Cross-Cultural Adaptation and Validation of the Methotrexate Intolerance Severity Score Questionnaire in Portuguese (Brazil) for Children and Adolescents with Juvenile Idiopathic Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Translation and Transcultural Adaptation
2.2. Statistical Analysis
3. Results
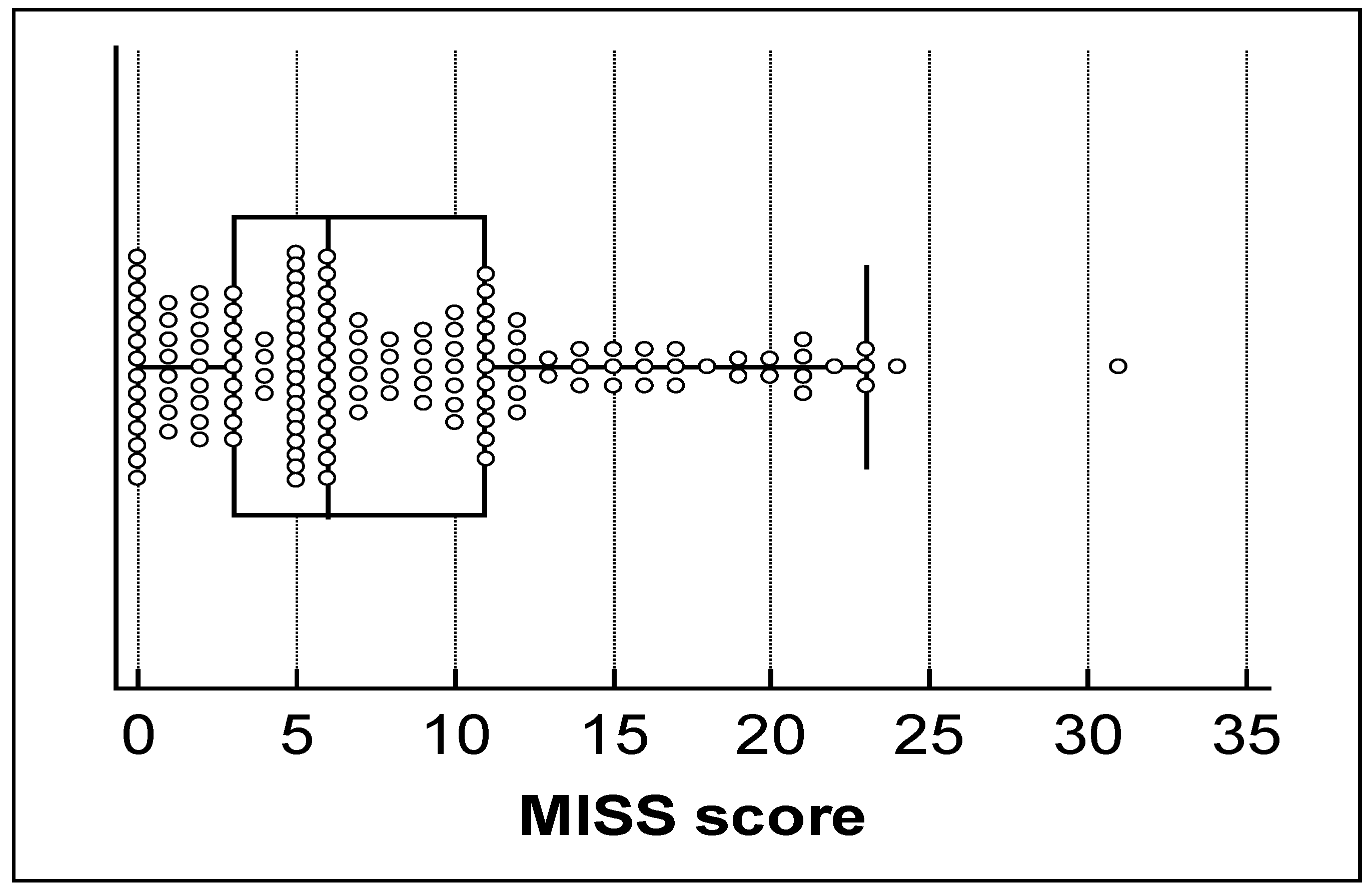
3.1. Descriptive Data
3.2. Cross-Cultural Adaptation
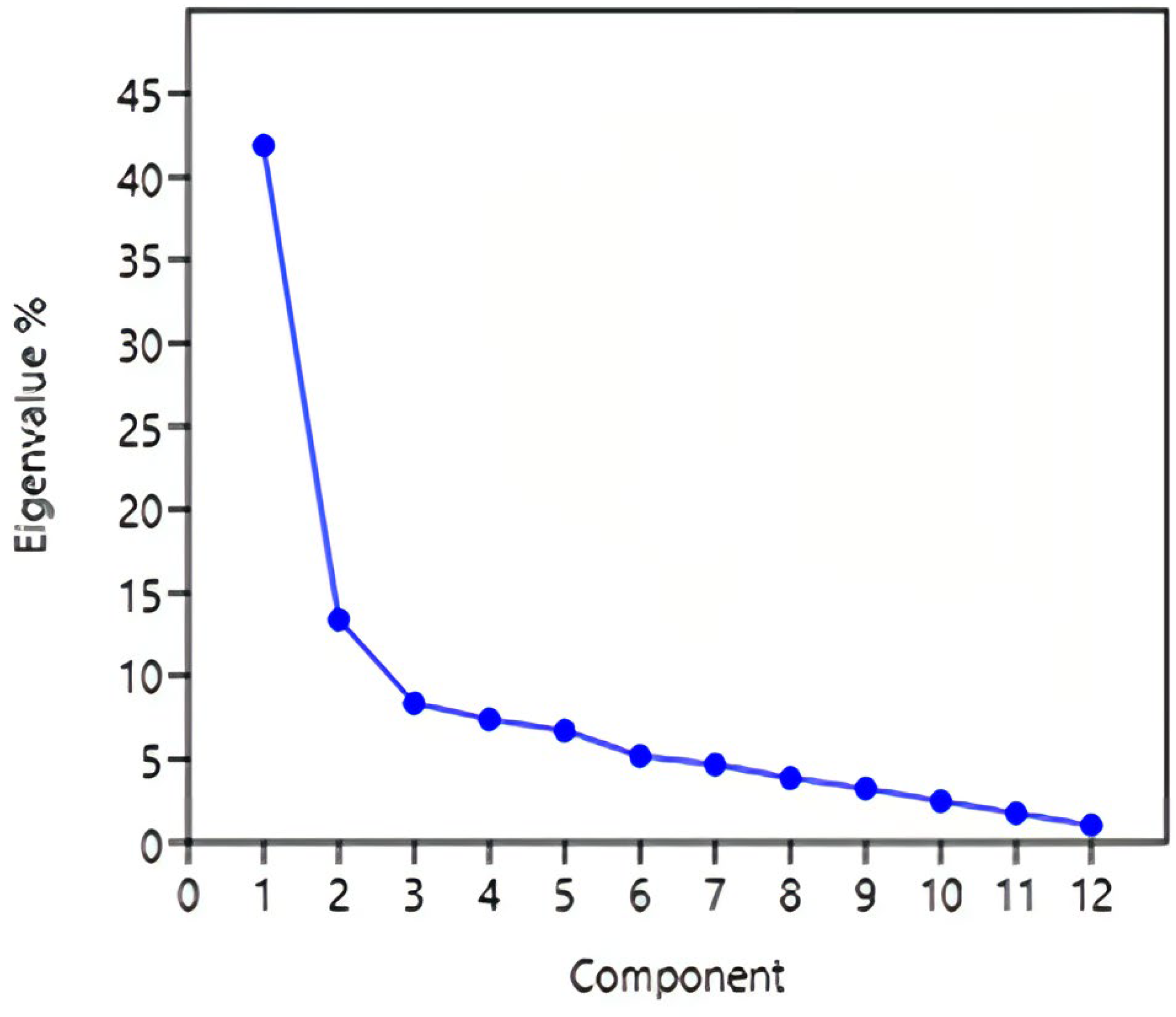
3.3. Psychometric Issues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barut, K.; Adrovic, A.; Şahin, S.; Kasapçopur, Ö. Juvenile Idiopathic Arthritis. Balkan Med. J. 2017, 34, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Hūgle, B.; Horneff, G. The role of synthetic drugs in the biologic era: Therapeutic strategies for treating juvenile idiopathic arthritis. Expert Opin. Pharmacother. 2016, 17, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Braun, J. Methotrexate: Optimizing the efficacy in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2011, 3, 151–158. [Google Scholar] [CrossRef]
- Ramanan, A.V.; Whitworth, P.; Baildam, E.M. Use of methotrexate in juvenile idiopathic arthritis. Arch. Dis. Child. 2003, 88, 197–200. [Google Scholar] [CrossRef]
- Canhão, H.; Fonseca, J.E.; Romão, V.C. Old drugs, old problems: Where do we stand in prediction of rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med. 2013, 11, 17. [Google Scholar]
- Blazina, S.; Markelj, G.; Avramovic, M.Z.; Toplak, N.; Avcin, T. Management of Juvenile Idiopathic Arthritis: A Clinical Guide. Paediatr. Drugs 2016, 18, 397–412. [Google Scholar] [CrossRef]
- Van der Meer, A.; Wulfraat, N.M.; Prakken, B.j.; Gijsbers, B.; Rademaker, C.M.; Sinnema, G. Psychological side effect of MTX treatment in juvenile idiopathic arthritis: A pilot study. Clin. Exp. Rheumatol. 2007, 25, 480–485. [Google Scholar]
- Bulatovic, M.; Heijstek, M.W.; Verkaaik, M.; van Dijkhuizen, E.H.; Ambrust, W.; Hoppenreijis, E.P.; Kamphuis, S.; Kuis, W.; Egberts, T.C.; Sinnema, G.; et al. High prevalence of methotrexate intolerance in juvenile idiopathic arthritis: Development and validation of a methotrexate intolerance severity score. Arthritis Rheum. 2011, 63, 2007–2013. [Google Scholar] [CrossRef]
- Ćalasan, M.B.; van den Bosch, O.F.; Creemers, M.C.; Custers, M.; Heurkens, A.H.; van Woerkom, J.M.; Wulffraat, N.M. Prevalence of methotrexate intolerance in rheumatoid arthritis and psoriatic arthritis. Arthritis Res Ther. 2013, 15, R217. [Google Scholar] [CrossRef]
- Van Dijkhuizen, E.H.P.; Wulffraat, N.M. Early predictors of prognosis in juvenile idiopathic arthritis: A systematic literature review. Ann. Rheum. Dis. 2014, 74, 12–51. [Google Scholar] [CrossRef]
- Van Dijkhuizen, E.H.; Ćalasan, M.B.; Pluijm, S.M.; de Rotte, M.C.; Vastert, S.; Kamphuis, S.; de Jonge, R.; Wulffraat, N. Prediction of methotrexate intolerance in juvenile idiopathic arthritis: A prospective, observational cohort study. Pediatr. Rheumatol. 2015, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, N.; Salim, B.; Nasim, A.; Hussain, K.; Gul, H.; Niazi, S. Frequency of methotrexate intolerance in rheumatoid arthritis patients using the metrotrexate intolerance severity score (MISS questionnaire). Clin. Rheumatol. 2016, 35, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Sceuern, A.; Tyrrel, P.N.; Haas, J.P.; Hugle, B. Countermeasures against methotrexate intolerance in juvenile idiopathic arthritis by parents show no effect. Rheumatology 2017, 58, 901–906. [Google Scholar] [CrossRef]
- Fránová, J.; Fingerhutová, S.; Kobrová, K.; Srp, R.; Nemcová, D.; Hoza, J.; Brunner, H.; Haas, J.P.; Hugle, B. Methotrexate efficacy, but not its intolerance, is associated with the dose and route of administration. Pediatr. Rheumatol. Online J. 2016, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Scheuern, A.; Fischer, N.; McDonald, J.; Haas, J.-P.; Hügle, B. Mutations in the MTHFR gene are not associated with methotrexate intolerance in patients with juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2016, 14, 11. [Google Scholar] [CrossRef]
- Chausset, A.; Fargeix, T.; Pereira, B.; Echaubard, S.; Duquesne, A.; Desjonquères, M. Miss questionnaire in French version: A good tool for children and parentes to assess methotrexate intolerance. Clin. Rheumatol. 2017, 36, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.M.; Brito, M.J.M.; Kakehasi, A.M. Cultural Adaptation and Validation of the Methotrexate Intolerance Severity Score in Brazilian Portuguese for Adults with Rheumatoid Arthritis. J. Clin. Rheumatol. 2021, 27, S168–S172. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390. [Google Scholar]
- Atilgan, H. Sample Size for Estimation of G and Phi Coefficients in Generalizability Theory. Eurasian J. Educ. Res. 2013, 51, 215–228. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis: A Global Perspective, 7th ed; Pearson Education: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Rejano-Campo, M.; Ferre-Peña, R.; Urraca-Gesto, M.A.; Gallego-Izquierdo, T.; Pecos-Martín, D.; Stuge, B.; Plaza-Manzano, G. Transcultural adaptation and psychometric validation of a Spanish-language version of the PelvicGirdle “Questionnaire”. Health Qual. Life Outcomes 2017, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Kothari, C.R. Research Methodology: Methods and Techniques, 2nd ed.; New Age International Publishers: New Delhi, India, 2004. [Google Scholar]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Kottner, J.; Audigé, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Kaya Akca, U.; Farisogullari, B.; Yardimci, G.K.; Sag, E.; Atalay, E.; Kasap Cuceoglu, M.; Basaran, O.; Kilic, L.; Ozen, S.; Bilginer, Y. Real-world data on MTX tolerance with regimens used in children versus adults. Clin. Rheumatol. 2021, 40, 5095–5102. [Google Scholar] [CrossRef]
- Van Dijkhuizen, E.H.; Pouw, J.N.; Scheuern, A.; Hügle, B.; Hardt, S.; Ganser, G.; Kümmerle-Deschner, J.B.; Horneff, G.; Holzinger, D.; Bulatović Ćalasan, M.; et al. Methotrexate intolerance in oral and subcutaneous administration in patients with juvenile idiopathic arthritis: A cross-sectional, observational study. Clin. Exp. Rheumatol. 2016, 34, 148–154. [Google Scholar] [CrossRef] [PubMed]

| Item | Total Item Correlation | Cronbach’s Alpha |
|---|---|---|
| 1 | 0.62 | 0.87 |
| 2 | 0.46 | 0.78 |
| 3 | 0.64 | 0.76 |
| 4 | 0.72 | 0.75 |
| 5 | 0.67 | 0.76 |
| 6 | 0.68 | 0.75 |
| 7 | 0.61 | 0.76 |
| 8 | 0.47 | 0.77 |
| 9 | 0.66 | 0.75 |
| 10 | 0.56 | 0.88 |
| 11 | 0.69 | 0.87 |
| 12 | 0.76 | 0.87 |
| Items | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 1 | 1 | |||||||||||
| 2 | 0.40 ** | 1 | ||||||||||
| 3 | 0.54 ** | 0.45 | 1 | |||||||||
| 4 | 0.56 ** | 0.32 | 0.41 | 1 | ||||||||
| 5 | 0.39 ** | 0.42 | 0.40 | 0.56 | 1 | |||||||
| 6 | 0.42 ** | 0.40 | 0.43 | 0.64 | 0.55 | 1 | ||||||
| 7 | 0.38 ** | 0.31 | 0.34 | 0.51 | 0.50 | 0.38 | 1 | |||||
| 8 | 0.24 ** | 0.28 | 0.20 | 0.18 | 0.39 | 0.26 | 0.43 | 1 | ||||
| 9 | 0.37 ** | 0.22 | 0.34 | 0.42 | 0.37 | 0.39 | 0.32 | 0.32 | 1 | |||
| 10 | 0.16 * | 0.16 | 0.29 | 0.26 | 0.32 | 0.28 | 0.41 | 0.50 | 0.43 | 1 | ||
| 11 | 0.30 ** | 0.23 | 0.38 | 0.43 | 0.40 | 0.43 | 0.30 | 0.34 | 0.69 | 0.57 | 1 | |
| 12 | 0.46 ** | 0.27 | 0.43 | 0.51 | 0.49 | 0.50 | 0.42 | 0.31 | 0.60 | 0.49 | 0.65 | 1 |
| Item | Kappa |
|---|---|
| 1 | 0.87 |
| 2 | 0.81 |
| 3 | 0.76 |
| 4 | 0.88 |
| 5 | 0.86 |
| 6 | 0.80 |
| 7 | 0.95 |
| 8 | 1.0 |
| 9 | 1.0 |
| 10 | 0.92 |
| 11 | 0.81 |
| 12 | 0.84 |
| Item | Kappa | Agreement Percentage (%) |
|---|---|---|
| 1 | 0.66 | 82 |
| 2 | 0.50 | 79 |
| 3 | 0.66 | 84 |
| 4 | 0.83 | 91 |
| 5 | 0.86 | 90 |
| 6 | 0.70 | 96 |
| 7 | 0.94 | 95 |
| 8 | 0.81 | 94 |
| 9 | 0.46 | 89 |
| 10 | 0.90 | 90 |
| 11 | 0.50 | 89 |
| 12 | 0.80 | 95 |
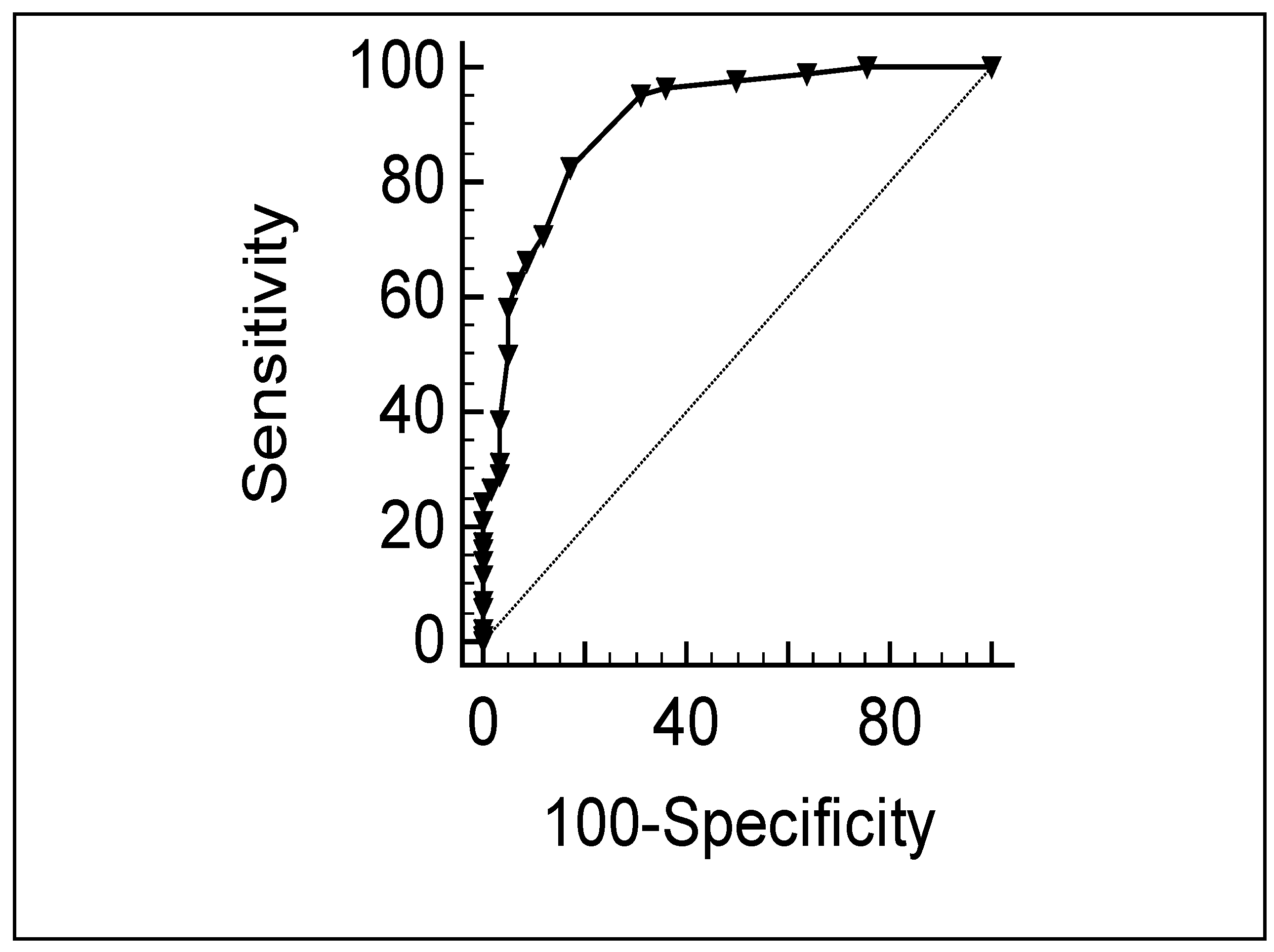
| Cut-Off Scores | Sensitivity | Specificity |
|---|---|---|
| 2 | 98 | 50 |
| 3 | 96 | 64 |
| 4 | 95 | 68 |
| 5 | 84 | 80 |
| 6 | 71 | 87 |
| 7 | 66 | 91 |
| 8 | 63 | 93 |
| 9 | 58 | 95 |
| 10 | 50 | 96 |
| 11 | 40 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Londe, A.C.; de Amorim, J.C.; Julio, P.R.; Wulffraat, N.M.; Marini, R.; Appenzeller, S. Cross-Cultural Adaptation and Validation of the Methotrexate Intolerance Severity Score Questionnaire in Portuguese (Brazil) for Children and Adolescents with Juvenile Idiopathic Arthritis. J. Clin. Med. 2023, 12, 1116. https://doi.org/10.3390/jcm12031116
Londe AC, de Amorim JC, Julio PR, Wulffraat NM, Marini R, Appenzeller S. Cross-Cultural Adaptation and Validation of the Methotrexate Intolerance Severity Score Questionnaire in Portuguese (Brazil) for Children and Adolescents with Juvenile Idiopathic Arthritis. Journal of Clinical Medicine. 2023; 12(3):1116. https://doi.org/10.3390/jcm12031116
Chicago/Turabian StyleLonde, Ana Carolina, Jaqueline Cristina de Amorim, Paulo Rogério Julio, Nico M. Wulffraat, Roberto Marini, and Simone Appenzeller. 2023. "Cross-Cultural Adaptation and Validation of the Methotrexate Intolerance Severity Score Questionnaire in Portuguese (Brazil) for Children and Adolescents with Juvenile Idiopathic Arthritis" Journal of Clinical Medicine 12, no. 3: 1116. https://doi.org/10.3390/jcm12031116
APA StyleLonde, A. C., de Amorim, J. C., Julio, P. R., Wulffraat, N. M., Marini, R., & Appenzeller, S. (2023). Cross-Cultural Adaptation and Validation of the Methotrexate Intolerance Severity Score Questionnaire in Portuguese (Brazil) for Children and Adolescents with Juvenile Idiopathic Arthritis. Journal of Clinical Medicine, 12(3), 1116. https://doi.org/10.3390/jcm12031116





