miR-197-3p Promotes Osteosarcoma Stemness and Chemoresistance by Inhibiting SPOPL
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Establishment of MTX-Resistant Osteosarcoma Subclones
2.2. Chemical and Reagents
2.3. Microarray-Based Analysis of microRNA Expression Profiles
2.4. MicroRNA Synthesis and Quantitative Real-Time PCR
2.5. MTT Assay for Cell Viability
2.6. Flow Cytometry Analysis
2.7. Transwell Migration Assay
2.8. Western Blots and Antibodies
2.9. Immunofluorescence Staining
2.10. Prediction of miRNA and mRNA Interaction
2.11. Luciferase Reporter Assay
2.12. Animal Models
2.13. Statistical Analysis
3. Results
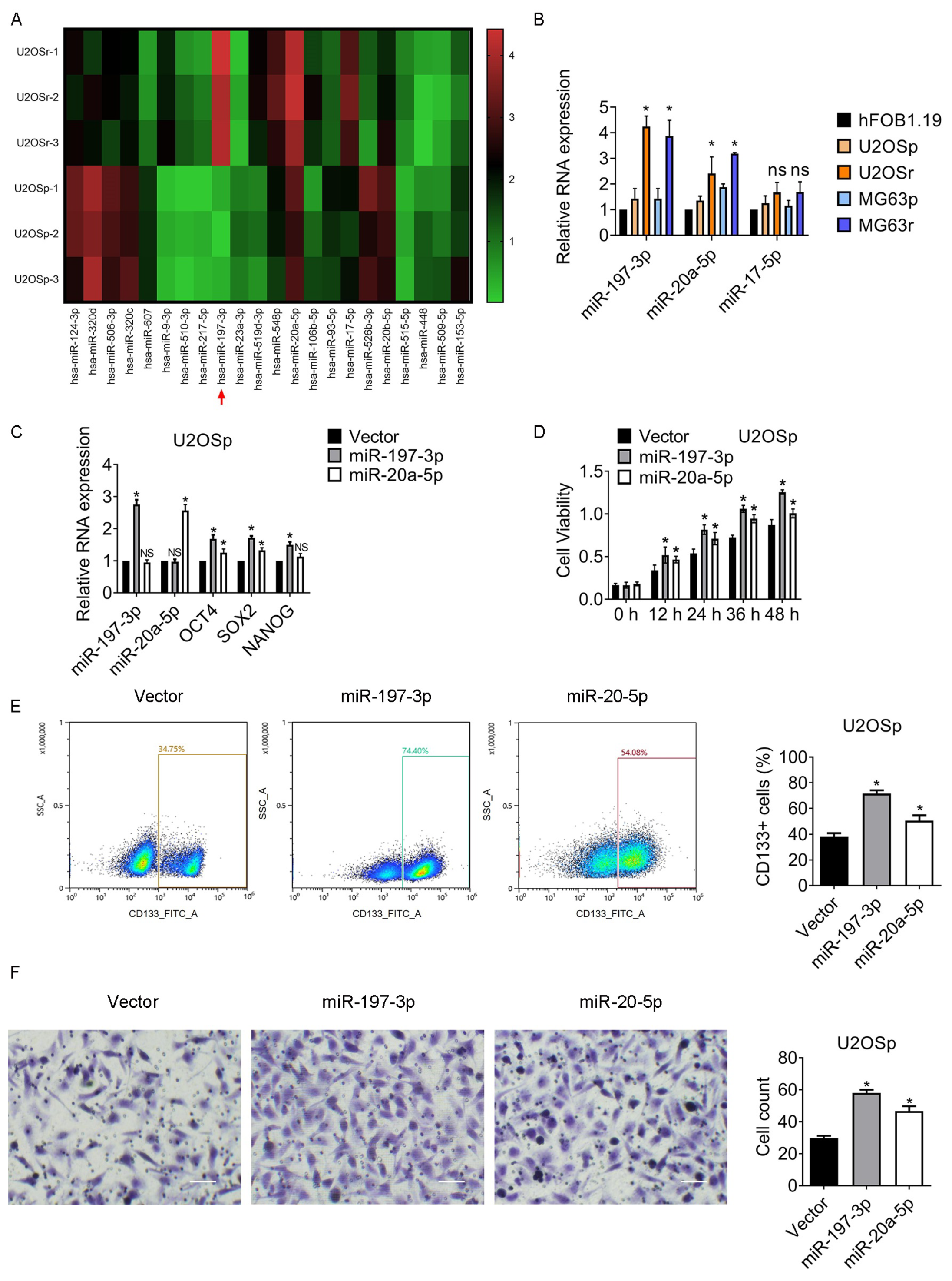
3.1. Subsection the CD133-Positive Populations Are Enriched in MTX-Resistant Osteosarcoma Clones
3.2. miR-197-3p and miR-20a-5p Increase in MTX-Resistant Subclones and Participate in the Regulation of Chemoresistance and Stemness
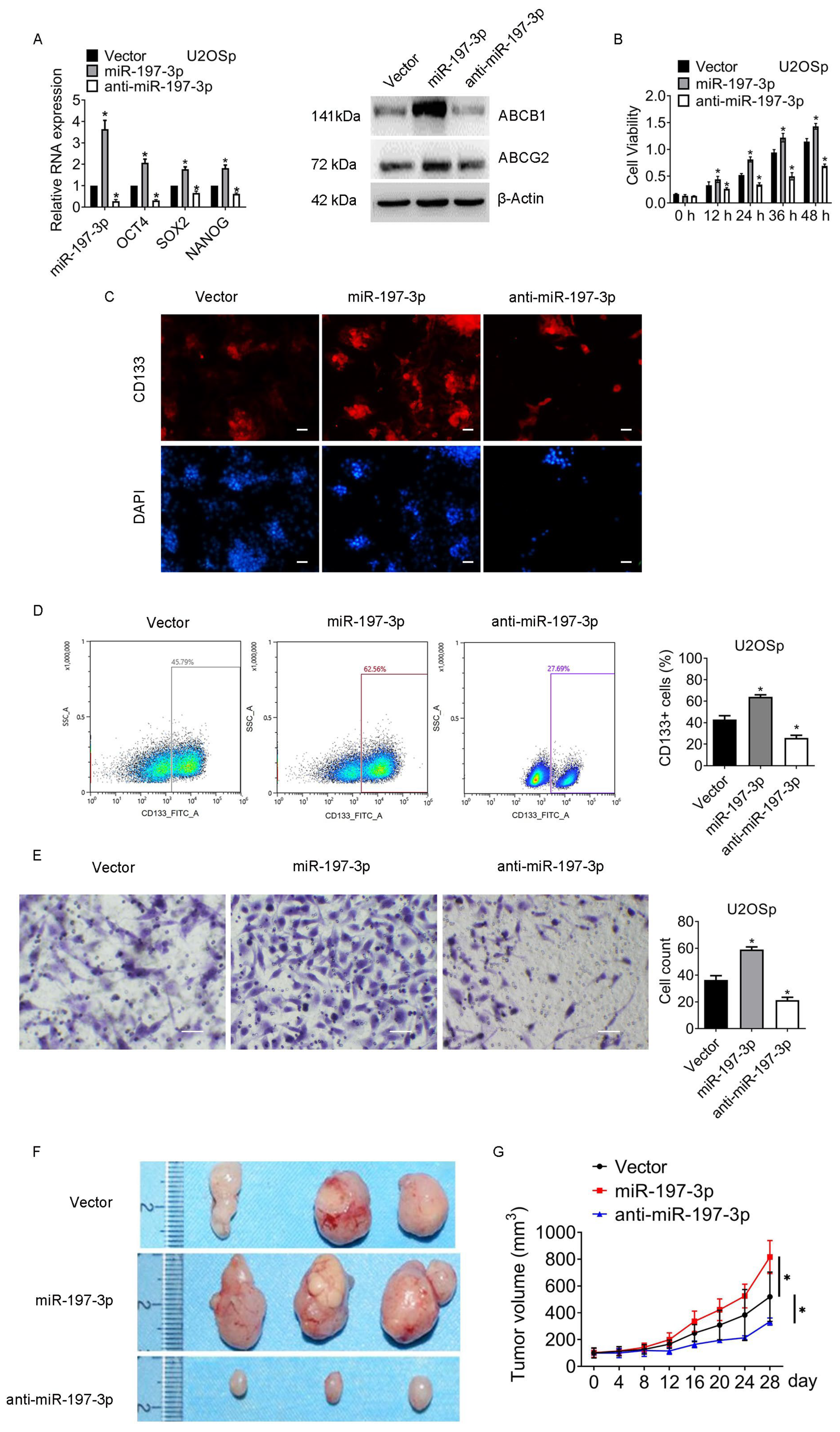
3.3. miR-197-3p Is Pivotal for Sustaining Chemoresistance and Stemness of Osteosarcoma
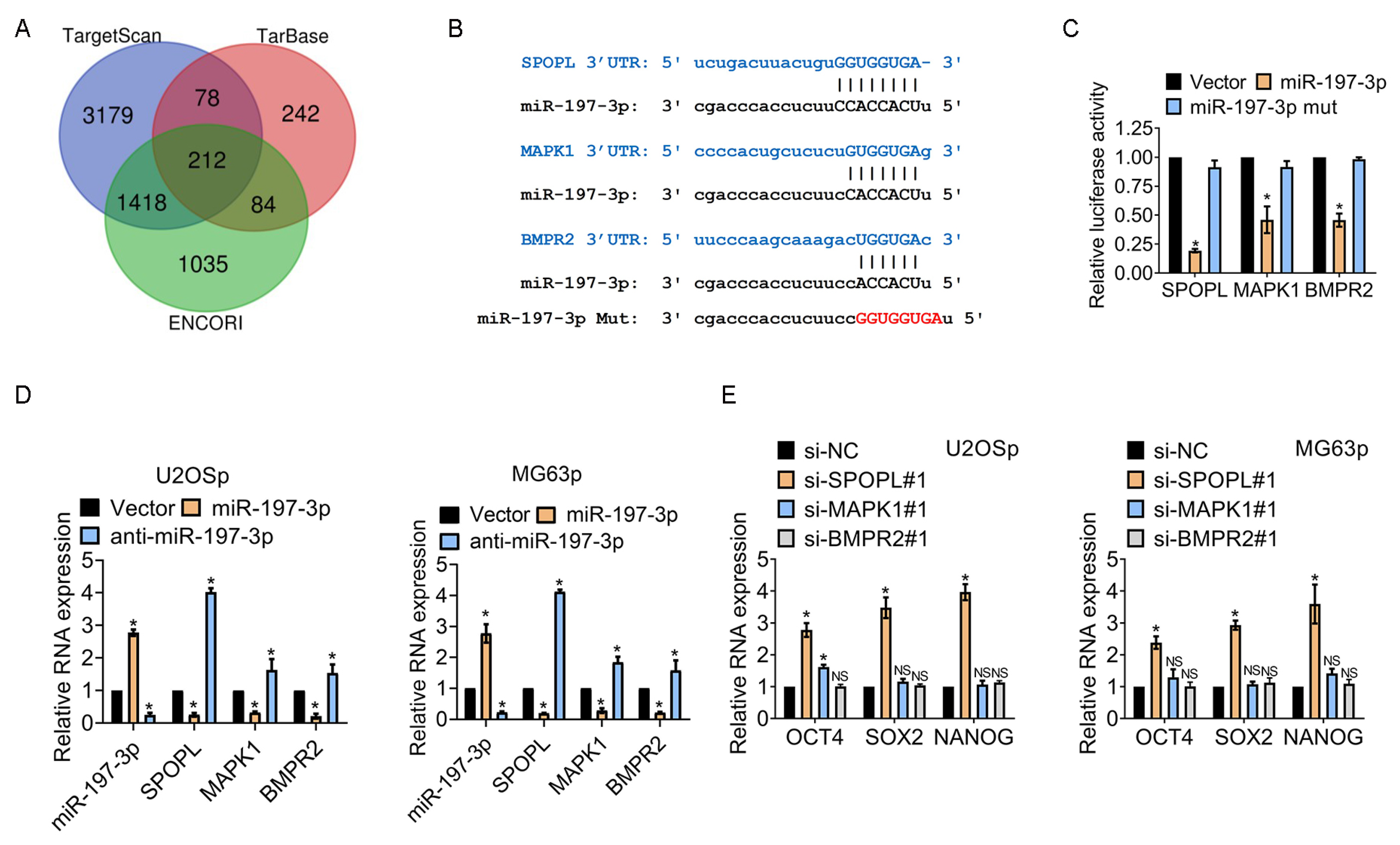
3.4. miR-197-3p Sustains Stemness of Osteosarcoma Cells by Targeting SPOPL
3.5. The miR-197-3p-SPOPL Axis Is Essential for Maintaining Osteosarcoma Chemoresistance and Stemness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hattinger, C.M.; Patrizio, M.P.; Fantoni, L.; Casotti, C.; Riganti, C.; Serra, M. Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers 2021, 13, 2878. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Menendez, S.T.; Gallego, B.; Murillo, D.; Rodriguez, A.; Rodriguez, R. Cancer Stem Cells as a Source of Drug Resistance in Bone Sarcomas. J. Clin. Med. 2021, 10, 2621. [Google Scholar] [CrossRef]
- Garcia-Ortega, D.Y.; Cabrera-Nieto, S.A.; Caro-Sanchez, H.S.; Cruz-Ramos, M. An overview of resistance to chemotherapy in osteosarcoma and future perspectives. Cancer Drug Resist. 2022, 5, 762–793. [Google Scholar] [CrossRef]
- Llobat, L.; Gourbault, O. Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines 2021, 9, 463. [Google Scholar] [CrossRef]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Drug resistance-related microRNAs in osteosarcoma: Translating basic evidence into therapeutic strategies. J. Cell. Mol. Med. 2019, 23, 2280–2292. [Google Scholar] [CrossRef]
- Ma, Y.; Yuwen, D.; Chen, J.; Zheng, B.; Gao, J.; Fan, M.; Xue, W.; Wang, Y.; Li, W.; Shu, Y.; et al. Exosomal Transfer of Cisplatin-Induced miR-425-3p Confers Cisplatin Resistance in NSCLC through Activating Autophagy. Int. J. Nanomed. 2019, 14, 8121–8132. [Google Scholar] [CrossRef]
- Azar, M.; Aghazadeh, H.; Mohammed, H.N.; Sara, M.R.S.; Hosseini, A.; Shomali, N.; Tamjidifar, R.; Tarzi, S.; Mansouri, M.; Sarand, S.P.; et al. miR-193a-5p as a promising therapeutic candidate in colorectal cancer by reducing 5-FU and Oxaliplatin chemoresistance by targeting CXCR4. Int. Immunopharm. 2021, 92, 107355. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Xu, D.; Ihira, K.; Konno, Y.; Kobayashi, N.; Todo, Y.; Watari, H. Long noncoding RNA NEAT1 drives aggressive endometrial cancer progression via miR-361-regulated networks involving STAT3 and tumor microenvironment-related genes. J. Exp. Clin. Cancer Res. 2019, 38, 295. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Burleson, M. SPOP and cancer: A systematic review. Am. J. Cancer Res. 2020, 10, 704–726. [Google Scholar] [PubMed]
- Palanisamy, N.; Yang, J.; Shepherd, P.D.A.; Li-Ning-Tapia, E.M.; Labanca, E.; Manyam, G.C.; Ravoori, M.K.; Kundra, V.; Araujo, J.C.; Efstathiou, E.; et al. The MD Anderson Prostate Cancer Patient-Derived Xenograft Series (MDA PCa PDX) Captures the Molecular Landscape of Prostate Cancer and Facilitates Marker-Driven Therapy Development. Clin. Cancer Res. 2020, 26, 4933–4946. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, L.; Zhang, M.; Huang, Z.; Lin, J.; Zhang, N. Expression and clinical relevance of SPOPL in medulloblastoma. Oncol. Lett. 2017, 14, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mi, M.; Li, X.; Zheng, X.; Wu, G.; Zhang, L. lncRNA OSTN-AS1 May Represent a Novel Immune-Related Prognostic Marker for Triple-Negative Breast Cancer Based on Integrated Analysis of a ceRNA Network. Front. Genet. 2019, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Reverter-Branchat, G.; Maurici, D.; Benini, S.; Shen, J.N.; Chano, T.; Hattinger, C.M.; Manara, M.C.; Pasello, M.; Scotlandi, K.; et al. Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann. Oncol. 2004, 15, 151–160. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Aoki, Y.; Tome, Y.; Han, Q.; Yamamoto, J.; Hamada, K.; Masaki, N.; Kubota, Y.; Bouvet, M.; Nishida, K.; Hoffman, R.M. Oral-recombinant Methioninase Converts an Osteosarcoma from Methotrexate-resistant to -sensitive in a Patient-derived Orthotopic-xenograft (PDOX) Mouse Model. Anticancer Res. 2022, 42, 731–737. [Google Scholar] [CrossRef]
- Di Fiore, R.; Santulli, A.; Ferrante, R.D.; Giuliano, M.; De Blasio, A.; Messina, C.; Pirozzi, G.; Tirino, V.; Tesoriere, G.; Vento, R. Identification and expansion of human osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide treatment. J. Cell. Physiol. 2009, 219, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neves, S.R.; Lopes, A.O.; do Carmo, A.; Paiva, A.A.; Simoes, P.C.; Abrunhosa, A.J.; Gomes, C.M. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer 2012, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Aslani, S.; Babaie, F.; Hemmatzadeh, M.; Pourmoghadam, Z.; Azizi, G.; Jadidi-Niaragh, F.; Mohammadi, H. MicroRNAs Implications in the Onset, Diagnosis, and Prognosis of Osteosarcoma. Curr. Mol. Med. 2021, 21, 573–588. [Google Scholar] [CrossRef]
- Zhao, F.; Pu, Y.; Cui, M.; Wang, H.; Cai, S. MiR-20a-5p represses the multi-drug resistance of osteosarcoma by targeting the SDC2 gene. Cancer Cell Int. 2017, 17, 100. [Google Scholar] [CrossRef]
- Pu, Y.; Yi, Q.; Zhao, F.; Wang, H.; Cai, W.; Cai, S. MiR-20a-5p represses multi-drug resistance in osteosarcoma by targeting the KIF26B gene. Cancer Cell Int. 2016, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, N.; Yang, Y.; Kleinerman, E.S. The Fas/FasL Signaling Pathway: Its Role in the Metastatic Process and as a Target for Treating Osteosarcoma Lung Metastases. Adv. Exp. Med. Biol. 2020, 1258, 177–187. [Google Scholar]
- Wang, W.; Zhou, L.; Li, Z.; Lin, G. Circ_0014130 is involved in the drug sensitivity of colorectal cancer through miR-197-3p/PFKFB3 axis. J. Gastroenterol. Hepatol. 2022, 37, 908–918. [Google Scholar] [CrossRef]
- Matuszyk, J. MALAT1-miRNAs network regulate thymidylate synthase and affect 5FU-based chemotherapy. Mol. Med. 2022, 28, 89. [Google Scholar] [CrossRef]
- Wang, M.; Hu, H.; Wang, Y.; Huang, Q.; Huang, R.; Chen, Y.; Ma, T.; Qiao, T.; Zhang, Q.; Wu, H.; et al. Long non-coding RNA TUG1 mediates 5-fluorouracil resistance by acting as a ceRNA of miR-197-3p in colorectal cancer. J. Cancer 2019, 10, 4603–4613. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; He, S. Hsa_circ_0025202 suppresses cell tumorigenesis and tamoxifen resistance via miR-197-3p/HIPK3 axis in breast cancer. World J. Surg. Oncol. 2021, 19, 39. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Li, Y. Silencing circular RNA-friend leukemia virus integration 1 restrained malignancy of CC cells and oxaliplatin resistance by disturbing dyskeratosis congenita 1. Open Life Sci. 2022, 17, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, P.; Zhao, W.; Zhu, L.; Sui, J.; Dai, Y.; Lai, Y. MiR-197-3p reduces bortezomib resistance in multiple myeloma by inhibiting IL-6 expression in a MEAF6-dependent manner. Leuk. Res. 2022, 114, 106785. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Ma, F.; Mi, L.; Gao, L.; Yu, W.; Li, M.; Feng, Z.; Huang, Y.; Wang, Q. Circ-CCS enhances autophagy during imatinib resistance of gastrointestinal stromal tumor by regulating miR-197-3p/ATG10 signaling. J. Cancer Res. Ther. 2022, 18, 1338–1345. [Google Scholar] [PubMed]
- Jiao, C.; Meng, T.; Zhou, C.; Wang, X.; Wang, P.; Lu, M.; Tan, X.; Wei, Q.; Ge, X.; Jin, J. TGF-beta signaling regulates SPOP expression and promotes prostate cancer cell stemness. Aging 2020, 12, 7747–7760. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, J.; Wan, F.; Zhao, L.; Chu, H.; Chen, C.; Liao, G.; Liu, J.; Yu, Y.; Teng, H.; et al. AMPK Promotes SPOP-Mediated NANOG Degradation to Regulate Prostate Cancer Cell Stemness. Dev. Cell 2019, 48, 345–360.e7. [Google Scholar] [CrossRef]
- Wang, L.Q.; Yu, P.; Li, B.; Guo, Y.H.; Liang, Z.R.; Zheng, L.L.; Yang, J.H.; Xu, H.; Liu, S.; Zheng, L.S.; et al. miR-372 and miR-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol. Oncol. 2018, 12, 1949–1964. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, S.; Bai, Y.; Ali, A.M.; Deng, J.; Chen, Y.; Fu, Y.; He, M. miR-197-3p Promotes Osteosarcoma Stemness and Chemoresistance by Inhibiting SPOPL. J. Clin. Med. 2023, 12, 1177. https://doi.org/10.3390/jcm12031177
Zhang J, Wang S, Bai Y, Ali AM, Deng J, Chen Y, Fu Y, He M. miR-197-3p Promotes Osteosarcoma Stemness and Chemoresistance by Inhibiting SPOPL. Journal of Clinical Medicine. 2023; 12(3):1177. https://doi.org/10.3390/jcm12031177
Chicago/Turabian StyleZhang, Jingyong, Shubao Wang, Yang Bai, Aasi Mohammad Ali, Jiewen Deng, Yushi Chen, Yonghui Fu, and Ming He. 2023. "miR-197-3p Promotes Osteosarcoma Stemness and Chemoresistance by Inhibiting SPOPL" Journal of Clinical Medicine 12, no. 3: 1177. https://doi.org/10.3390/jcm12031177
APA StyleZhang, J., Wang, S., Bai, Y., Ali, A. M., Deng, J., Chen, Y., Fu, Y., & He, M. (2023). miR-197-3p Promotes Osteosarcoma Stemness and Chemoresistance by Inhibiting SPOPL. Journal of Clinical Medicine, 12(3), 1177. https://doi.org/10.3390/jcm12031177




