The sFlt-1/PlGF Ratio in Pregnant Patients Affected by COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction
2.4. Bias Assessment
2.5. Data synthesis and Statistical Analysis
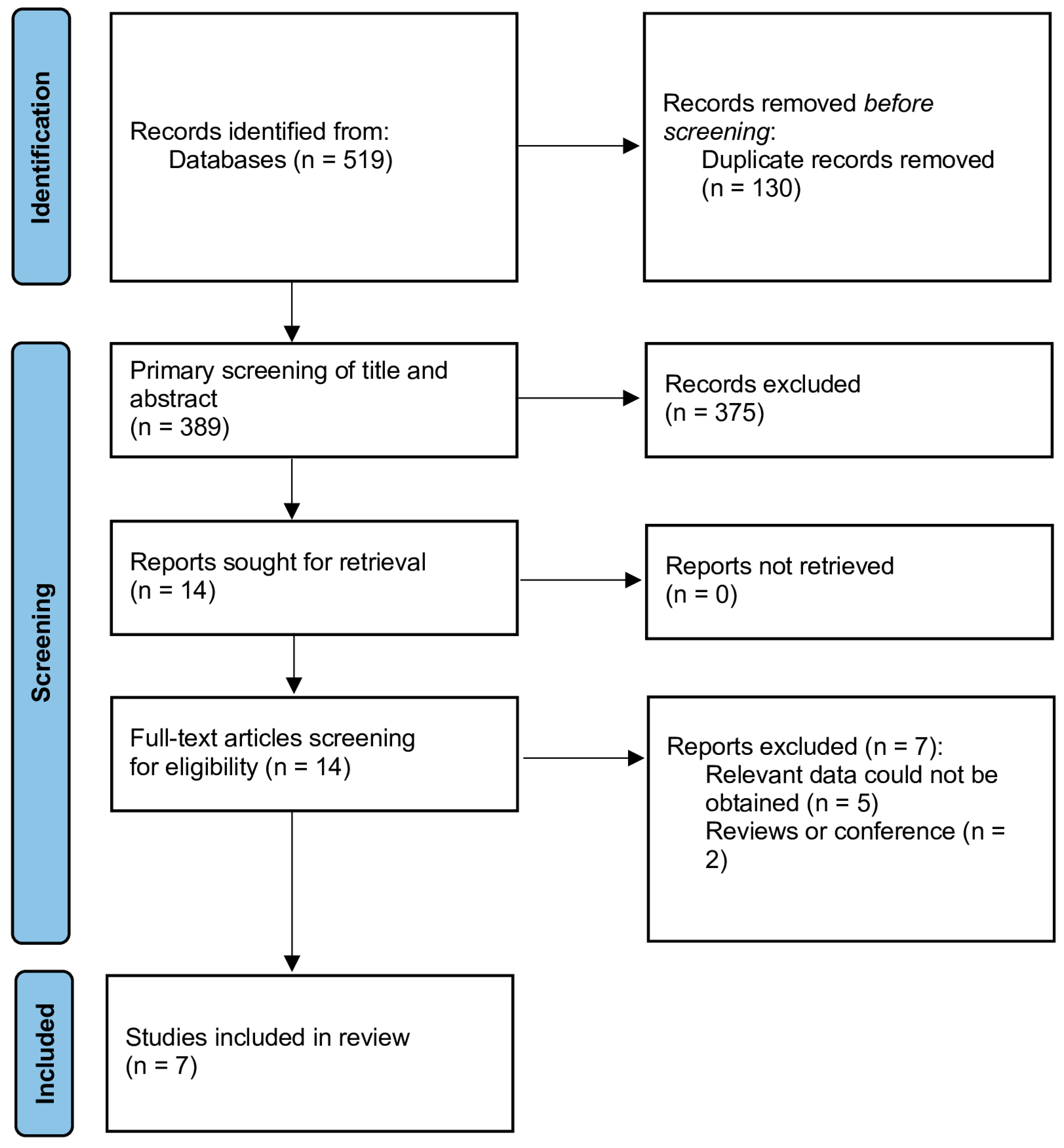
3. Results
3.1. Study Selection and Characteristics
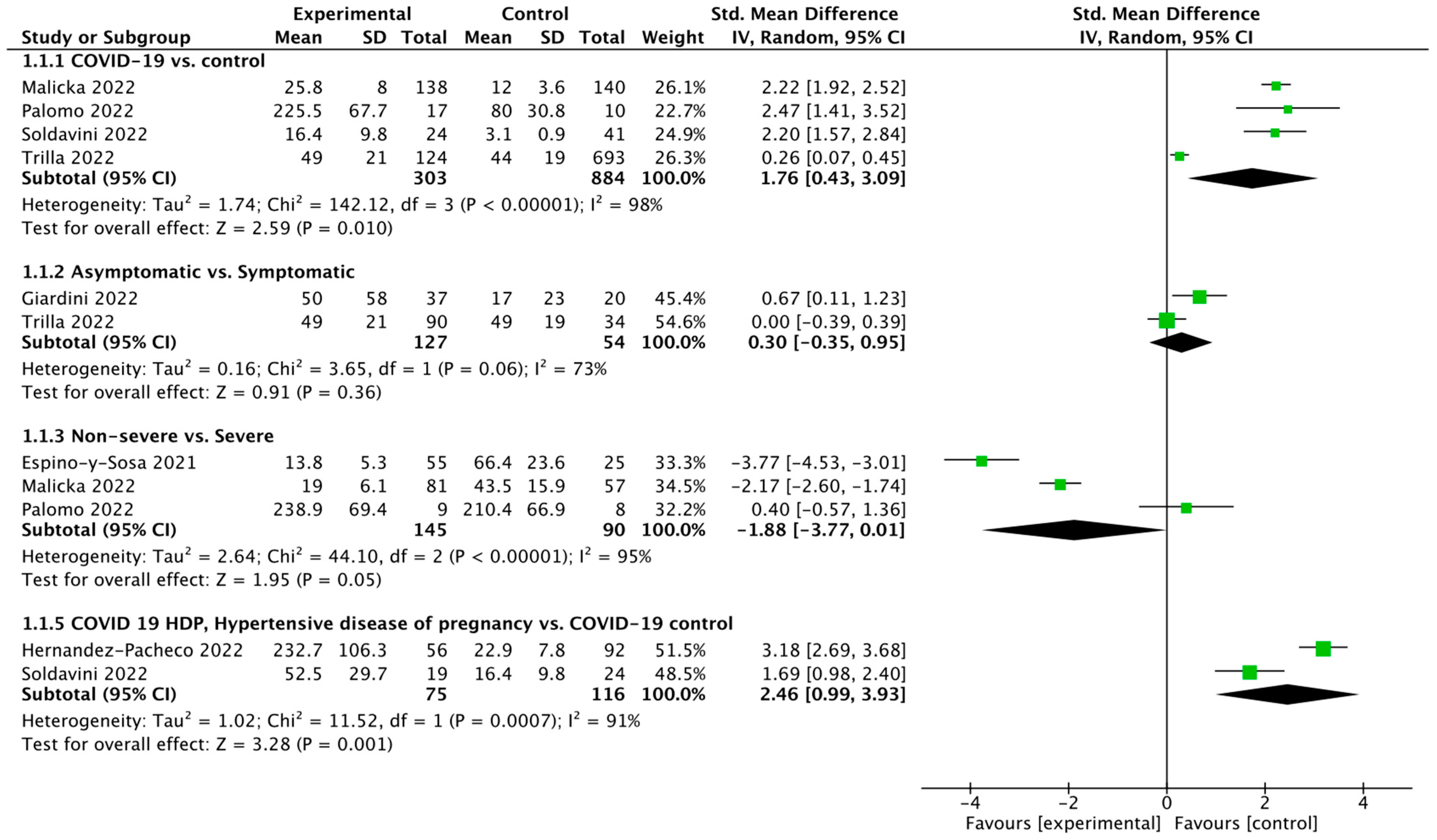
3.2. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smereka, J.; Szarpak, L.; Filipiak, K.J. Modern Medicine in COVID-19 Era. Disaster Emerg. Med. J. 2020, 5, 103–105. [Google Scholar] [CrossRef]
- Feduniw, S.; Modzelewski, J.; Kajdy, A.; Sys, D.; Kwiatkowski, S.; Makomaska-Szaroszyk, E.; Rabijewski, M. Anxiety of Pregnant Women in Time of Catastrophic Events, Including COVID-19 Pandemic: A Systematic Review and Meta-Analysis. J. Psychosom. Obstet. Gynecol. 2021, 43, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 Infection with Serious Maternal Morbidity and Mortality from Obstetric Complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef]
- Calvert, C.; John, J.; Nzvere, F.P.; Cresswell, J.A.; Fawcus, S.; Fottrell, E.; Say, L.; Graham, W.J. Maternal Mortality in the Covid-19 Pandemic: Findings from a Rapid Systematic Review. Glob. Health Action 2021, 14, 1974677. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Bappy, M.H.; Desai, S.; Chowdhury, S.; Patel, V.; Chowdhury, M.d.S.; Fonseca, A.; Sekzer, C.; Zahid, S.; Patousis, A.; et al. COVID-19 and Pregnancy. Discoveries 2022, 10, e147. [Google Scholar] [PubMed]
- Ahlberg, M.; Neovius, M.; Saltvedt, S.; Söderling, J.; Pettersson, K.; Brandkvist, C.; Stephansson, O. Association of SARS-CoV-2 Test Status and Pregnancy Outcomes. JAMA 2020, 324, 1782–1785. [Google Scholar] [CrossRef]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 Pandemic on Maternal and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- Bobei, T.-I.; Sima, R.-M.; Gorecki, G.-P.; Poenaru, M.-O.; Olaru, O.-G.; Bobirca, A.; Cirstoveanu, C.; Chicea, R.; Topirceanu-Andreoiu, O.-M.; Ples, L. Placenta, the Key Witness of COVID-19 Infection in Premature Births. Diagnostics 2022, 12, 2323. [Google Scholar] [CrossRef] [PubMed]
- Gesaka, S.R.; Obimbo, M.M.; Wanyoro, A. Coronavirus Disease 2019 and the Placenta: A Literature Review. Placenta 2022, 126, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Solorzano, J.; Jaisingh, K.; Ahmad, J.; Thevuthasan, S.; Foster, A.; Watts, M.; Kolluru, G.K.; Orr, A.W.; Kevil, C.G.; Dominic, P. Vascular endothelial growth factor is associated with the severe COVID-19 disease. J. Am. Coll. Cardiol. 2022, 79, 2063. [Google Scholar] [CrossRef]
- McKeeman, G.C.; Ardill, J.E.S.; Caldwell, C.M.; Hunter, A.J.; McClure, N. Soluble Vascular Endothelial Growth Factor Receptor-1 (SFlt-1) Is Increased throughout Gestation in Patients Who Have Preeclampsia Develop. Am. J. Obstet. Gynecol. 2004, 191, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Bednarek-Jędrzejek, M.; Kwiatkowski, S.; Ksel-Hryciów, J.; Tousty, P.; Nurek, K.; Kwiatkowska, E.; Cymbaluk-Płoska, A.; Torbé, A. The SFlt-1/PlGF Ratio Values within the 85 Brackets as Compared to Perinatal Outcomes. J. Perinat. Med. 2019, 47, 732–740. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s and Children’s Health (UK). Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy; National Institute for Health and Clinical Excellence: Guidance; RCOG Press: London, UK, 2010. [Google Scholar]
- Kwiatkowski, S.; Bednarek-Jędrzejek, M.; Kwiatkowska, E.; Cymbaluk-Płoska, A.; Torbè, A. Diagnosis of Placental Insufficiency Independently of Clinical Presentations Using SFlt-1/PLGF Ratio, Including SGA Patients. Pregnancy Hypertens. 2021, 25, 244–248. [Google Scholar] [CrossRef]
- Espino-Y-Sosa, S.; Martinez-Portilla, R.J.; Torres-Torres, J.; Solis-Paredes, J.M.; Estrada-Gutierrez, G.; Hernandez-Pacheco, J.A.; Espejel-Nuñez, A.; Mateu-Rogell, P.; Juarez-Reyes, A.; Lopez-Ceh, F.E.; et al. Novel Ratio Soluble Fms-like Tyrosine Kinase-1/Angiotensin-II (SFlt-1/ANG-II) in Pregnant Women Is Associated with Critical Illness in COVID-19. Viruses 2021, 13, 1906. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-H.; Deng, W.; Tong, Z.; Liu, Y.-X.; Zhang, L.-F.; Zhu, H.; Gao, H.; Huang, L.; Liu, Y.-L.; Ma, C.-M.; et al. Mice Transgenic for Human Angiotensin-Converting Enzyme 2 Provide a Model for SARS Coronavirus Infection. Comp. Med. 2007, 57, 450–459. [Google Scholar] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Malicka, E.; Szymusik, I.; Rebizant, B.; Dąbrowski, F.; Brawura-Biskupski-Samaha, R.; Kosińska-Kaczyńska, K. SFlt-1/PlGF Ratio Is not a Good Predictor of Severe COVID-19 nor of Adverse Outcome in Pregnant Women with SARS-CoV-2 Infection—A Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 15054. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Giardini, V.; Ornaghi, S.; Gambacorti-Passerini, C.; Casati, M.; Carrer, A.; Acampora, E.; Vasarri, M.V.; Arienti, F.; Vergani, P. Imbalanced Angiogenesis in Pregnancies Complicated by SARS-CoV-2 Infection. Viruses 2022, 14, 2207. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pacheco, J.A.; Torres-Torres, J.; Martinez-Portilla, R.J.; Solis-Paredes, J.M.; Estrada-Gutierrez, G.; Mateu-Rogell, P.; Nares-Torices, M.A.; Lopez-Marenco, M.E.; Escobedo-Segura, K.R.; Posadas-Nava, A.; et al. SFlt-1 Is an Independent Predictor of Adverse Maternal Outcomes in Women With SARS-CoV-2 Infection and Hypertensive Disorders of Pregnancy. Front. Med. 2022, 9, 894633. [Google Scholar] [CrossRef]
- Palomo, M.; Youssef, L.; Ramos, A.; Torramade-Moix, S.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Bonastre, L.; Pino, M.; Gomez-Ramirez, P.; Martin, L.; et al. Differences and Similarities in Endothelial and Angiogenic Profiles of Preeclampsia and COVID-19 in Pregnancy. Am. J. Obstet. Gynecol. 2022, 227, 277.e1–277.e16. [Google Scholar] [CrossRef]
- Soldavini, C.M.; Di Martino, D.; Sabattini, E.; Ornaghi, S.; Sterpi, V.; Erra, R.; Invernizzi, F.; Tine’, G.; Giardini, V.; Vergani, P.; et al. SFlt-1/PlGF Ratio in Hypertensive Disorders of Pregnancy in Patients Affected by COVID-19. Pregnancy Hypertens. 2022, 27, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Trilla, C.; Mora, J.; Crovetto, F.; Crispi, F.; Gratacos, E.; Llurba, E.; on behalf of the KidsCorona Pregnancy COVID-19 Group. First-Trimester SARS-CoV-2 Infection: Clinical Presentation, Inflammatory Markers, and Obstetric Outcomes. Fetal Diagn. Ther. 2022, 49, 67–76. [Google Scholar] [CrossRef]
- Mendoza, M.; Garcia-Ruiz, I.; Maiz, N.; Rodo, C.; Garcia-Manau, P.; Serrano, B.; Lopez-Martinez, R.; Balcells, J.; Fernandez-Hidalgo, N.; Carreras, E.; et al. Pre-eclampsia-like Syndrome Induced by Severe COVID-19: A Prospective Observational Study. BJOG 2020, 127, 1374–1380. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The Impact of COVID-19 on Pregnancy Outcomes: A Systematic Review and Meta-Analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Pantazi, I.; Alhamlan, F.S.; Alothaid, H.; Matou-Nasri, S.; Sourvinos, G.; Vergadi, E.; Tsatsanis, C. SARS-CoV-2 Modulates Inflammatory Responses of Alveolar Epithelial Type II Cells via PI3K/AKT Pathway. Front. Immunol. 2022, 13, 1020624. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 Spike Protein Induces Inflammation via TLR2-Dependent Activation of the NF-ΚB Pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.; Verdin-Terán, S.L.; Camacho, A.; Moreno-Fierros, L. Expression of Toll-like Receptor TLR-2, TLR-3, TLR-4 and TLR-9 Is Increased in Placentas from Patients with Preeclampsia. Arch. Med. Res. 2011, 42, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Panda, A.; Ueda, I.; Abrahams, V.M.; Norwitz, E.R.; Stanic, A.K.; Young, B.C.; Ecker, J.L.; Altfeld, M.; Shaw, A.C.; et al. Dendritic Cells in the Circulation of Women with Preeclampsia Demonstrate a Pro-Inflammatory Bias Secondary to Dysregulation of TLR Receptors. J. Reprod. Immunol. 2012, 94, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Borczuk, A.C.; Yantiss, R.K. The Pathogenesis of Coronavirus-19 Disease. J. Biomed. Sci. 2022, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Todros, T.; Masturzo, B.; De Francia, S. COVID-19 Infection: ACE2, Pregnancy and Preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 330. [Google Scholar] [CrossRef]
- Szarpak, Ł.; Nowak, B.; Kosior, D.; Zaczynski, A.; Filipiak, K.J.; Jaguszewski, M.J. Cytokines as Predictors of COVID-19 Severity: Evidence from a Meta-Analysis. Pol. Arch. Intern. Med. 2021, 131, 98–99. [Google Scholar] [CrossRef]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef]
- Wang, C.-L.; Liu, Y.-Y.; Wu, C.-H.; Wang, C.-Y.; Wang, C.-H.; Long, C.-Y. Impact of COVID-19 on Pregnancy. Int. J. Med. Sci. 2021, 18, 763–767. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency from COVID-19 Causes Stillbirth and Neonatal Death from Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Zuin, M.; Gentili, V.; Cervellati, C.; Rizzo, R.; Zuliani, G. Viral Load Difference between Symptomatic and Asymptomatic COVID-19 Patients: Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2021, 13, 645–653. [Google Scholar] [CrossRef]
- Van Doorn, R.; Mukhtarova, N.; Flyke, I.P.; Lasarev, M.; Kim, K.; Hennekens, C.H.; Hoppe, K.K. Dose of Aspirin to Prevent Preterm Preeclampsia in Women with Moderate or High-Risk Factors: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0247782. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Borowski, D.; Kajdy, A.; Poon, L.C.; Rokita, W.; Wielgos, M. Why We Should Not Stop Giving Aspirin to Pregnant Women during the COVID-19 Pandemic. Ultrasound Obstet. Gynecol. 2020, 55, 841–843. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study Design | Research Group | No. of Patients | Maternal Age | NOS Score |
|---|---|---|---|---|---|---|
| Espino-y-Sosa et al. 2021 [15] | Mexico | Prospective cohort study | Severe | 25 | 30.8 ± 1.3 | 9 |
| Non-severe | 55 | 29.1 ± 2.1 | ||||
| Giardini et al. 2022 [24] | Italy | Retrospective trial | Asymptomatic | 37 | 33 ± 5 | 8 |
| Symptomatic | 20 | 33 ± 4 | ||||
| Hernandez-Pacheco et al. 2022 [25] | Mexico | Multicenter retrospective cohort study | COVID-19 HDP | 56 | 31.6 ± 2.4 | 7 |
| COVID-19 Non-HDP | 92 | 30.2 ± 2.0 | ||||
| Malicka et al. 2022 [18] | Poland | Case-Control Study | COVID-19 (+) | 138 | 32.0 ± 1.3 | 8 |
| COVID-19 (−) | 140 | 31.8 ± 1.5 | ||||
| Palomo et al. 2022 [26] | Spain | Multicenter prospective population-based cohort study | Severe | 8 | 33.6 ± 4.1 | 8 |
| Non-severe | 9 | 35.1 ± 2.0 | ||||
| COVID-19 (+) | 17 | 34.4 ± 3.2 | ||||
| COVID-19 (−) | 10 | 36.0 ± 2.0 | ||||
| Soldavini et al. 2022 [27] | Italy | Multicenter prospective population-based cohort study | COVID-19 HDP | 19 | 34.8 ± 1.8 | 9 |
| COVID-19 Non-HDP | 24 | 31.0 ± 1.5 | ||||
| COVID-19 (+) | 24 | 16.4 ± 9.8 | ||||
| COVID-19 (−) | 41 | 3.1 ± 0.9 | ||||
| Trilla et al. 2022 [28] | Spain | Multicenter prospective population-based cohort study | COVID-19 (+) | 124 | 33.1 ± 5.1 | 9 |
| COVID-19 (−) | 693 | 33.9 ± 5.2 | ||||
| Asymptomatic | 90 | NS | ||||
| Symptomatic | 34 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosinska-Kaczynska, K.; Malicka, E.; Szymusik, I.; Dera, N.; Pruc, M.; Feduniw, S.; Rafique, Z.; Szarpak, L. The sFlt-1/PlGF Ratio in Pregnant Patients Affected by COVID-19. J. Clin. Med. 2023, 12, 1059. https://doi.org/10.3390/jcm12031059
Kosinska-Kaczynska K, Malicka E, Szymusik I, Dera N, Pruc M, Feduniw S, Rafique Z, Szarpak L. The sFlt-1/PlGF Ratio in Pregnant Patients Affected by COVID-19. Journal of Clinical Medicine. 2023; 12(3):1059. https://doi.org/10.3390/jcm12031059
Chicago/Turabian StyleKosinska-Kaczynska, Katarzyna, Ewa Malicka, Iwona Szymusik, Norbert Dera, Michal Pruc, Stepan Feduniw, Zubaid Rafique, and Lukasz Szarpak. 2023. "The sFlt-1/PlGF Ratio in Pregnant Patients Affected by COVID-19" Journal of Clinical Medicine 12, no. 3: 1059. https://doi.org/10.3390/jcm12031059
APA StyleKosinska-Kaczynska, K., Malicka, E., Szymusik, I., Dera, N., Pruc, M., Feduniw, S., Rafique, Z., & Szarpak, L. (2023). The sFlt-1/PlGF Ratio in Pregnant Patients Affected by COVID-19. Journal of Clinical Medicine, 12(3), 1059. https://doi.org/10.3390/jcm12031059








