10.810 [9.200, 13.400] | 0.264 | |
| CHO, mean (±SD) | 4.346 ± 0.938 | 4.398 ± 0.924 | 4.296 ± 0.949 | 0.438 | |
| TG, median[IQR] | 1.220 [0.860, 1.590] | 1.220 [0.820, 1.720] | 1.220 [0.910, 1.530] | 0.728 | |
| UA, median[IQR] | 305.600 [253.200, 367.600] | 301.200 [244.500, 363.400] | 307.800 [274.000, 380.000] | 0.064 | |
| Urea, median[IQR] | 4.700 [3.800, 5.800] | 4.700 [3.800, 5.800] | 4.600 [3.800, 5.600] | 0.801 | |
| Glu, median[IQR] | 5.120 [4.710, 5.640] | 5.060 [4.740, 5.490] | 5.150 [4.710, 5.860] | 0.175 | |
| IL-6, median[IQR] | 2.430 [1.000, 3.990] | 2.200 [1.000, 3.520] | 2.980 [1.000, 4.190] | 0.051 | |
| IL-6, n (%) | |||||
| G1 (≤2.00) | 69 (33.824) | 40 (39.604) | 29 (28.155) | 0.035 | |
| G2 (2.00–3.40) | 69 (33.824) | 35 (34.653) | 34 (33.010) | ||
| G3 (3.40–4.83) | 33 (16.176) | 9 (8.911) | 24 (23.301) | ||
| G4 (>4.83) | 33 (16.176) | 17 (16.832) | 16 (15.534) | ||
| TNF-α, median[IQR] | 6.790 [5.460, 8.140] | 6.350 [5.290, 7.650] | 7.130 [6.060, 8.470] | 0.007 | |
| TNF-α, n (%) | |||||
| G1 (≤6.24) | 75 (36.765) | 46 (45.545) | 29 (28.155) | 0.058 | |
| G2 (6.24–8.10) | 76 (37.255) | 34 (33.663) | 42 (40.777) | ||
| G3 (8.10–9.26) | 27 (13.235) | 12 (11.881) | 15 (14.563) | ||
| G4 (>9.26) | 26 (12.745) | 9 (8.911) | 17 (16.505) | ||
| IL-1β, n (%) | |||||
| NORMAL (0–5.00) | 162 (79.412) | 81 (80.198) | 81 (78.641) | 0.783 | |
| HIGH (>5.00) | 42 (20.588) | 20 (19.802) | 22 (21.359) | ||
| IL2R, mean (±SD) | 332.564 ± 103.813 | 341.386 ± 110.318 | 323.913 ± 96.229 | 0.231 | |
| IL8, median[IQR] | 16.700 [11.500, 26.700] | 15.400 [11.300, 26.300] | 17.000 [12.300, 27.400] | 0.461 | |
| Surgical approach, n (%) | |||||
| indirect | 85 (41.667) | 44 (43.564) | 41 (39.806) | 0.586 | |
| direct | 119 (58.333) | 57 (56.436) | 62 (60.194) |
| Marker and Levels | Events [no., (%)] | Crude Model | Model 2 | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| IL-6 (ng/L) | G1 (≤2.00) | 42.029 | Reference | – | Reference | – | Reference | – |
| G2 (2.00–3.40) | 49.275 | 1.34 [0.684, 2.623] | 0.393 | 1.367 [0.664, 2.817] | 0.396 | 1.321 [0.605, 2.886] | 0.485 | |
| G3 (3.40–4.83) | 72.727 | 3.678 [1.491, 9.074] | 0.005 | 4.522 [1.671, 12.234] | 0.003 | 4.794 [1.669, 13.775] | 0.004 | |
| G4 (>4.83) | 48.485 | 1.298 [0.564, 2.987] | 0.539 | 0.994 [0.401, 2.463] | 0.989 | 0.982 [0.371, 2.595] | 0.97 | |
| P for trend | - | - | 1.232 [0.948, 1.601] | 0.119 | 1.179 [0.889, 1.564] | 0.252 | 1.187 [0.880, 1.600] | 0.261 |
| TNF-α (ng/L) | G1 (≤6.24) | 38.667 | Reference | – | Reference | – | Reference | – |
| G2 (6.24–8.10) | 55.263 | 1.959 [1.025, 3.747] | 0.042 | 2.017 [1.000, 4.067] | 0.05 | 2.023 [0.957, 4.274] | 0.065 | |
| G3 (8.10–9.26) | 55.556 | 1.983 [0.814, 4.829] | 0.132 | 1.271 [0.490, 3.300] | 0.622 | 1.2 [0.438, 3.288] | 0.722 | |
| G4 (>9.26) | 65.385 | 2.996 [1.180, 7.610] | 0.021 | 3.393 [1.211, 9.505] | 0.02 | 4.616 [1.485, 14.355] | 0.008 | |
| P for trend | - | - | 1.432 [1.077, 1.903] | 0.013 | 1.385 [1.021, 1.877] | 0.036 | 1.467 [1.055, 2.040] | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Sun, J.; Shi, Z.; Huang, Z.; Yu, L.; Du, H.; Ge, P.; Zhang, D. Circulating Inflammatory Cytokine Associated with Poor Prognosis in Moyamoya Disease: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 823. https://doi.org/10.3390/jcm12030823
Liu W, Sun J, Shi Z, Huang Z, Yu L, Du H, Ge P, Zhang D. Circulating Inflammatory Cytokine Associated with Poor Prognosis in Moyamoya Disease: A Prospective Cohort Study. Journal of Clinical Medicine. 2023; 12(3):823. https://doi.org/10.3390/jcm12030823
Chicago/Turabian StyleLiu, Wei, Jian Sun, Zhiyong Shi, Zheng Huang, Lebao Yu, Haibin Du, Peicong Ge, and Dong Zhang. 2023. "Circulating Inflammatory Cytokine Associated with Poor Prognosis in Moyamoya Disease: A Prospective Cohort Study" Journal of Clinical Medicine 12, no. 3: 823. https://doi.org/10.3390/jcm12030823
APA StyleLiu, W., Sun, J., Shi, Z., Huang, Z., Yu, L., Du, H., Ge, P., & Zhang, D. (2023). Circulating Inflammatory Cytokine Associated with Poor Prognosis in Moyamoya Disease: A Prospective Cohort Study. Journal of Clinical Medicine, 12(3), 823. https://doi.org/10.3390/jcm12030823





