Clinical and Quality of Life Benefits for End-Stage Workers’ Compensation Chronic Pain Claimants following H-Wave® Device Stimulation: A Retrospective Observational Study with Mean 2-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. H-Wave® Stimulation Intensities and Paradigms
2.2. Data Source and Study Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Cohort and Exclusion
3.2. Safety
3.3. Demographics and Clinical Presentation
3.4. Quality of Life-Related Assessments
3.5. Work- and Medication-Related Findings
3.6. Overall Outcome/Satisfaction and Effects of Chronicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chronic Pain and High-Impact Chronic Pain among U.S. Adults, 2019. Available online: https://www.cdc.gov/nchs/products/databriefs/db390.htm#:~:text=Interview%20Survey%2C%202019.-,Summary,65%20and%20over%20(30.8%25) (accessed on 1 October 2022).
- Horppu, R.; Väänänen, A.; Kausto, J. Evaluation of a guidelines implementation intervention to reduce work disability and sick leaves related to chronic musculoskeletal pain: A theory-informed qualitative study in occupational health care. BMC Musculoskelet. Disord. 2022, 23, 272. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hillner, B.E. The Cost of Pain. JAMA Netw. Open 2019, 2, e191532. [Google Scholar] [CrossRef] [PubMed]
- Pharmacologic Management of Chronic Non-Cancer Pain in Adults. Available online: https://www-uptodate-com.proxy.ulib.uits.iu.edu/contents/pharmacologic-management-of-chronic-non-cancer-pain-in-adults?search=Management%20of%20chronic%20pain&topicRef=126111&source=see_link#H1470711919 (accessed on 1 October 2022).
- Daoust, R.; Paquet, J.; Cournoyer, A.; Piette, É.; Morris, J.; Lessard, J.; Castonguay, V.; Lavigne, G.; Huard, V.; Chauny, J.M. Opioid and non-opioid pain relief after an emergency department acute pain visit. CJEM. 2021, 23, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017, 37, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Khademi, H.; Kamangar, F.; Brennan, P.; Malekzadeh, R. Opioid Therapy and its Side Effects: A Review. Arch. Iran. Med. 2016, 19, 870–876. [Google Scholar] [PubMed]
- Miller, J.; MacDermid, J.C.; Walton, D.M.; Richardson, J. Chronic Pain Self-Management Support With Pain Science Education and Exercise (COMMENCE) for People With Chronic Pain and Multiple Comorbidities: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 101, 750–761. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jones, M.R.; Kaye, A.M.; Ripoll, J.G.; Galan, V.; Beakley, B.D.; Calixto, F.; Bolden, J.L.; Urman, R.D.; Manchikanti, L. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: Part 1. Pain Physician 2017, 20, S93–S109. [Google Scholar] [CrossRef]
- Swedish Council on Health Technology Assessment. Methods of Treating Chronic Pain: A Systematic Review; SBU Yellow Report No. 177/1+2; Swedish Council on Health Technology Assessment (SBU): Stockholm, Sweden, 2006. [Google Scholar]
- Ekholm, O.; Diasso, P.D.K.; Davidsen, M.; Kurita, G.P.; Sjøgren, P. Increasing prevalence of chronic non-cancer pain in Denmark from 2000 to 2017: A population-based survey. Eur. J. Pain 2022, 26, 624–633. [Google Scholar] [CrossRef]
- Eklund, K.; Stålnacke, B.M.; Stenberg, G.; Enthoven, P.; Gerdle, B.; Sahlén, K.G. A cost-utility analysis of multimodal pain rehabilitation in primary healthcare. Scand. J. Pain 2020, 21, 48–58. [Google Scholar] [CrossRef]
- Williamson, T.K.; Rodriguez, H.C.; Gonzaba, A.; Poddar, N.; Norwood, S.M.; Gupta, A. H-Wave® Device Stimulation: A Critical Review. J. Pers. Med. 2021, 11, 1134. [Google Scholar] [CrossRef]
- Blum, K.; Ho, C.K.; Chen, A.L.; Fulton, M.; Fulton, B.; Westcott, W.L.; Reinl, G.; Braverman, E.R.; Dinubile, N.; Chen, T.J. The H-Wave((R)) Device Induces NODependent Augmented Microcirculation and Angiogenesis, Providing Both Analgesia and Tissue Healing in Sports Injuries. Physician Sportsmed. 2008, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Thiese, M.S.; Hughes, M.; Biggs, J. Electrical stimulation for chronic non-specific low back pain in a working-age population: A 12-week double blinded randomized controlled trial. BMC Musculoskelet. Disord. 2013, 14, 117. [Google Scholar] [CrossRef]
- Blum, K.; Chen, A.L.; Chen, T.J.; Prihoda, T.J.; Schoolfield, J.; DiNubile, N.; Waite, R.L.; Arcuri, V.; Kerner, M.; Braverman, E.R.; et al. The H-Wave device is an effective and safe non-pharmacological analgesic for chronic pain: A meta-analysis. Adv. Ther. 2008, 25, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Blum, K.; DiNubile, N.A.; Chen, T.J.H.; Waite, R.L.; Schoolfield, J.; Martinez-Pons, M.; Callahan, M.F.; Smith, T.L.; Mengucci, J.; Blum, S.H.; et al. H-Wave, a nonpharmacologic alternative for the treatment of patients with chronic soft tissue inflammation and neuropathic pain: A preliminary statistical outcome study. Adv. Ther. 2006, 23, 446–455. [Google Scholar] [CrossRef]
- Blum, K.; Chen, A.L.C.; Chen, T.J.H.; Waite, R.L.; Downs, B.W.; Braverman, E.R.; Kerner, M.M.; Savarimuthu, S.M.; DiNubile, N. Repetitive H-Wave device stimulation and program induces significant increases in the range of motion of post operative rotator cuff reconstruction in a double-blinded randomized placebo controlled human study. BMC Musculoskelet. Disord. 2009, 10, 132. [Google Scholar] [CrossRef]
- Blum, K.; DiNubile, N.A.; Chen, T.J.H.; Waite, R.L.; Schoolfield, J.; Martinez-Pons, M.; Callahan, M.F.; Smith, T.L.; Mengucci, J.; Blum, S.H.; et al. The H-Wave small muscle fiber stimulator, a nonpharmacologic alternative for the treatment of chronic soft-tissue injury and neuropathic pain: An extended population observational study. Adv. Ther. 2006, 23, 739–749. [Google Scholar] [CrossRef]
- Hooten, W.M. Chronic Pain and Mental Health Disorders: Shared Neural Mechanisms, Epidemiology, and Treatment. Mayo Clin. Proc. 2016, 91, 955–970. [Google Scholar] [CrossRef]
- Dueñas, M.; Ojeda, B.; Salazar, A.; Mico, J.A.; Failde, I. A review of chronic pain impact on patients, their social environment and the health care system. J. Pain Res. 2016, 9, 457–467. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Kodumudi, G.; Babayan, K.; Fontes, M.; Burg, M.M. Pain and Psychology—A Reciprocal Relationship. Ochsner J. 2017, 17, 173–180. [Google Scholar]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Park, H.; Kim, D.W.; Jeon, E.K.; Rhee, C.M.; Kalantar-Zadeh, K.; Kang, E.W.; Kang, S.W.; Han, S.H. Polypharmacy, hospitalization, and mortality risk: A nationwide cohort study. Sci. Rep. 2020, 10, 18964. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Yang, L.; Yang, Y.; Qiao, G.; Lu, C.; Liu, K. Association between polypharmacy and mortality in the older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2022, 100, 104630. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician. 2008, 11, S105–S120. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Blum, K.; Callahan, M.F.; DiNubile, N.A.; Chen, T.J.; Waite, R.L. H-Wave induces arteriolar vasodilation in rat striated muscle via nitric oxide-mediated mechanisms. J. Orthop. Res. 2009, 27, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Overdose Death Rates. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates#:~:text=Drug%20overdose%20deaths%20involving%20prescription,increase%20to%2016%2C416%20in%202020 (accessed on 1 October 2022).
- Workers’ Compensation: Keeping Injured and Ill Workers in the Workforce. Available online: https://www.ncsl.org/research/labor-and-employment/workers-compensation-report.aspx (accessed on 1 October 2022).
- Williamson, T.K.; Rodriguez, H.C.; Han, D.; Norwood, S.M.; Gupta, A. Job-Related Performance and Quality of Life Benefits in First Responders Given Access to H-Wave® Device Stimulation: A Retrospective Cohort Study. J. Pers. Med. 2022, 12, 1674. [Google Scholar] [CrossRef]
- ODG by MCG. Available online: https://www.mcg.com/odg/ (accessed on 1 October 2022).


| Characteristic | Proportion |
|---|---|
| Gender | |
| Male | 48.7% |
| Female | 51.3% |
| Ethnicity | |
| White | 82.4% |
| Black | 14.9% |
| Hispanic | 1.4% |
| Asian | 1.4% |
| Weight (lbs.) | Mean: 206 ± 55 |
| BMI (kg/m2) | Mean: 31.5 ± 7.2 |
| Age (years) | Mean: 54.4 ± 10.6 |
| DOI to trial (months) | Mean: 93.0 ± 73.4 |
| Workers’ compensation | 98.7% |
| Attorney involved | 24.3% |
| Painful Body Part | Proportion (%) |
|---|---|
| Low back | 68.9 |
| Neck/Upper back | 20.3 |
| Leg | 20.3 |
| Shoulder | 13.5 |
| Hip | 4.1 |
| Knee | 4.1 |
| Ankle/Foot | 4.1 |
| Pelvis/Groin | 2.8 |
| Chest | 2.7 |
| Quality of Life Patient-Reported Outcome Measures | ||||
|---|---|---|---|---|
| Pre | Post | Difference | p-Value | |
| VAS | 6.3 ± 1.7 | 4.2 ± 1.8 | −2.1 ± 1.9 | <0.0001 * |
| BPI | 45.1 ± 14.6 | 32.9 ± 17.3 | −12.7 ± 15.8 | <0.0001 * |
| PDQ | 96.2 ± 28.0 | 63.0 ± 37.2 | −33.5 ± 27.7 | <0.0001 * |
| PHQ-9 | 9.7 ± 6.7 | 7.3 ± 6.2 | −2.4 ± 6.5 | 0.0096 * |
| GAD-7 | 7.9 ± 6.2 | 6.3 ± 5.5 | −2.5 ± 5.0 | 0.0024 * |
| Functional Improvement | ||
|---|---|---|
| Count | Proportion of Total | |
| None | 8 | 11.0% |
| Mild | 11 | 15.0% |
| Moderate | 54 | 74.0% |
| Count | Returned to Work | ||
|---|---|---|---|
| No | Yes | Total | |
| Not Working Before Trial | 46 (63.0%) | 3 (4.1%) | 49 (67.1%) |
| Working Before Trial | 3 (4.1%) | 21 (28.8%) | 24 (32.9%) |
| Total | 49 (67.1%) | 24 (32.9%) | 73 (100%) |
| Count | Post-Trial Opioid Use | ||||
|---|---|---|---|---|---|
| Stopped After Trial | Reduced After Trial | No Change | Increased After Trial | Total | |
| No Opioid Before Trial | NA | NA | 30 (41.1%) | 2 (2.7%) | 32 (43.8%) |
| On Opioid Before Trial | 17 (23.3%) | 3 (4.1%) | 18 (24.7%) | 3 (4.1%) | 41 (56.2%) |
| Total | 17 (23.3%) | 3 (4.1%) | 48 (65.8%) | 5 (6.9%) | 73 (100%) |
| Count | Post-Trial Polypharmacy Use | ||||
| Stopped After Trial | Reduced After Trial | No Change | Increased After Trial | Total | |
| None Before Trial | NA | NA | 21 (28.8%) | 7 (9.6%) | 28 (38.4%) |
| On Drugs Before Trial | 11 (15.1%) | 6 (8.2%) | 22 (30.1%) | 6 (8.2%) | 45 (61.6%) |
| Total | 11 (15.1%) | 6 (8.2%) | 43 (58.9%) | 13 (17.8%) | 73 (100%) |
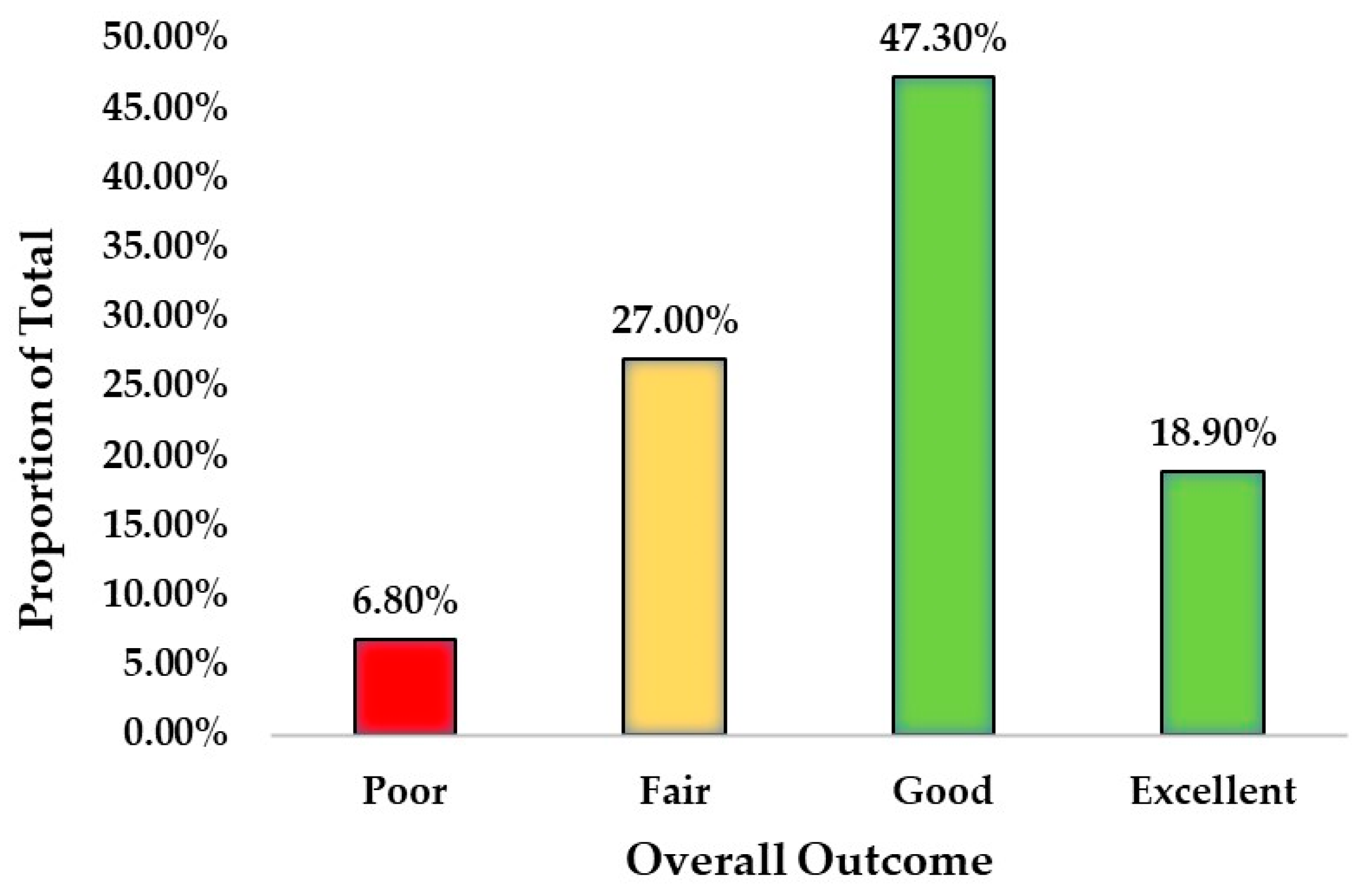
| Overall Outcome | ||
|---|---|---|
| Count | Proportion of Total | |
| Poor | 5 | 6.8% |
| Fair | 20 | 27.0% |
| Good | 35 | 47.3% |
| Excellent | 14 | 18.9% |
| Time Between (Months) | Study Group | Trial Failure |
|---|---|---|
| Average ± S.D. | 92.96 ± 73.41 | 124.51 ± 83.03 |
| 95% Interval | (75.95, 109.97) | (103.60, 145.42) |
| Sample Size | 74 | * 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, A.; Williamson, T.K.; Han, D.; Hazlewood, J.E.; Norwood, S.M.; Gupta, A. Clinical and Quality of Life Benefits for End-Stage Workers’ Compensation Chronic Pain Claimants following H-Wave® Device Stimulation: A Retrospective Observational Study with Mean 2-Year Follow-Up. J. Clin. Med. 2023, 12, 1148. https://doi.org/10.3390/jcm12031148
Trinh A, Williamson TK, Han D, Hazlewood JE, Norwood SM, Gupta A. Clinical and Quality of Life Benefits for End-Stage Workers’ Compensation Chronic Pain Claimants following H-Wave® Device Stimulation: A Retrospective Observational Study with Mean 2-Year Follow-Up. Journal of Clinical Medicine. 2023; 12(3):1148. https://doi.org/10.3390/jcm12031148
Chicago/Turabian StyleTrinh, Alan, Tyler K. Williamson, David Han, Jeffrey E. Hazlewood, Stephen M. Norwood, and Ashim Gupta. 2023. "Clinical and Quality of Life Benefits for End-Stage Workers’ Compensation Chronic Pain Claimants following H-Wave® Device Stimulation: A Retrospective Observational Study with Mean 2-Year Follow-Up" Journal of Clinical Medicine 12, no. 3: 1148. https://doi.org/10.3390/jcm12031148
APA StyleTrinh, A., Williamson, T. K., Han, D., Hazlewood, J. E., Norwood, S. M., & Gupta, A. (2023). Clinical and Quality of Life Benefits for End-Stage Workers’ Compensation Chronic Pain Claimants following H-Wave® Device Stimulation: A Retrospective Observational Study with Mean 2-Year Follow-Up. Journal of Clinical Medicine, 12(3), 1148. https://doi.org/10.3390/jcm12031148







