A Nomogram Model Based on Neuroendocrine Markers for Predicting the Prognosis of Neuroendocrine Carcinoma of Cervix
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Population
2.2. Immunohistochemistry
2.3. Follow-Up and Recurrence
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of Patints
3.1.1. Factors Related to Recurrence of Cervical Neuroendocrine Carcinoma
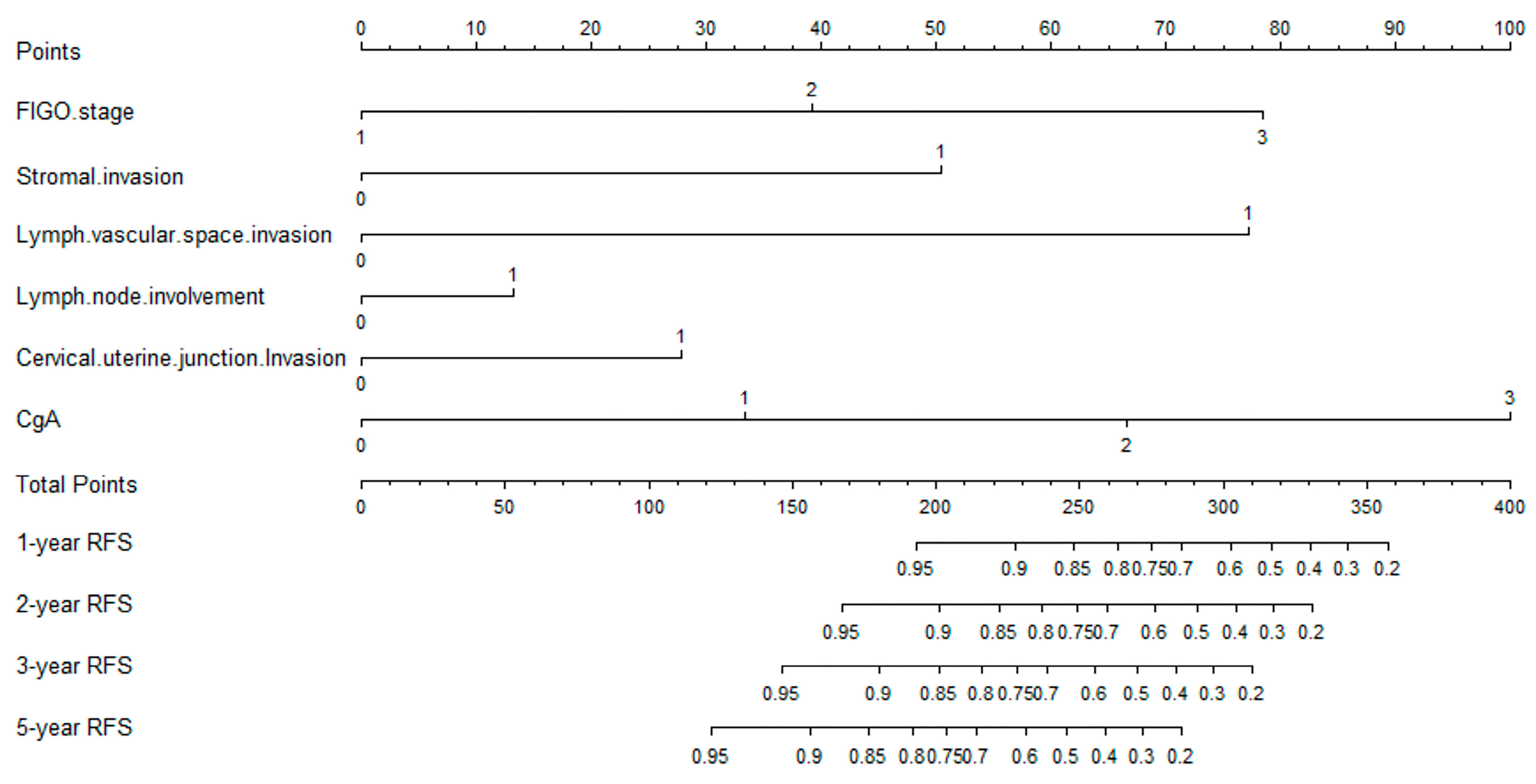
3.1.2. Predictive Nomogram Model for Cancer Recurrence
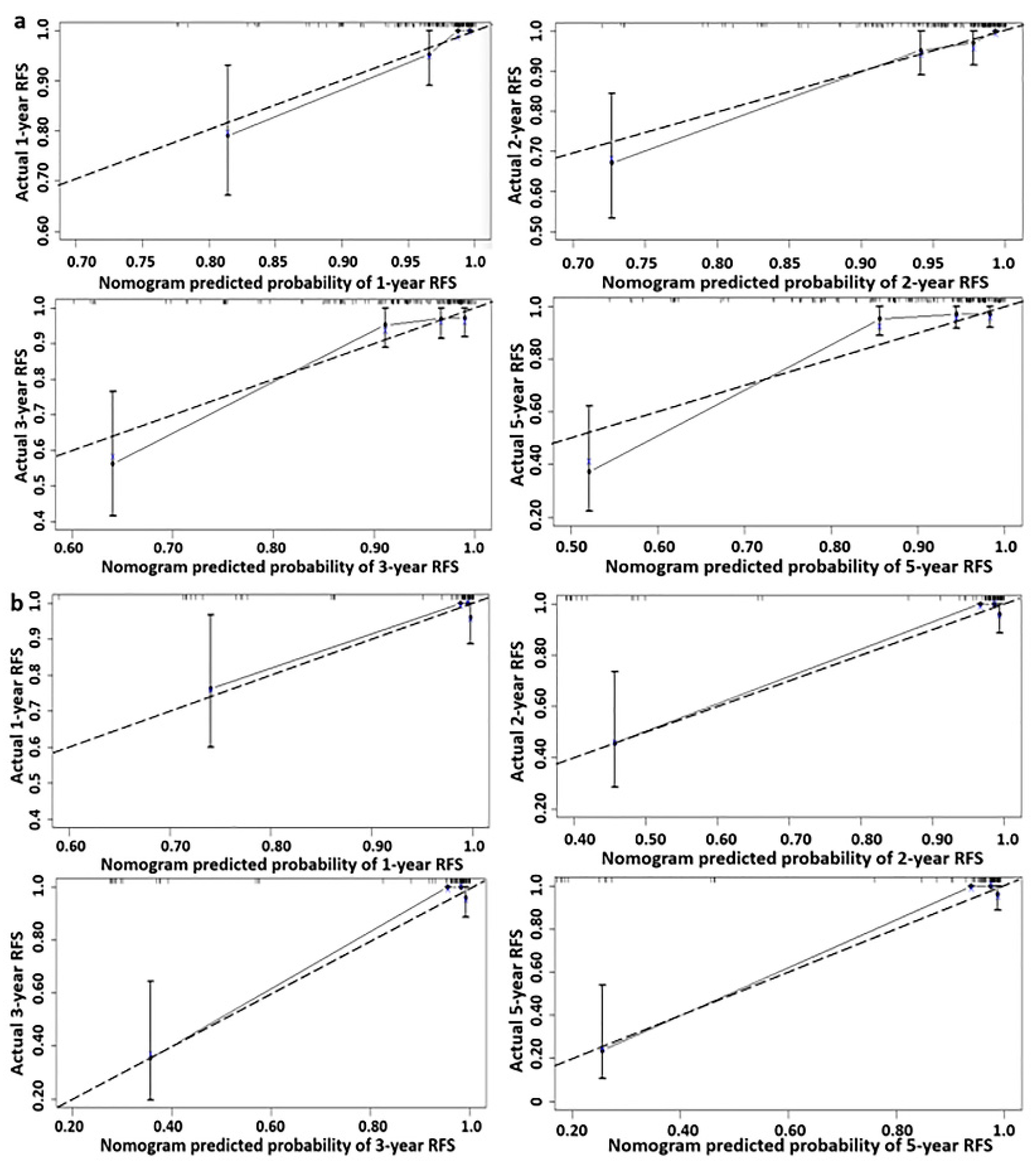
3.1.3. Optimal Threshold of Recurrence-Free Survival Rate of Nomogram Model
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margolis, B.; Tergas, A.I.; Chen, L.; Hou, J.Y.; Burke, W.M.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Natural history and outcome of neuroendocrine carcinoma of the cervix. Gynecol. Oncol. 2016, 141, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Song, L.; Yang, F.; Tang, C.; Yang, S.; He, J.; Pan, X. Enhanced efficacy of adjuvant chemotherapy and radiotherapy in selected cases of surgically resected neuroendocrine carcinoma of the uterine cervix: A retrospective cohort study. Medicine 2017, 96, e6361. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Zheng, G.; Schoolmeester, J.K.; Li, Z.; Pallavajjala, A.; Haley, L.; Conner, M.G.; Vang, R.; Hung, C.-F.; Wu, T.-C.; et al. Next-generation Sequencing Reveals Recurrent Somatic Mutations in Small Cell Neuroendocrine Carcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2018, 42, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Hirschowitz, L.; Dawson, P.; Askew, S.; Pearmain, P.; Jones, P.W.; Singh, K.; Chan, K.K.; Moss, E.L. Neuroendocrine Carcinoma of the Cervix: Review of a Series of Cases and Correlation With Outcome. Int. J. Surg. Pathol. 2016, 24, 490–496. [Google Scholar] [CrossRef]
- Chen, T.-C.; Huang, H.-J.; Wang, T.-Y.; Yang, L.-Y.; Chen, C.-H.; Cheng, Y.-M.; Liou, W.-H.; Hsu, S.-T.; Wen, K.-C.; Ou, Y.-C.; et al. Primary surgery versus primary radiation therapy for FIGO stages I–II small cell carcinoma of the uterine cervix: A retrospective Taiwanese Gynecologic Oncology Group study. Gynecol. Oncol. 2015, 137, 468–473. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Jia, Y.; Zhou, J.; Ma, D.; Li, S. Prognostic risk factors for small cell carcinoma of the cervix and impact of platinum-based neoadjuvant chemotherapy. Int. J. Gynecol. Obstet. 2015, 130, 31–35. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Thomas, B.; Behrens, C.; Onuki, N.; Lindberg, G.; Albores-Saavedra, J.; Gazdar, A.F. Molecular Abnormalities Associated with Endocrine Tumors of the Uterine Cervix. Gynecol. Oncol. 1999, 72, 3–9. [Google Scholar] [CrossRef]
- Salvo, G.; Gonzalez Martin, A.; Gonzales, N.R.; Frumovitz, M. Updates and management algorithm for neuroendocrine tumors of the uterine cervix. Int. J. Gynecol. Cancer 2019, 29, 986–995. [Google Scholar] [CrossRef]
- Howitt, B.; Kelly, P.; McCluggage, W.G. Pathology of Neuroendocrine Tumours of the Female Genital Tract. Curr. Oncol. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Dongol, S.; Tai, Y.; Shao, Y.; Jiang, J.; Kong, B. A retrospective clinicopathological analysis of small-cell carcinoma of the uterine cervix. Mol. Clin. Oncol. 2014, 2, 71–75. [Google Scholar] [CrossRef]
- Li, J.; Ouyang, Y.; Tao, Y.; Wang, L.; Li, M.; Gao, L.; Cao, X. Small cell carcinoma of the uterine cervix: A multi-institutional experience. Int. J. Gynecol. Cancer 2020, 30, 174–180. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, H.; Li, X.-M.; Yin, C.-H.; Wu, Y.-M. A clinical analysis of small-cell neuroendocrine carcinoma of the gynecologic tract: Report of 20 cases. Arch. Gynecol. Obstet. 2018, 299, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Carinelli, S.; Aletti, G. Neuroendrocrine tumors of the uterine cervix: A therapeutic challenge for gynecologic oncologists. Gynecol. Oncol. 2017, 144, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Gan, Q.; Cheng, J. Prognostic Factors and Local Treatment Modalities of Small-Cell Carcinoma of the Cervix: An Analysis According to the International Federation of Gynecology and Obstetrics Stage. Cancer Manag. Res. 2020, 12, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-W.; Luo, R.-Z.; Sun, X.-Y.; Yang, X.; Yang, H.-X.; Xiong, S.-P.; Liu, L.-L. Co-expression of SOX2 and HR-HPV RISH predicts poor prognosis in small cell neuroendocrine carcinoma of the uterine cervix. BMC Cancer 2021, 21, 332. [Google Scholar] [CrossRef]
- Giorgadze, T.; Kanhere, R.; Pang, C.; Ganote, C.; Miller, L.E.; Tabaczka, P.; Brown, E.; Husain, M. Small cell carcinoma of the cervix in liquid-based Pap test: Utilization of split-sample immunocytochemical and molecular analysis. Diagn. Cytopathol. 2010, 40, 214–219. [Google Scholar] [CrossRef]
- Chavez-Blanco, A.; Taja-Chayeb, L.; Cetina, L.; Chanona-Vilchis, G.; Trejo-Becerril, C.; Perez-Cardenas, E.; Segura-Pacheco, B.; Acuña-González, C.; Dueñas-Gonzalez, A. Neuroendocrine Marker Expression in Cervical Carcinomas of Non-Small Cell Type. Int. J. Gynecol. Pathol. 2002, 21, 368–374. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Q.; Liu, J.; Chu, K.; Huang, Y.; Zhang, Z.; Li, T.; Dai, Y.; Li, J. Prognostic factors and treatment comparison in small cell neuroendocrine carcinoma of the uterine cervix based on population analyses. Cancer Med. 2020, 9, 6524–6532. [Google Scholar] [CrossRef]
- Peng, P.; Ming, W.; Jiaxin, Y.; Keng, S. Neuroendocrine tumor of the uterine cervix: A clinicopathologic study of 14 cases. Arch. Gynecol. Obstet. 2012, 286, 1247–1253. [Google Scholar] [CrossRef]
- Burzawa, J.; Gonzales, N.; Frumovitz, M. Challenges in the diagnosis and management of cervical neuroendocrine carcinoma. Expert Rev. Anticancer. Ther. 2015, 15, 805–810. [Google Scholar] [CrossRef]
- Lee, J.-M.; Lee, K.-B.; Nam, J.-H.; Ryu, S.-Y.; Bae, D.-S.; Park, J.-T.; Kim, S.-C.; Cha, S.-D.; Kim, K.-R.; Song, S.-Y.; et al. Prognostic factors in FIGO stage IB–IIA small cell neuroendocrine carcinoma of the uterine cervix treated surgically: Results of a multi-center retrospective Korean study. Ann. Oncol. 2007, 19, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.J.; Reidy-Lagunes, D.; Gehrig, P.A. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011, 122, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Takei, Y.; Treilleux, I.; Devouassoux-Shisheboran, M.; Ledermann, J.; Viswanathan, A.N.; Mahner, S.; Provencher, D.M.; Mileshkin, L.; Åvall-Lundqvist, E.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Small Cell Carcinoma of the Cervix. Int. J. Gynecol. Cancer 2014, 24 (Suppl. 3), S102–S108. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.G.; Kapp, D.S.; Shin, J.Y.; Urban, R.; Sherman, A.E.; Chen, L.-M.; Osann, K.; Chan, J.K. Small cell carcinoma of the cervix: Treatment and survival outcomes of 188 patients. Am. J. Obstet. Gynecol. 2010, 203, 347.e1–347.e6. [Google Scholar] [CrossRef]
- Huang, L.; Liao, L.-M.; Liu, A.-W.; Wu, J.-B.; Cheng, X.-L.; Lin, J.-X.; Zheng, M. Analysis of the impact of platinum-based combination chemotherapy in small cell cervical carcinoma: A multicenter retrospective study in Chinese patients. BMC Cancer 2014, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Gordhandas, S.; Schlappe, B.A.; Zhou, Q.; Iasonos, A.; Leitao, M.M.; Park, K.J.; de Brot, L.; Alektiar, K.M.; Sabbatini, P.J.; Aghajanian, C.A.; et al. Small cell neuroendocrine carcinoma of the cervix: Analysis of prognostic factors and patterns of metastasis. Gynecol. Oncol. Rep. 2022, 43, 101058. [Google Scholar] [CrossRef]
- Zivanovic, O.; Leitao, M.; Park, K.; Zhao, H.; Diaz, J.; Konner, J.; Alektiar, K.; Chi, D.; Abu-Rustum, N.; Aghajanian, C. Small cell neuroendocrine carcinoma of the cervix: Analysis of outcome, recurrence pattern and the impact of platinum-based combination chemotherapy. Gynecol. Oncol. 2009, 112, 590–593. [Google Scholar] [CrossRef]
- Rickman, D.S.; Beltran, H.; Demichelis, F.; Rubin, M.A. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med. 2017, 23, 664–673. [Google Scholar] [CrossRef]


| Variable | Training Cohort | % | Validation Cohort | % | p Value |
|---|---|---|---|---|---|
| N = 171 | N = 86 | ||||
| Age median (years); | 0.399 | ||||
| Mean ± SD | 45.92 ± 9.991 | 44.91 ± 10.104 | |||
| Median (range) | 46 (25–75) | 45 (25–75) | |||
| BMI (kg/m2); | 0.781 | ||||
| Mean ± SD | 22.81 ± 2.976 | 22.73 ± 2.605 | |||
| Median (range) | 22.30 (18–39) | 22 (18–32) | |||
| FIGO stage | 0.582 | ||||
| I | 111 | 64.9 | 51 | 59.3 | |
| II | 24 | 14.0 | 16 | 18.6 | |
| III | 36 | 21.1 | 19 | 22.1 | |
| Pathological type | 0.527 | ||||
| LG-NECC | 21 | 12.3 | 13 | 15.1 | |
| HG-NECC | 150 | 87.7 | 73 | 84.9 | |
| Stromal invasion | 0.567 | ||||
| <1/2 | 86 | 50.3 | 40 | 46.5 | |
| ≥1/2 | 85 | 49.7 | 46 | 53.5 | |
| Endometrial invasion | 0.912 | ||||
| Yes | 23 | 13.5 | 12 | 14.0 | |
| No | 148 | 86.5 | 74 | 86.0 | |
| Nerve invasion | |||||
| Yes | 14 | 8.2 | 8 | 9.3 | 0.763 |
| No | 157 | 91.8 | 78 | 90.7 | |
| C-UJI | 0.168 | ||||
| Yes | 40 | 23.4 | 27 | 31.4 | |
| No | 131 | 76.6 | 59 | 68.6 | |
| LVI | 0.681 | ||||
| Yes | 45 | 26.3 | 22 | 25.6 | |
| No | 126 | 73.7 | 64 | 74.4 | |
| LVSI | 0.135 | ||||
| Yes | 123 | 71.9 | 54 | 62.8 | |
| No | 48 | 28.1 | 32 | 37.2 | |
| HPV | |||||
| negative | 81 | 47.4 | 37 | 43.0 | 0.509 |
| positive | 90 | 52.6 | 49 | 57.0 | |
| P16 | 0.681 | ||||
| 0 | 21 | 12.3 | 13 | 15.1 | |
| 1+ | 80 | 46.8 | 44 | 51.2 | |
| 2+ | 12 | 7.0 | 4 | 4.7 | |
| 3+ | 58 | 33.9 | 25 | 29.1 | |
| Syn | 0.293 | ||||
| 0 | 17 | 9.9 | 14 | 16.3 | |
| 1+ | 94 | 55.0 | 48 | 55.8 | |
| 2+ | 41 | 24.0 | 19 | 22.1 | |
| 3+ | 19 | 11.1 | 5 | 5.8 | |
| CgA | 0.523 | ||||
| 0 | 53 | 31.0 | 20 | 23.3 | |
| 1+ | 72 | 42.1 | 42 | 48.8 | |
| 2+ | 21 | 12.3 | 9 | 10.5 | |
| 3+ | 25 | 14.6 | 15 | 17.4 | |
| CD56 | 0.722 | ||||
| 0 | 46 | 26.9 | 20 | 23.3 | |
| 1+ | 71 | 41.5 | 42 | 48.8 | |
| 2+ | 14 | 8.2 | 7 | 8.1 | |
| 3+ | 40 | 23.4 | 17 | 19.8 | |
| Recurrence | 0.122 | ||||
| Yes | 23 | 13.5 | 18 | 20.9 | |
| No | 148 | 86.5 | 68 | 79.1 | |
| Death | 0.669 | ||||
| Yes | 21 | 12.3 | 9 | 10.5 | |
| No | 150 | 87.7 | 77 | 89.5 | |
| RFS (months) | 0.815 | ||||
| Median | 42 | 37.5 | |||
| Mean ± SD | 52.73 ± 42.892 | 54.51 ± 43.767 | |||
| Range | 2–150 | 4–145 | |||
| Follow-up (months) | 0.803 | ||||
| Median | 44 | 38 | |||
| Mean ± SD | 54.19 ± 41.232 | 54.51 ± 43.763 | |||
| Range | 2–150 | 4–145 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| FIGO stage | ||||||
| I | 1.000 | p < 0.001 | 1.000 | 0.023 | ||
| II | 3.903 | 1.100–13.847 | 0.035 | 8.868 | 1.523–15.652 | 0.015 |
| III | 8.718 | 3.305–15.996 | p < 0.001 | 5.628 | 1.126–12.128 | 0.035 |
| Stromal invasion (<1/2 vs. ≥1/2) | 3.715 | 1.132–3.176 | 0.009 | 9.898 | 2.309–42.429 | 0.002 |
| Nerve invasion (Yes vs. No) | 3.367 | 1.131–10.024 | 0.029 | 1.185 | 0.144–9.776 | 0.875 |
| LVSI (Yes vs. No) | 4.857 | 1.138–20.727 | 0.033 | 7.077 | 1.099–5.564 | 0.039 |
| LVI (Yes vs. No) | 3.848 | 1.693–8.748 | 0.001 | 6.235 | 1.360–8.576 | 0.018 |
| C-UJI (Yes vs. No) | 3.466 | 1.513–7.938 | 0.003 | 8.693 | 2.606–15.445 | 0.005 |
| CgA | ||||||
| 0 | 1.000 | 0.003 | 1.000 | 0.021 | ||
| 1+ | 6.300 | 0.788–5.380 | 0.083 | 6.302 | 1.143–6.841 | 0.040 |
| 2+ | 8.442 | 1.570–15.095 | 0.018 | 7.772 | 1.149–7.462 | 0.040 |
| 3+ | 9.673 | 2.933–17.216 | 0.003 | 9.362 | 4.304–10.180 | 0.003 |
| Variable | Training Cohort | Validation Cohort | ||
|---|---|---|---|---|
| C-Index | 95% CI | C-Index | 95% CI | |
| FIGO stage, stromal invasion, lymph vascular space invasion, lymph node involvement, cervical uterine junction invasion | 0.829 | 0.747–0.911 | 0.883 | 0.756–1.010 |
| FIGO stage, stromal invasion, lymph vascular space invasion, lymph node involvement, cervical uterine junction invasion, CgA | 0.863 | 0.784–0.942 | 0.884 | 0.758–1.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Pi, J.; Zou, J.; Feng, M.; Chen, H.; Lin, C.; Yang, S.; Deng, Y.; Xiao, X. A Nomogram Model Based on Neuroendocrine Markers for Predicting the Prognosis of Neuroendocrine Carcinoma of Cervix. J. Clin. Med. 2023, 12, 1227. https://doi.org/10.3390/jcm12031227
Jia M, Pi J, Zou J, Feng M, Chen H, Lin C, Yang S, Deng Y, Xiao X. A Nomogram Model Based on Neuroendocrine Markers for Predicting the Prognosis of Neuroendocrine Carcinoma of Cervix. Journal of Clinical Medicine. 2023; 12(3):1227. https://doi.org/10.3390/jcm12031227
Chicago/Turabian StyleJia, Mingzhu, Jiangchuan Pi, Juan Zou, Min Feng, Huiling Chen, Changsheng Lin, Shuqi Yang, Ying Deng, and Xue Xiao. 2023. "A Nomogram Model Based on Neuroendocrine Markers for Predicting the Prognosis of Neuroendocrine Carcinoma of Cervix" Journal of Clinical Medicine 12, no. 3: 1227. https://doi.org/10.3390/jcm12031227
APA StyleJia, M., Pi, J., Zou, J., Feng, M., Chen, H., Lin, C., Yang, S., Deng, Y., & Xiao, X. (2023). A Nomogram Model Based on Neuroendocrine Markers for Predicting the Prognosis of Neuroendocrine Carcinoma of Cervix. Journal of Clinical Medicine, 12(3), 1227. https://doi.org/10.3390/jcm12031227





