Influence of Guanine-Based Purines on the Oxidoreductive Reactions Involved in Normal or Altered Brain Functions
Abstract
1. Introduction
2. Outline of the Role of Guanine Base Purines in the Brain
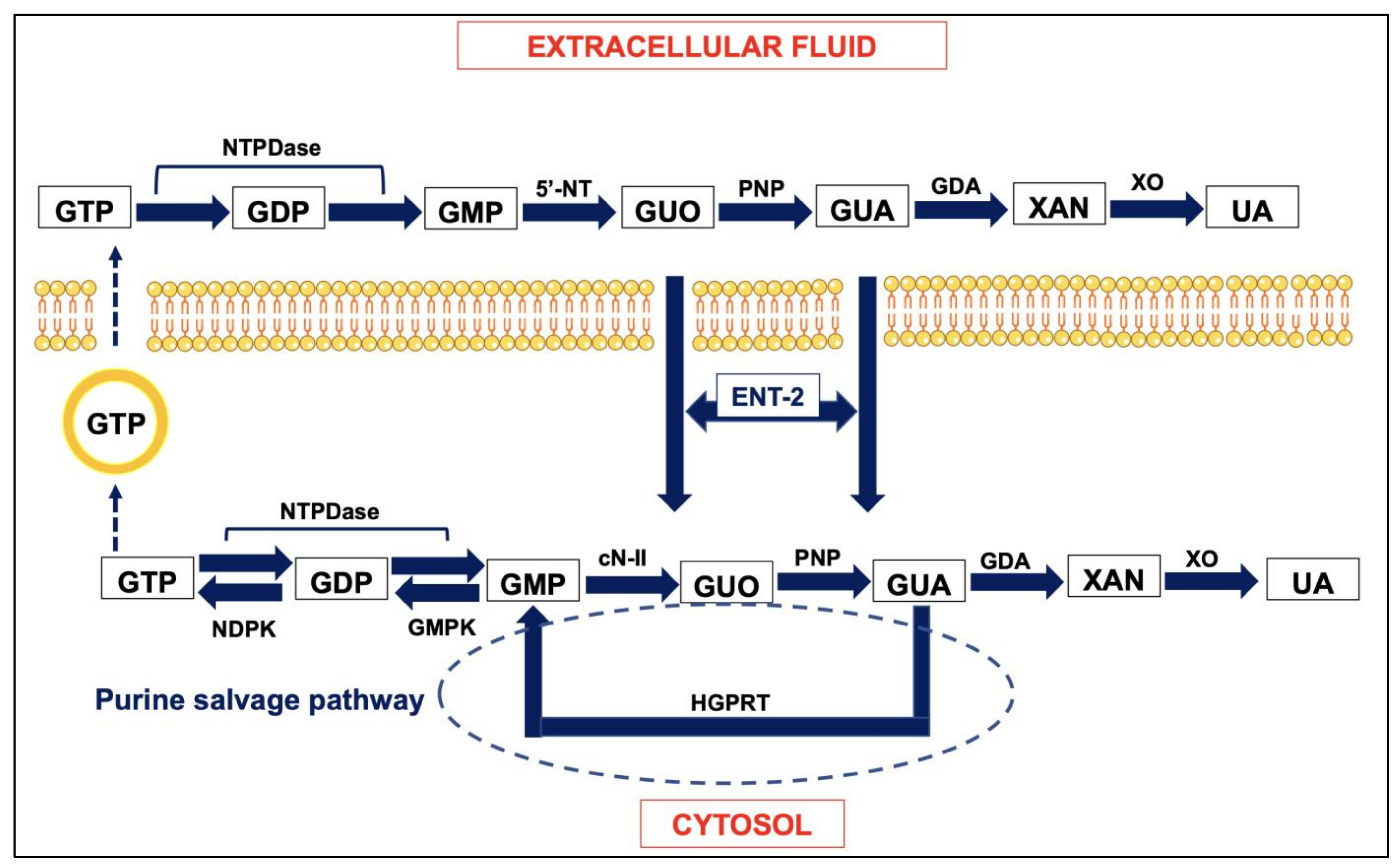
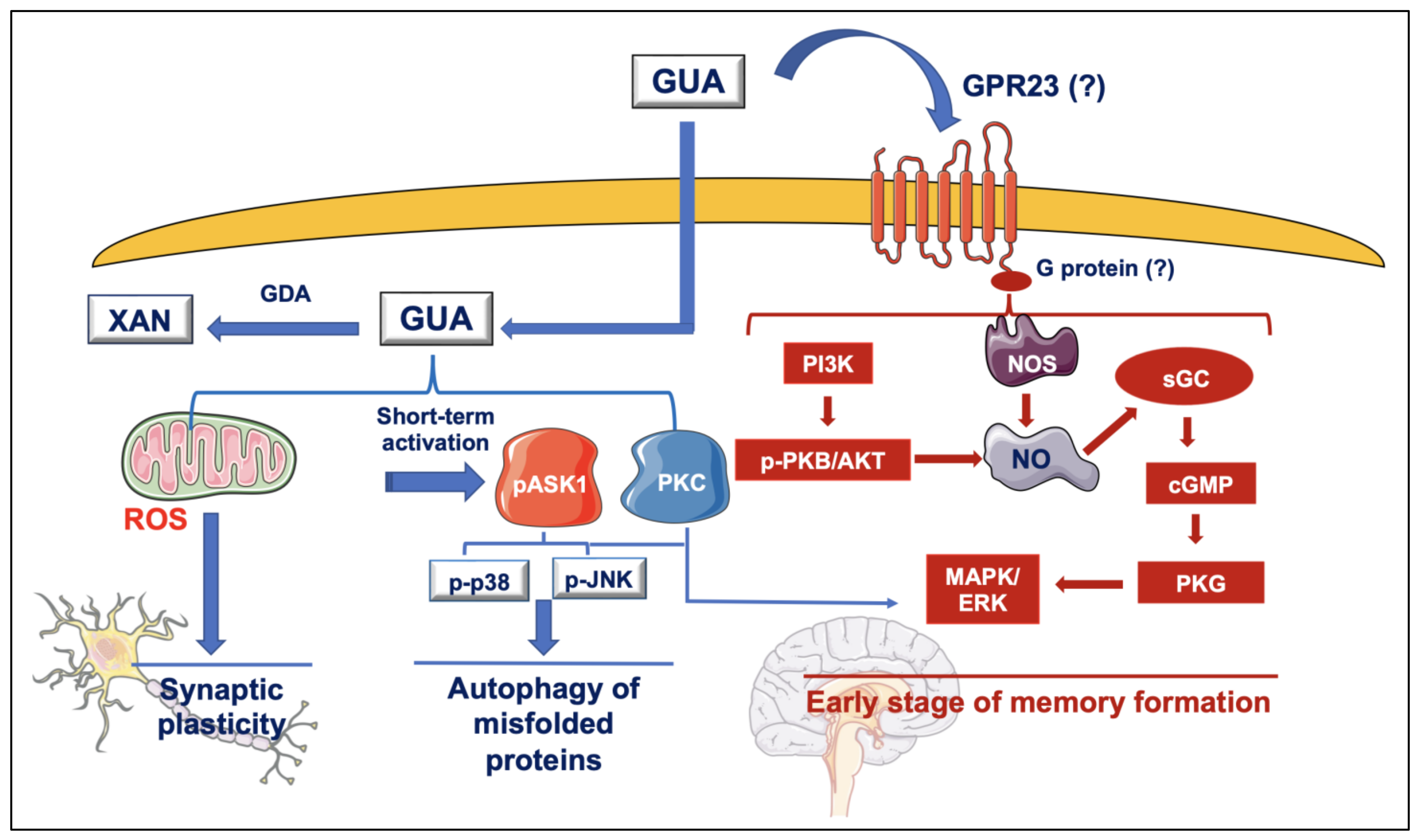
3. Neuroprotective Effects of GBPs, Mainly GUO, against Brain Oxidative Injury
| Experimental Model | Principal Mechanisms | Effect(s) | Ref. |
|---|---|---|---|
| Astrocyte cell lines under OGD | Activation of PI3K, PKC, MAPK/ERK | Reduction in oxidative stress | [61] |
| Hippocampal slices under OGD | Activation of MAPK/ERK in cooperation with adenosine A1R | Reduction in oxidative stress, promotion of Glu uptake and inhibition of nNOS | [66] |
| Hippocampal slices exposed to Glu | Activation of PI3K/GSK3β pathway | iNOS suppression but only at [GUO] = 100 µM | [67] |
| Hippocampal slices under OGD | Inhibition of nNOS activity | Mitochondria protection. Inhibition of ROS/radical species production | [68] |
| Hippocampal slices subjected to OGD and then reoxygenation | Inhibition of NOS activity | Prevention of the impairment of ATP production in neural cells and of lactate release and Glu uptake from astrocytes | [69] |
| Astrocytes from aged rats | HO-1 activation | Anti-inflammatory effects | [80] |
| Rat C6 glioma cells exposed to azide | HO-1 activation | Inhibition of oxidative/nitrosative stress | [81] |
| SH-SY5Y neuroblastoma cells subjected to mitochondrial oxidative stress with rotenone+ oligomycin Rat cortical neurons and astrocytes | HO-1 activation and involvement of PI3K/GSK3β pathway Modulation of SUMOylation (short-time effect = 1 h) | Protection against mitochondrial oxidative stress Possible neuroprotection | [82] [83] |
| SH-SY5Y neuroblastoma cells exposed to Aβ peptide | Inhibition of ROS production Inhibition of β-secretase | Inhibition of cell apoptosis | [95,96] |
| SH-SY5Y neuroblastoma cells exposed to MPP+ | Involvement of PI3K pathway | Inhibition of cell apoptosis | [99] |
| Rat striatal slices exposed to 6-OHDA | Prevention of mitochondria dysfunction as for ATP depletion and ROS production | Protection against oxidative damage | [100] |
| Experimental Model | Principal Mechanisms | Effect(s) | Ref. |
|---|---|---|---|
| Acute ammonia intoxication in adult rats | Decreased Glu and alanine levels in CSF and oxidative stress in cerebral cortex | Reduction in lethality and coma duration; improvement of EEG traces | [86] |
| Chronic hepatic encephalopathy obtained by bile duct ligation in rats | Reduction in Glu and other metabolite levels in the CSF and of oxidative brain stress parameters | Attenuation of behavioral and EEG impairment | [87] |
| Ischemia induced by thermocoagulation in rat cortical brain | Prevention of ROS production and lipid peroxidation | Reduction in infarcted area, inflammation and neurodegeneration; improvement of forelimb dysfunction | [88,89] |
| Traumatic injury in rat brain induced by fluid percussion AD mouse model obtained by i.c.v. injection of Aβ oligomers | Reduction in mitochondrial dysfunction and glutamate activity | Protection against locomotor and behavioral impairments Recovery of object recognition short-term memory | [90,91] |
| Involvement of adenosine A1R | [57] | ||
| Restoration of glutamate uptake and pre-synaptic Ca2+ homeostasis; partial protection of mitochondrial swelling | [97] | ||
| Rat cecal ligation inducing oxidative stress in different brain regions | Reduction in lipid peroxidation | Neuroprotection; improvement of cognitive impairment | [101] |
4. Neuroprotective Effects of GBPs against Learning and Memory Impairment
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magistretti, P.J.; Pellerin, L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1155–1163. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration; from a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef]
- Kishida, K.T.; Klann, E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid. Redox Signal. 2007, 9, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, C.; Carrasco, M.A.; Muñoz, P.; Núñez, M.T. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid. Redox Signal. 2007, 9, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.; Day, A. Hydrogen peroxide as a signaling molecule. Antioxid. Redox Signal. 2011, 15, 147–151. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative eustress; On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Neurovascular-neuroenergetic coupling axis in the brain: Master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic Biol. Med. 2017, 108, 668–682. [Google Scholar] [CrossRef]
- Lushchak, V.I. Interplay between bioenergetics and oxidative stress at normal brain aging. Aging as a result of increasing disbalance in the system oxidative stress-energy provision. Pflugers Arch. 2021, 473, 713–722. [Google Scholar] [CrossRef]
- Garaschuk, O.; Semchyshyn, H.M.; Lushchak, V.I. Healthy brain aging: Interplay between reactive species, inflammation and energy supply. Ageing Res. Rev. 2018, 43, 26–45. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy; Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy; Interactions and Molecular Regulatory Mechanisms. Cell Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Frati, A.; Santoro, A.; Frati, P.; Fineschi, V.; Pesce, A. Diffuse Axonal Injury; Clinical Prognostic Factors; Molecular Experimental Models and the Impact of the Trauma Related Oxidative Stress. An Extensive Review Concerning Milestones and Advances. Int. J. Mol. Sci. 2021, 22, 10865. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress; A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative Stress; Inflammation; and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef]
- Parsons, A.L.M.; Bucknor, E.M.V.; Castroflorio, E.; Soares, T.R.; Oliver, P.L.; Rial, D. The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants 2022, 11, 157. [Google Scholar] [CrossRef]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. Adv. Neurobiol. 2020, 24, 193–206. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia. Brain Sci. 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef]
- Kandezi, N.; Mohammadi, M.; Ghaffari, M.; Gholami, M.; Motaghinejad, M.; Safari, S. Novel Insight to Neuroprotective Potential of Curcumin: A Mechanistic Review of Possible Involvement of Mitochondrial Biogenesis and PI3/Akt/GSK3 or PI3/Akt/CREB/BDNF Signaling Pathways. Int. J. Mol. Cell Med. 2020, 9, 1–32. [Google Scholar] [CrossRef]
- Tsai, I.C.; Hsu, C.W.; Chang, C.H.; Tseng, P.T.; Chang, K.V. The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Ghanaatfar, F.; Ghanaatfar, A.; Isapour, P.; Farokhi, N.; Bozorgniahosseini, S.; Javadi, M.; Gholami, M.; Ulloa, L.; Coleman-Fuller, N.; Motaghinejad, M. Is lithium neuroprotective? An updated mechanistic illustrated review. Fundam. Clin. Pharmacol. 2022, 37, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Zarneshan, S.N.; Fakhri, S.; Khan, H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res. 2022, 177, 106099. [Google Scholar] [CrossRef]
- Khan, N.; Shah, P.P.; Ban, D.; Trigo-Mouriño, P.; Carneiro, M.G.; DeLeeuw, L.; Dean, W.L.; Trent, J.O.; Beverly, L.J.; Konrad, M.; et al. Solution structure and functional investigation of human guanylate kinase reveals allosteric networking and a crucial role for the enzyme in cancer. J. Biol. Chem. 2019, 294, 11920–11933. [Google Scholar] [CrossRef] [PubMed]
- Boissan, M.; Schlattner, U.; Lacombe, M.L. The NDPK/NME superfamily: State of the art. Lab. Investig. 2018, 98, 164–174. [Google Scholar] [CrossRef]
- Mancinelli, R.; Fanò-Illic, G.; Pietrangelo, T.; Fulle, S. Guanosine-Based Nucleotides; the Sons of a Lesser God in the Purinergic Signal Scenario of Excitable Tissues. Int. J. Mol. Sci. 2020, 21, 1591. [Google Scholar] [CrossRef]
- Tasca, C.I.; Lanznaster, D.; Oliveira, K.A.; Fernández-Dueñas, V.; Ciruela, F. Neuromodulatory Effects of Guanine-Based Purines in Health and Disease. Front. Cell Neurosci. 2018, 12, 376. [Google Scholar] [CrossRef]
- Lanznaster, D.; Dal-Cim, T.; Piermartiri, T.C.; Tasca, C.I. Guanosine: A Neuromodulator with Therapeutic Potential in Brain Disorders. Aging Dis. 2016, 7, 657–679. [Google Scholar] [CrossRef]
- Kasai, H. What causes human cancer? Approaches from the chemistry of DNA damage. Genes Environ. 2016, 38, 19. [Google Scholar] [CrossRef]
- Zhou, X.E.; Melcher, K.; Xu, H.E. Understanding the GPCR biased signaling through G protein and arrestin complex structures. Curr. Opin. Struct. Biol. 2017, 45, 150–159. [Google Scholar] [CrossRef]
- Droppelmann, C.A.; Campos-Melo, D.; Volkening, K.; Strong, M.J. The emerging role of guanine nucleotide exchange factors in ALS and other neurodegenerative diseases. Front. Cell Neurosci. 2014, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F. The CGMP System: Components and Function. Biol. Chem. 2020, 401, 447–469. [Google Scholar] [CrossRef]
- Rutten, K.; Van Donkelaar, E.L.; Ferrington, L.; Blokland, A.; Bollen, E.; Steinbusch, H.W.M.; Kelly, P.A.T.; Prickaerts, J.H. Phosphodiesterase Inhibitors Enhance Object Memory Independent of Cerebral Blood Flow and Glucose Utilization in rats. Neuropsychopharmacology 2009, 34, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Carlson, S.S.; Kelly, R.B. Chemical and physical characterization of cholinergic synaptic vesicles. Biochemistry 1978, 17, 1199–1206. [Google Scholar] [CrossRef]
- Santos, T.G.; Souza, D.O.; Tasca, C.I. GTP uptake into rat brain synaptic vesicles. Brain Res. 2006, 1070, 71–76. [Google Scholar] [CrossRef]
- Ciccarelli, R.; Di Iorio, P.; Giuliani, P.; D’Alimonte, I.; Ballerini, P.; Caciagli, F.; Rathbone, M.P. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia 1999, 25, 93–98. [Google Scholar] [CrossRef]
- Di Liberto, V.; Mudò, G.; Garozzo, R.; Frinchi, M.; Fernandez-Dueñas, V.; Di Iorio, P.; Ciccarelli, R.; Caciagli, F.; Condorelli, D.F.; Ciruela, F.; et al. The Guanine-Based Purinergic System: The Tale of An Orphan Neuromodulation. Front. Pharmacol. 2016, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Naes, S.M.; Ab-Rahim, S.; Mazlan, M.; Abdul Rahman, A. Equilibrative Nucleoside Transporter 2: Properties and Physiological Roles. Biomed. Res. Int. 2020, 2020, 5197626. [Google Scholar] [CrossRef]
- Zuccarini, M.; Giuliani, P.; Frinchi, M.; Mudò, G.; Serio, R.M.; Belluardo, N.; Buccella, S.; Carluccio, M.; Condorelli, D.F.; Caciagli, F.; et al. Uncovering the Signaling Pathway behind Extracellular Guanine-Induced Activation of NO System: New Perspectives in Memory-Related Disorders. Front. Pharmacol. 2018, 9, 110. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 2398212818817494. [Google Scholar] [CrossRef]
- Svensson, E.; Apergis-Schoute, J.; Burnstock, G.; Nusbaum, M.P.; Parker, D.; Schiöth, H.B. General Principles of Neuronal Co-transmission: Insights from Multiple Model Systems. Front. Neural Circuits 2019, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Introduction to Purinergic Signalling in the Brain. Adv. Exp. Med. Biol. 2020, 1202, 1–12. [Google Scholar] [CrossRef]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- IJzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine Receptors: A Further Update. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Borea, P.A.; Varani, K.; Vincenzi, F.; Jacobson, K.A.; Gessi, S. A2A Adenosine Receptor Antagonists in Neurodegenerative Diseases. Curr. Med. Chem. 2022, 29, 4138–4151. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Long, K.D.; Rosse, R.B.; Mastropaolo, J.; Eller, J. Hypothesized deficiency of guanine-based purines may contribute to abnormalities of neurodevelopment; neuromodulation; and neurotransmission in Lesch-Nyhan syndrome. Clin. Neuropharmacol. 2005, 28, 28–37. [Google Scholar] [CrossRef]
- Schmidt, A.P.; Lara, D.R.; Souza, D.O. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol. Ther. 2007, 116, 401–416. [Google Scholar] [CrossRef]
- Bettio, L.E.; Gil-Mohapel, J.; Rodrigues, A.L. Guanosine and its role in neuropathologies. Purinergic Signal. 2016, 12, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, P.; Beggiato, S.; Ronci, M.; Nedel, C.B.; Tasca, C.I.; Zuccarini, M. Unfolding New Roles for Guanine-Based Purines and Their Metabolizing Enzymes in Cancer and Aging Disorders. Front. Pharmacol. 2021, 12, 653549. [Google Scholar] [CrossRef] [PubMed]
- Gysbers, J.W.; Guarnieri, S.; Mariggiò, M.A.; Pietrangelo, T.; Fanò, G.; Rathbone, M.P. Extracellular guanosine 5′ triphosphate enhances nerve growth factor-induced neurite outgrowth via increases in intracellular calcium. Neuroscience 2000, 96, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, R.; Zuccarini, M.; Giuliani, P.; Di Liberto, V.; Mudò, G.; Caciagli, F.; Ciccarelli, R.; Ciruela, F.; Di Iorio, P.; Condorelli, D.F. Guanine inhibits the growth of human glioma and melanoma cell lines by interacting with GPR23. Front. Pharmacol. 2022, 13, 970891. [Google Scholar] [CrossRef] [PubMed]
- Traversa, U.; Bombi, G.; Di Iorio, P.; Ciccarelli, R.; Werstiuk, E.S.; Rathbone, M.P. Specific [(3)H]-guanosine binding sites in rat brain membranes. Br. J. Pharmacol. 2002, 135, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.F.; Comasseto, D.D.; Ramos, D.B.; Hansel, G.; Zimmer, E.R.; Loureiro, S.O.; Ganzella, M.; Souza, D.O. Guanosine Anxiolytic-Like Effect Involves Adenosinergic and Glutamatergic Neurotransmitter Systems. Mol. Neurobiol. 2017, 54, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Massari, C.M.; Constantino, L.C.; Marques, N.F.; Binder, L.B.; Valle-León, M.; López-Cano, M.; Fernández-Dueñas, V.; Ciruela, F.; Tasca, C.I. Involvement of adenosine A1 and A2A receptors on guanosine-mediated anti-tremor effects in reserpinized mice. Purinergic Signal. 2020, 16, 379–387. [Google Scholar] [CrossRef]
- Dobrachinski, F.; Gerbatin, R.R.; Sartori, G.; Golombieski, R.M.; Antoniazzi, A.; Nogueira, C.W.; Royes, L.F.; Fighera, M.R.; Porciúncula, L.O.; Cunha, R.A.; et al. Guanosine Attenuates Behavioral Deficits After Traumatic Brain Injury by Modulation of Adenosinergic Receptors. Mol. Neurobiol. 2019, 56, 3145–3158. [Google Scholar] [CrossRef]
- Gerbatin, R.R.; Dobrachinski, F.; Cassol, G.; Soares, F.A.A.; Royes, L.F.F. A 1 rather than A 2A adenosine receptor as a possible target of Guanosine effects on mitochondrial dysfunction following Traumatic Brain Injury in rats. Neurosci. Lett. 2019, 704, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Lanznaster, D.; Massari, C.M.; Marková, V.; Símková, T.; Duroux, R.; Jacobson, K.A.; Fernández-Dueñas, V.; Tasca, C.I.; Ciruela, F. Adenosine A1-A2A Receptor-Receptor Interaction: Contribution to Guanosine-Mediated Effects. Cells 2019, 8, 1630. [Google Scholar] [CrossRef]
- Dal-Cim, T.; Ludka, F.K.K.; Martins, W.C.; Reginato, C.; Parada, E.; Egea, J.; López, M.G.; Tasca, C.I. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J. Neurochem. 2013, 126, 437–450. [Google Scholar] [CrossRef]
- Frinchi, M.; Verdi, V.; Plescia, F.; Ciruela, F.; Grillo, M.; Garozzo, R.; Condorelli, D.F.; Di Iorio, P.; Caciagli, F.; Ciccarelli, R.; et al. Guanosine-Mediated Anxiolytic-Like Effect: Interplay with Adenosine A 1 and A 2A Receptors. Int. J. Mol. Sci. 2020, 21, 9281. [Google Scholar] [CrossRef]
- Chojnowski, K.; Opielka, M.; Nazar, W.; Kowianski, P.; Smolenski, R.T. Neuroprotective Effects of Guanosine in Ischemic Stroke-Small Steps towards Effective Therapy. Int. J. Mol. Sci. 2021, 22, 6898. [Google Scholar] [CrossRef]
- Massari, C.M.; Zuccarini, M.; Di Iorio, P.; Tasca, C.I. Guanosine Mechanisms of Action: Toward Molecular Targets. Front. Pharmacol. 2021, 12, 653146. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.F.; Hascup, E.R.; Bartke, A.; Hascup, K.N. Friend or Foe? Defining the Role of Glutamate in Aging and Alzheimer’s Disease. Front. Aging 2022, 3, 929474. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Elfeki, N.; Ballerini, P.; D’Alimonte, I.; Bau, C.; Ciccarelli, R.; Caciagli, F.; Gabriele, J.; Jiang, S. Guanosine improves motor behavior, reduces apoptosis, and stimulates neurogenesis in rats with parkinsonism. J. Neurosci. Res. 2009, 87, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Bettio, L.E.; Neis, V.B.; Pazini, F.L.; Brocardo, P.S.; Patten, A.R.; Gil-Mohapel, J.; Christie, B.R.; Rodrigues, A.L. The antidepressant-like effect of chronic guanosine treatment is associated with increased hippocampal neuronal differentiation. Eur. J. Neurosci. 2016, 43, 1006–1015. [Google Scholar] [CrossRef]
- Dal-Cim, T.; Poluceno, G.G.; Lanznaster, D.; Oliveira, K.A.; DeNedel, C.B.; Tasca, C.I. Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: Involvement of A(1) and A(2A) adenosine receptors and PI3K, MEK, and PKC pathways. Purinergic Signal. 2019, 15, 465–476. [Google Scholar] [CrossRef]
- Egea, J.; Romero, A.; Barrio, L.; Rodrigues, A.L.S.; Tasca, C.I.; Molz, S.; Dal-Cim, T.; Budni, J.; Martín-de-Saavedra, M.D.; Egea, J.; et al. Neuroprotective effect of guanosine against glutamate-induced cell death in rat hippocampal slices is mediated by the phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase 3β pathway activation and inducible nitric oxide synthase inhibition. J. Neurosci. Res. 2011, 89, 1400–1408. [Google Scholar] [CrossRef]
- Thomaz, D.T.; Dal-Cim, T.A.; Martins, W.C.; Cunha, M.P.; Lanznaster, D.; Bem, A.F.; Tasca, C.I. Guanosine prevents nitroxidative stress and recovers mitochondrial membrane potential disruption in hippocampal slices subjected to oxygen/glucose deprivation. Purinergic Signal. 2016, 12, 707–718. [Google Scholar] [CrossRef]
- Thomaz, D.T.; Rafognatto, R.; Luisa, A.; Binder, B.; Scheffer, L.; Willms, A.; Fátima, C.; Mena, R.; Silva, B.; Tasca, C.I. Guanosine Neuroprotective Action in Hippocampal Slices Subjected to Oxygen and Glucose Deprivation Restores ATP Levels, Lactate Release and Glutamate Uptake Impairment: Involvement of Nitric Oxide. Neurochem. Res. 2020, 45, 2217–2229. [Google Scholar] [CrossRef]
- Albrecht, P.; Henke, N.; Tien, M.L.; Issberner, A.; Bouchachia, I.; Maher, P.; Lewerenz, J.; Methner, A. Extracellular cyclic GMP and its derivatives GMP and guanosine protect from oxidative glutamate toxicity. Neurochem. Int. 2013, 62, 610–619. [Google Scholar] [CrossRef]
- Courtes, A.A.; de Carvalho, N.R.; Gonçalves, D.F.; Hartmann, D.D.; da Rosa, P.C.; Dobrachinski, F.; Franco, J.L.; de Souza, D.O.G.; Soares, F.A.A. Guanosine protects against Ca2+-induced mitochondrial dysfunction in rats. Biomed. Pharm. Ther. 2019, 111, 1438–1446. [Google Scholar] [CrossRef]
- Pozo Devoto, V.M.; Lacovich, V.; Feole, M.; Bhat, P.; Chovan, J.; Čarna, M.; Onyango, I.G.; Dragišić, N.; Sűsserová, M.; Barrios-Llerena, M.E.; et al. Unraveling axonal mechanisms of traumatic brain injury. Acta Neuropathol. Commun. 2022, 10, 140. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Liu, J.; Xie, F.; Su, B.; Wang, X. Abnormalities of Mitochondrial Dynamics in Neurodegenerative Diseases. Antioxidant 2017, 6, 25. [Google Scholar] [CrossRef]
- Stocker, R.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Antioxidant activities of bile pigments: Biliverdin and bilirubin. Methods Enzym. Ther. 1990, 186, 301–309. [Google Scholar] [CrossRef]
- Cazuza, R.A.; Pol, O.; Leite-Panissi, C.R.A. Enhanced expression of heme oxygenase-1 in the locus coeruleus can be associated with anxiolytic-like effects. Behav. Brain Res. 2018, 336, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ya, B.L.; Liu, Q.; Li, H.F.; Cheng, H.J.; Yu, T.; Chen, L.; Wang, Y.; Yuan, L.L.; Li, W.J.; Liu, W.Y.; et al. Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression. Oxid. Med. Cell Longev. 2018, 2018, 6069150. [Google Scholar] [CrossRef]
- Scapagnini, G.; Butterfield, D.A.; Colombrita, C.; Sultana, R.; Pascale, A.; Calabrese, V. Ethylferulate, alipophilic polyphenol induces HO-1 and protects rat neurons against oxidative stress. Antioxid. Redox Signal. 2004, 6, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gu, R.; Hu, W.; Sun, Z.; Wang, G.; Wang, L.; Xu, Y. Upregulation of hemeoxygenase-1 protected against brain damage induced by transient cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2018, 15, 4629–4636. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kim, Y.M. Beneficial and Detrimental Roles of Heme Oxygenase-1 in the Neurovascular System. Int. J. Mol. Sci. 2022, 23, 7041. [Google Scholar] [CrossRef]
- Souza, D.G.; Bellaver, B.; Bobermin, L.D.; Souza, D.O.; Quincozes-Santos, A. Anti-aging effects of guanosine in glial cells. Purinergic Signal. 2016, 12, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Bobermin, L.G.; Souza, D.; Bellaver, B.; Gonçalves, C.-A.; Souza, D.O. Guanosine protects C6 astroglial cells against azide-induced oxidative damage: A putative role of heme oxygenase 1. J. Neurochem. 2014, 130, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Dal-Cim, T.; Molz, S.; Egea, J.; Parada, E.; Romero, A.; Budni, J.; Martín, M.D.; Saavedra, D.; Tasca, C.I.; López, M.G. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3 b pathway. Neurochem. Int. 2012, 61, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zanella, C.A.; Tasca, C.I.; Henley, J.M.; Wilkinson, K.A. Guanosine modulates SUMO2/3-ylation in neurons and astrocytes via adenosine receptors. Purinergic Signal. 2020, 16, 439–450. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Henley, J.M. Mechanisms, regulation and consequences of protein SUMOylation. Biochem. J. 2010, 428, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.M.; Craig, T.J.; Wilkinson, K.A. Neuronal SUMOylation: Mechanisms, physiology, and roles in neuronal dysfunction. Physiol. Rev. 2014, 94, 1249–1285. [Google Scholar] [CrossRef]
- Cittolin-Santos, G.F.; de Assis, A.M.; Guazzelli, P.A.; Pniz, L.G.; da Silva, J.S.; Calcagnotto, M.E.; Hansel, G.; Zenki, G.K.; Kalinine, E.; Duarte, M.M.; et al. Guanosine Exerts Neuroprotective Effect in an Experimental Model of Acute Ammonia Intoxication. Mol. Neurobiol. 2017, 54, 3137–3148. [Google Scholar] [CrossRef]
- Paniz, L.G.; Calcagnotto, M.E.; Pandolfo, P.; Machado, D.G.; Santos, G.F.; Hansel, G.; Almeida, R.F.; Bruch, R.S.; Torres, F.V.; de Assis, A.M.; et al. Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab. Brain Dis. 2014, 29, 645–654. [Google Scholar] [CrossRef]
- Hansel, G.; Tonon, A.C.; Guella, F.L.; Pettenuzzo, L.F.; Duarte, T.; Duarte, M.M.; Oses, J.P.; Achaval, M.; Souza, D.O. Guanosine protects against cortical focal ischemia. Involvement of inflammatory response. Mol. Neurobiol. 2015, 52, 1791–1803. [Google Scholar] [CrossRef]
- Hansel, G.; Ramos, D.B.; Delgado, C.A.; Souza, D.G.; Almeida, R.F.; Portela, L.V.; Quincozes-Santos, A.; Souza, D.O. The potential therapeutic effect of guanosine after cortical focal ischemia in rats. PLoS ONE 2014, 9, e90693. [Google Scholar] [CrossRef]
- Courtes, A.A.; Gonçalves, D.F.; Hartmann, D.D.; da Rosa, P.C.; Cassol, G.; Royes, L.F.F.; de Carvalho, N.R.; Soares, F.A.A. Guanosine protects against behavioural and mitochondrial bioenergetic alterations after mild traumatic brain injury. Brain Res. Bull. 2020, 163, 31–39. [Google Scholar] [CrossRef]
- Gerbatin, R.R.; Cassol, G.; Dobrachinski, F.; Ferreira, A.P.O.; Quines, C.B.; Della Pace, I.D.; Busanello, G.L.; Gutierres, J.M.; Nogueira, C.W.; Oliveira, M.S.; et al. Guanosine Protects Against Traumatic Brain Injury-Induced Functional Impairments and Neuronal Loss by Modulating Excitotoxicity, Mitochondrial Dysfunction, and Inflammation. Mol. Neurobiol. 2017, 54, 7585–7596. [Google Scholar] [CrossRef]
- Morella, I.M.; Brambilla, R.; Morè, L. Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders. Neurosci. Biobehav Rev. 2022, 142, 104892. [Google Scholar] [CrossRef]
- Singh, P.; Barman, B.; Thakur, M.K. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front. Aging Neurosci. 2022, 14, 944697. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.H.; Lee, J. A New Insight into an Alternative Therapeutic Approach to Restore Redox Homeostasis and Functional Mitochondria in Neurodegenerative Diseases. Antioxidants 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Pettifer, K.M.; Kleywegt, S.; Bau, C.J.; Ramsbottom, J.D.; Vertes, E.; Ciccarelli, R.; Caciagli, F.; Werstiuk, E.S.; Rathbone, M.P. Guanosine protects SH-SY5Y cells against β-amyloid-induced apoptosis. Neuroreport 2004, 15, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Merlicco, A.; Morroni, F.; Bolondi, C.; Di Iorio, P.; Ciccarelli, R.; Romano, S.; Giuliani, P.; Hrelia, R. Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by Amyloid-β peptide oligomers. J. Biol. Regul. Homeost. Agents 2010, 24, 297–306. [Google Scholar] [PubMed]
- da Silva, J.S.; Nonose, Y.; Rohden, F.; Lukasewicz Ferreira, P.C.; Fontella, F.U.; Rocha, A.; Brochier, A.W.; Apel, R.V.; de Lima, T.M.; Seminotti, B.; et al. Guanosine Neuroprotection of Presynaptic Mitochondrial Calcium Homeostasis in a Mouse Study with Amyloid-β Oligomers. Mol. Neurobiol. 2020, 57, 4790–4809. [Google Scholar] [CrossRef]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef]
- Pettifer, K.M.; Jiang, S.; Bau, C.; Ballerini, P.; D’Alimonte, I.; Werstiuk, E.S.; Rathbone, M.P. MPP+-induced cytotoxicity in neuroblastoma cells: Antagonism and reversal by guanosine. Purinergic Signal. 2007, 3, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.F.; Massari, C.M.; Tasca, C.I. Guanosine Protects Striatal Slices Against 6-OHDA-Induced Oxidative Damage, Mitochondrial Dysfunction, and ATP Depletion. Neurotox. Res. 2019, 35, 475–483. [Google Scholar] [CrossRef]
- Petronilho, F.; Périco, S.R.; Vuolo, F.; Mina, F.; Constantino, L.; Comim, C.M.; Quevedo, J.; Souza, D.O.; Dal-Pizzo, F. Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav. Immun. 2012, 26, 904–910. [Google Scholar] [CrossRef]
- Lanznaster, D.; Mack, J.M.; Coelho, V.; Ganzella, M.; Almeide, R.F.; Dal-Cim, T.; Hansel, G.; Zimmer, E.R.; Souza, D.O.; Prediger, R.D.; et al. Guanosine Prevents Anhedonic-Like Behavior and Impairment in Hippocampal Glutamate Transport Following Amyloid-β1–40 Administration in Mice. Mol. Neurobiol. 2017, 54, 5482–5496. [Google Scholar] [CrossRef]
- Vinadé, E.R.; Schmidt, A.P.; Frizzo, M.E.; Izquierdo, I.; Elisabetsky, E.; Souza, D.O. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003, 977, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Vinadé, E.R.; Izquierdo, I.; Lara, D.R.; Schmidt, A.P.; Souza, D.O. Oral administration of guanosine impairs inhibitory avoidance performance in rats and mice. Neurobiol. Learn. Mem. 2004, 81, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ganzella, M.; de Oliveira, E.D.A.; Comassetto, D.D.; Cechetti, F.; Cereser, V.M., Jr.; Moreira, J.D.; Hansel, G.; Almeida, R.F.; Barbosa Ramos, D.; Figueredo, Y.N.; et al. Effects of chronic guanosine treatment on hippocampal damage and cognitive impairment of rats submitted to chronic cerebral hypoperfusion. Neurol. Sci. 2012, 33, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, L.; Feng, S.; Zhu, L.; Yang, L.; Liu, T.C.; Duan, R. Therapeutic non-invasive brain treatments in Alzheimer’s disease: Recent advances and challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef]
- Pandey, S.N.; Singh, G.; Semwal, B.C.; Gupta, G.; Alharbi, K.S.; Almalki, W.H.; Albratty, M.; Najmi, A.; Meraya, A.M. Therapeutic approaches of nutraceuticals in the prevention of Alzheimer’s disease. J. Food Biochem. 2022, 46, e14426. [Google Scholar] [CrossRef]
- Alhowail, A.; Alsikhan, R.; Alsaud, M.; Aldubayan, M.; Rabbani, S.I. Protective Effects of Pioglitazone on Cognitive Impairment and the Underlying Mechanisms: A Review of Literature. Drug Des. Dev. Ther. 2022, 16, 2919–2931. [Google Scholar] [CrossRef]
- Ferrari, F.; Moretti, A.; Villa, R.F. Incretin-based drugs as potential therapy for neurodegenerative diseases: Current status and perspectives. Pharmacol. Ther. 2022, 239, 108277. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Sharma, N.; Singh, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Bungau, S.G. Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders. Molecules 2022, 27, 4970. [Google Scholar] [CrossRef]
- Giuliani, P.; Buccella, S.; Ballerini, P.; Ciccarelli, R.; D’Alimonte, I.; Cicchitti, S.; Petragnani, N.; Natale, S.; Iacovella, G.; Caciagli, F.; et al. Guanine-based purines modulate the effect of L-NAME on learning and memory in rats. Panminerva Med. 2012, 54 (Suppl. 4), 53–58. [Google Scholar]
- Giuliani, P.; Ballerini, P.; Ciccarelli, R.; Buccella, S.; Romano, S.; D’Alimonte, I.; Poli, A.; Beraudi, A.; Peña, E.; Jiang, S.; et al. Tissue distribution and metabolism of guanosine in rats following intraperitoneal injection. J. Biol. Regul. Homeost. Agents 2012, 26, 51–65. [Google Scholar] [PubMed]
- Giuliani, P.; Zuccarini, M.; Buccella, S.; Rossini, M.; D’Alimonte, I.; Ciccarelli, R.; Marzo, M.; Marzo, A.; Di Iorio, P.; Caciagli, F. Development of a new HPLC method using fluorescence detection without derivatization for determining purine nucleoside phosphorylase activity in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1009–1010, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, P.; Zuccarini, M.; Buccella, S.; Peña-Altamira, L.E.; Polazzi, E.; Virgili, M.; Monti, B.; Poli, A.; Rathbone, M.P.; Di Iorio, P.; et al. Evidence for purine nucleoside phosphorylase (PNP) release from rat C6 glioma cells. J. Neurochem. 2017, 141, 208–221. [Google Scholar] [CrossRef]
- Peña-Altamira, L.E.; Polazzi, E.; Giuliani, P.; Beraudi, A.; Massenzio, F.; Mengoni, I.; Poli, A.; Zuccarini, M.; Ciccarelli, R.; Di Iorio, P.; et al. Release of soluble and vesicular purine nucleoside phosphorylase from rat astrocytes and microglia induced by pro-inflammatory stimulation with extracellular ATP via P2X7 receptors. Neurochem. Int. 2018, 115, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.R.; Welsh, W.J.; Firestein, B.L. Structural characterization of the zinc binding domain in cytosolic PSD-95 interactor (cypin): Role of zinc binding in guanine deamination and dendrite branching. Proteins 2008, 70, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.V.; Swiatwoski, P.; Kwon, M.; Rodriguez, A.R.; Campagno, K.; Firestein, B.L. A novel short isoform of cytosolic PSD-95 interactor (Cypin) regulates neuronal development. Mol. Neurobiol. 2018, 55, 6269–6281. [Google Scholar] [CrossRef]
- Swiatkowski, P.; Sewell, E.; Sweet, E.S.; Dickson, S.; Swanson, R.A.; McEwan, S.A.; Cuccolo, N.; McDonnell, M.E.; Patel, M.V.; Varghese, N.; et al. Cypin: A novel target for traumatic brain injury. Neurobiol. Dis. 2018, 119, 13–25. [Google Scholar] [CrossRef]
- Llull, L.; Amaro, S.; Chamorro, Á. Administration of uric acid in the emergency treatment of acute ischemic stroke. Curr. Neurol. Neurosci. Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, C.P.; Tseng, C.Y.; Eisenberg, Y.; Firestein, B.L. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia 2007, 55, 463–472. [Google Scholar] [CrossRef]
- De Vera, M.; Rahman, M.M.; Rankin, J.; Kopec, J.; Gao, X.; Choi, H. Gout and the risk of Parkinson’s disease: A cohort study. Arthritis Rheum. 2008, 59, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Milton, V.J.; Sweeney, S.T. Oxidative stress in synapse development and function. Dev. Neurobiol. 2012, 72, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Dailah, H.G. Potential of Therapeutic Small Molecules in Apoptosis Regulation in the Treatment of Neurodegenerative Diseases: An Updated Review. Molecules 2022, 27, 7207. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Hu, Y.; Schultz, C.; Kandel, E.R.; Hawkins, R.D. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature 1994, 368, 635–639. [Google Scholar] [CrossRef]
- Fedele, E.; Ricciarelli, R. Memory Enhancers for Alzheimer’s Dementia: Focus on cGMP. Pharmaceuticals 2021, 14, 61. [Google Scholar] [CrossRef]
- Peixoto, C.A.; Nunes, A.K.; Garcia-Osta, A. Phosphodiesterase-5 Inhibitors: Action on the Signaling Pathways of Neuroinflammation, Neurodegeneration, and Cognition. Mediat. Inflamm. 2015, 2015, 940207. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Lefkimmiatis, K.; Pozzan, T. The basics of mitochondrial cAMP signalling: Where, when, why. Cell Calcium 2021, 93, 102320. [Google Scholar] [CrossRef]
- Song, T.; Hatano, N.; Horii, M.; Tokumitsu, H.; Yamaguchi, F.; Tokuda, M.; Watanabe, S. Calcium/calmodulin-dependent protein kinase I inhibits neuronal nitric-oxide synthase activity through serine 741 phosphorylation. FEBS Lett. 2004, 570, 133–137. [Google Scholar] [CrossRef]
- Takata, T.; Araki, S.; Tsuchiya, Y.; Watanabe, Y. Oxidative Stress Orchestrates MAPK and Nitric-Oxide Synthase Signal. Int. J. Mol. Sci. 2020, 21, 8750. [Google Scholar] [CrossRef]
- Miyamoto, E. Molecular mechanism of neuronal plasticity: Induction and maintenance of long-term potentiation in the hippocampus. J. Pharmacol. Sci. 2006, 100, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Loureiro, S.O.; Pettenuzzo, L.F.; Almeida, R.F.; Ynumaru, E.Y.; Guazzelli, P.A.; Meyer, F.S.; Pasquetti, M.V.; Ganzella, M.; Calcagnotto, M.E.; et al. Effects of intranasal guanosine administration on brain function in a rat model of ischemic stroke. Purinergic Signal. 2021, 17, 255–271. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccarini, M.; Pruccoli, L.; Balducci, M.; Giuliani, P.; Caciagli, F.; Ciccarelli, R.; Di Iorio, P. Influence of Guanine-Based Purines on the Oxidoreductive Reactions Involved in Normal or Altered Brain Functions. J. Clin. Med. 2023, 12, 1172. https://doi.org/10.3390/jcm12031172
Zuccarini M, Pruccoli L, Balducci M, Giuliani P, Caciagli F, Ciccarelli R, Di Iorio P. Influence of Guanine-Based Purines on the Oxidoreductive Reactions Involved in Normal or Altered Brain Functions. Journal of Clinical Medicine. 2023; 12(3):1172. https://doi.org/10.3390/jcm12031172
Chicago/Turabian StyleZuccarini, Mariachiara, Letizia Pruccoli, Martina Balducci, Patricia Giuliani, Francesco Caciagli, Renata Ciccarelli, and Patrizia Di Iorio. 2023. "Influence of Guanine-Based Purines on the Oxidoreductive Reactions Involved in Normal or Altered Brain Functions" Journal of Clinical Medicine 12, no. 3: 1172. https://doi.org/10.3390/jcm12031172
APA StyleZuccarini, M., Pruccoli, L., Balducci, M., Giuliani, P., Caciagli, F., Ciccarelli, R., & Di Iorio, P. (2023). Influence of Guanine-Based Purines on the Oxidoreductive Reactions Involved in Normal or Altered Brain Functions. Journal of Clinical Medicine, 12(3), 1172. https://doi.org/10.3390/jcm12031172








