Quality of Life in COVID-Related ARDS Patients One Year after Intensive Care Discharge (Odissea Study): A Multicenter Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Design
2.2. Patients’ Characteristics
2.3. Outcomes
2.4. Data Collection
2.5. Statistical Analysis
2.6. Sample Size
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization (WHO). Coronavirus COVID-19 Dashboard; World Health Organization: Geneva, Switzerland, 2020; Available online: https://covid19.who.int (accessed on 19 January 2023).
- Hajjar, L.A.; Costa, I.B.S.D.S.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; de Oliveira, G.Q.; de Almeida, J.P.; Bello, M.V.D.O.; Garzillo, C.; et al. Intensive care management of patients with COVID-19: A practical approach. Ann. Intensiv. Care 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- The Recovery Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-intensive care syndrome: Its pathophysiology, prevention, and future directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-Year Outcomes in Survivors of the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Hatch, R.; Young, D.; Barber, V.; Griffiths, J.; Harrison, D.A.; Watkinson, P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: A UK-wide prospective cohort study. Crit. Care 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Kerckhoffs, M.C.; Kosasi, F.F.L.; Soliman, I.W.; van Delden, J.J.M.; Cremer, O.L.; de Lange, D.W.; Slooter, A.J.C.; Kesecioglu, J.; van Dijk, D. Determinants of self-reported unacceptable outcome of intensive care treatment 1 year after discharge. Intensiv. Care Med. 2019, 45, 806–814. [Google Scholar] [CrossRef]
- Bein, T.; Weber-Carstens, S.; Apfelbacher, C. Long-term outcome after the acute respiratory distress syndrome. Curr. Opin. Crit. Care 2018, 24, 35–40. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of COVID-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef]
- Gamberini, L.; Mazzoli, C.A.; Sintonen, H.; Colombo, D.; Scaramuzzo, G.; Allegri, D.; Tonetti, T.; Zani, G.; Capozzi, C.; Giampalma, E.; et al. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual. Life Res. 2021, 30, 2805–2817. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Vlake, J.H.B.; Van Bommel, J.; Hellemons, M.E.; Wils, E.-J.; Bienvenu, O.J.; Schut, A.F.C.; Klijn, E.; Van Bavel, M.P.; Gommers, D.; Van Genderen, M.E. Psychologic Distress and Quality of Life After ICU Treatment for Coronavirus Disease 2019: A Multicenter, Observational Cohort Study. Crit. Care Explor. 2021, 3, e0497. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinski, M.; Bayliss, M.S.; McHorney, C.A.; Rogers, W.H.; Raczek, A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med. Care 1995, 33, AS264–AS279. [Google Scholar]
- Weiss, D.S. The Impact of Event Scale: Revised. In Cross-cultural Assessment of Psychological Trauma and PTSD; Wilson, J.P., Tang, C.S., Eds.; Springer: New York, NY, USA, 2007; pp. 219–238. [Google Scholar]
- Murray, J.F.; Matthay, M.A.; Luce, J.M.; Flick, M.R. An Expanded Definition of the Adult Respiratory Distress Syndrome. Am. Rev. Respir. Dis. 1988, 138, 720–723, Erratum in Am. Rev. Respir. Dis. 1989, 139, 1065. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eberst, G.; Claudé, F.; Laurent, L.; Meurisse, A.; Roux-Claudé, P.; Barnig, C.; Vernerey, D.; Paget-Bailly, S.; Bouiller, K.; Chirouze, C.; et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann. Intensiv. Care 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.M.; Tansey, C.M.; Tomlinson, G.; Diaz-Granados, N.; Matté, A.; Barr, A.; Mehta, S.; Mazer, C.D.; Guest, C.B.; Stewart, T.E.; et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 538–544. [Google Scholar] [CrossRef]
- Bienvenu, J.; Colantuoni, E.; Mendez-Tellez, P.A.; Shanholtz, C.; Himmelfarb, C.D.; Pronovost, P.J.; Needham, D.M. Cooccurrence of and Remission From General Anxiety, Depression, and Posttraumatic Stress Disorder Symptoms After Acute Lung Injury. Crit. Care Med. 2015, 43, 642–653. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef]
- Marti, J.; Hall, P.; Hamilton, P.; Lamb, S.; McCabe, C.; Lall, R.; Darbyshire, J.; Young, D.; Hulme, C. One-year resource utilisation, costs and quality of life in patients with acute respiratory distress syndrome (ARDS): Secondary analysis of a randomised controlled trial. J. Intensive Care 2016, 4, 56. [Google Scholar] [CrossRef]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef]
- Cui, J.M.; Yuan, B.M.; Li, Y.B.; Li, Z.M.; Yuan, Y. The clinical characters and prognosis of COVID-19 patients with multiple organ dysfunction. Medicine 2021, 100, e27400. [Google Scholar] [CrossRef] [PubMed]
- Deana, C.; Bagatto, D. Severe stroke in patients admitted to intensive care unit after COVID-19 infection: Pictorial essay of a case series. Brain Hemorrhages 2022, 3, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Deana, C.; Vetrugno, L.; Fabris, M.; Curcio, F.; Sozio, E.; Tascini, C.; Bassi, F. Pericardial Cytokine “Storm” in a COVID-19 Patient: The Confirmation of a Hypothesis. Inflammation 2022, 45, 1–5. [Google Scholar] [CrossRef]
- DePace, N.L.; Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Curr. Cardiol. Rep. 2022, 24, 1711–1726. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J. Med. Virol. 2021, 94, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, C.S.B.; Mytton, O.T.; Gentry, S.V.; Thomas-Meyer, M.; Allen, J.L.Y.; Narula, A.A.; McGrath, B.; Lupton, M.; Broadbent, J.; Ahmed, A.; et al. Managing intensive care admissions when there are not enough beds during the COVID-19 pandemic: A systematic review. Thorax 2020, 76, 302–312. [Google Scholar] [CrossRef]
- Deana, C.; Rovida, S.; Orso, D.; Bove, T.; Bassi, F.; De Monte, A.; Vetrugno, L. Learning from the Italian experience during COVID-19 pandemic waves: Be prepared and mind some crucial aspects. Acta Bio-Med. Atenei Parm. 2021, 92, e2021097. [Google Scholar] [CrossRef]
- Dennis, J.M.; McGovern, A.P.M.; Vollmer, S.J.; Mateen, B.A.M. Improving Survival of Critical Care Patients With Coronavirus Disease 2019 in England: A National Cohort Study, March to June 2020. Crit. Care Med. 2021, 49, 209–214. [Google Scholar] [CrossRef]
- Sprung, C.L.; Joynt, G.M.; Christian, M.D.; Truog, R.D.; Rello, J.; Nates, J.L. Adult ICU Triage During the Coronavirus Disease 2019 Pandemic: Who Will Live and Who Will Die? Recommendations to Improve Survival. Crit. Care Med. 2020, 48, 1196–1202. [Google Scholar] [CrossRef]
- Lombardi, Y.; Azoyan, L.; Szychowiak, P.; Bellamine, A.; Lemaitre, G.; Bernaux, M.; Daniel, C.; Leblanc, J.; Riller, Q.; Steichen, O.; et al. External validation of prognostic scores for COVID-19: A multicenter cohort study of patients hospitalized in Greater Paris University Hospitals. Intensiv. Care Med. 2021, 47, 1426–1439. [Google Scholar] [CrossRef]
- Vetrugno, L.; Castaldo, N.; Fantin, A.; Deana, C.; Cortegiani, A.; Longhini, F.; Forfori, F.; Cammarota, G.; Grieco, D.L.; Isola, M.; et al. Ventilatory associated barotrauma in COVID-19 patients: A multicenter observational case control study (COVI-MIX-study). Pulmonology 2022. [CrossRef] [PubMed]
- Brown, S.M.; Wilson, E.; Presson, A.P.; Zhang, C.; Dinglas, V.D.; Greene, T.; Hopkins, R.O.; Needham, D.M. Predictors of 6-month health utility outcomes in survivors of acute respiratory distress syndrome. Thorax 2017, 72, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Lim, M.A.; Huang, I.; Raharjo, S.B.; Lukito, A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. J. Renin-Angiotensin-Aldosterone Syst. 2020, 21, 1470320320926899. [Google Scholar] [CrossRef]
- Trevisol, D.J.; Moreira, L.B.; Kerkhoff, A.; Fuchs, S.C.; Fuchs, F. Health-related quality of life and hypertension: A systematic review and meta-analysis of observational studies. J. Hypertens. 2011, 29, 179–188. [Google Scholar] [CrossRef]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 1702. [Google Scholar] [CrossRef]
- Prasad, M.; Leon, M.; Lerman, L.O.; Lerman, A. Viral Endothelial Dysfunction: A Unifying Mechanism for COVID-19. Mayo Clin. Proc. 2021, 96, 3099–3108. [Google Scholar] [CrossRef]
- Harvey, M.A.; Davidson, J.E. Postintensive Care Syndrome. Crit. Care Med. 2016, 44, 381–385. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Lee, D.T.S.; Wing, Y.K.; Leung, H.C.M.; Sung, J.J.Y.; Ng, Y.K.; Yiu, G.C.; Chen, R.Y.L.; Chiu, H.F.K. Factors Associated with Psychosis among Patients with Severe Acute Respiratory Syndrome: A Case-Control Study. Clin. Infect. Dis. 2004, 39, 1247–1249. [Google Scholar] [CrossRef]
- Righy, C.; Rosa, R.G.; da Silva, R.T.A.; Kochhann, R.; Migliavaca, C.B.; Robinson, C.C.; Teche, S.P.; Teixeira, C.; Bozza, F.A.; Falavigna, M. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: A systematic review and meta-analysis. Crit. Care 2019, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; Salve, G.; Tavazzi, G.; Politi, P.; Mojoli, F. High prevalence of acute stress disorder and persisting symptoms in ICU survivors after COVID-19. Intensiv. Care Med. 2021, 47, 616. [Google Scholar] [CrossRef] [PubMed]
- Carenzo, L.; Protti, A.; Corte, F.D.; Aceto, R.; Iapichino, G.; Milani, A.; Santini, A.; Chiurazzi, C.; Ferrari, M.; Heffler, E.; et al. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann. Intensiv. Care 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.-F.; Minguet, P.; Colson, C.; Kellens, I.; Chaabane, S.; Delanaye, P.; Cavalier, E.; Chase, J.G.; Lambermont, B.; Misset, B. Post-intensive care syndrome after a critical COVID-19: Cohort study from a Belgian follow-up clinic. Ann. Intensiv. Care 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deana, C.; Verriello, L.; Pauletto, G.; Corradi, F.; Forfori, F.; Cammarota, G.; Bignami, E.; Vetrugno, L.; Bove, T. Insights into neurological dysfunction of critically ill COVID-19 patients. Trends Anaesth. Crit. Care 2021, 36, 30–38. [Google Scholar] [CrossRef]
- Zante, B.; Erne, K.; Grossenbacher, J.; Camenisch, S.A.; Schefold, J.C.; Jeitziner, M.-M. Symptoms of post-traumatic stress disorder (PTSD) in next of kin during suspension of ICU visits during the COVID-19 pandemic: A prospective observational study. BMC Psychiatry 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Moss, S.J.; Rosgen, B.K.; Lucini, F.; Krewulak, K.D.; Soo, A.; Doig, C.J.; Patten, S.B.; Stelfox, H.T.; Fiest, K.M. Psychiatric Outcomes in ICU Patients With Family Visitation. Chest 2022, 162, 578–587. [Google Scholar] [CrossRef]
- Lettinga, K.D.; Verbon, A.; Nieuwkerk, P.; Jonkers, R.E.; Gersons, B.P.R.; Prins, J.M.; Speelman, P. Health-Related Quality of Life and Posttraumatic Stress Disorder among Survivors of an Outbreak of Legionnaires Disease. Clin. Infect. Dis. 2002, 35, 11–17. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Rahman, L.; del Campo, C.M.Z.-M.; Maldonado, J.C.; Jafri, N.; Cummings, M.A.; Maurer, S.; Kozlakidis, Z. PTSD as an Endothelial Disease: Insights From COVID-19. Front. Cell. Neurosci. 2021, 15, 770387. [Google Scholar] [CrossRef]
- Tarsitani, L.; Vassalini, P.; Koukopoulos, A.; Borrazzo, C.; Alessi, F.; Di Nicolantonio, C.; Serra, R.; Alessandri, F.; Ceccarelli, G.; Mastroianni, C.M.; et al. Post-traumatic Stress Disorder Among COVID-19 Survivors at 3-Month Follow-up After Hospital Discharge. J. Gen. Intern. Med. 2021, 36, 1702–1707. [Google Scholar] [CrossRef]
- Kubzansky, L.D.; Bordelois, P.; Jun, H.J.; Roberts, A.; Cerda, M.; Bluestone, N.; Koenen, K. The Weight of Traumatic Stress. JAMA Psychiatry 2014, 71, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Li, Y.; Li, L.; He, J.; Ouyang, F.; Xiao, S. Prevalence of post-traumatic stress symptoms among people influenced by coronavirus disease 2019 outbreak: A meta-analysis. Eur. Psychiatry 2021, 64, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef]
- Vahia, I.V.; Jeste, D.V.; Reynolds, C.F. Older Adults and the Mental Health Effects of COVID-19. JAMA 2020, 324, 2253. [Google Scholar] [CrossRef]
- Dmytriw, A.A.; Chibbar, R.; Chen, P.P.Y.; Traynor, M.D.; Kim, D.W.; Bruno, F.P.; Cheung, C.C.; Pareek, A.; Chou, A.C.C.; Graham, J.; et al. Outcomes of acute respiratory distress syndrome in COVID-19 patients compared to the general population: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2021, 15, 1347–1354. [Google Scholar] [CrossRef]
- Fazzini, B.; Battaglini, D.; Carenzo, L.; Pelosi, P.; Cecconi, M.; Puthucheary, Z. Physical and psychological impairment in survivors of acute respiratory distress syndrome: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 129, 801–814. [Google Scholar] [CrossRef]
- Latronico, N.; Peli, E.; Calza, S.; Rodella, F.; Novelli, M.P.; Cella, A.; Marshall, J.; Needham, D.M.; Rasulo, F.A.; Piva, S. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 2022, 77, 300–303. [Google Scholar] [CrossRef]


| n = 343 | |
|---|---|
| Age, median (IQR) | 63 (57–70) |
| Men, N (%) | 272 (79.3) |
| Weight at baseline (Kg), median (IQR) | 86 (78–98) |
| Weight at follow-up (Kg), median (IQR) | 85 (75–95) |
| Weight variation (Kg) median (IQR) | −2.5 (0;−7) |
| Cardiovascular disease, N (%) | |
| Arterial hypertension | 147 (42.9) |
| Chronic artery disease | 9 (2.6) |
| Other | 11 (3.2) |
| Pulmonary disease, N (%) | |
| COPD | 21 (6.1) |
| Pulmonary fibrosis | 5 (1.5) |
| Asthma | 10 (2.9) |
| Emphysema | 2 (0.6) |
| Other | 15 (4.4) |
| Kidney disease, N (%) | |
| Chronic renal failure | 12 (3.5) |
| Other | 5 (1.5) |
| Liver disease, N (%) | |
| Cirrhosis | 3 (0.9) |
| Other | 7 (2.0) |
| Diabetes, N (%) | 49 (14.2) |
| Level of education, N (%) | |
| Low | 157 (46) |
| High | 186 (54) |
| Marital status, N (%) | |
| Single | 36 (10.5) |
| Married/cohabiting | 273 (79.6) |
| Separated/divorced | 20 (5.8) |
| Widowed | 14 (4.1) |
| Living alone, N (%) | 58 (17) |
| Employment, N (%) | |
| Jobless | 28 (8.2) |
| Active | 159 (46.3) |
| Retired | 156 (45.5) |
| n = 343 | |
|---|---|
| APACHE II score at ICU admission, median (IQR) | 11 (8–14) |
| Lung injury score at ICU admission, median (IQR) | 3 (2.3–3) |
| Mechanical ventilation, N (%) | 343 (100) |
| Noninvasive | 32 (9) |
| Endotracheal intubation | 311 (91) |
| MV duration (days), median (IQR) | 10 (2–20) |
| NMB administration | 111 (32.3) |
| Duration of NMB (hours), median (IQR) | 96 (36–160) |
| Steroid administration | 194 (56.5) |
| Duration of steroid treatment (days), median (IQR) | 10 (7–13) |
| RRT in ICU, N (%) | 23 (6.7) |
| Tracheostomy in ICU, N (%) | 87 (25.4) |
| Day of ICU stay at tracheostomy execution, median (IQR) | 11 (6–17) |
| Length of hospital stay before ICU admission, (days) median (IQR) | 2 (1–4) |
| LOSICU, (days) median (IQR) | 13 (6–25) |
| LOSHOSP, (days) median (IQR) | 32 (19–47) |
| Discharge ward after ICU, N (%) | |
| Medical | 83 (24.1) |
| Pulmonology | 82 (24) |
| HDU | 91 (26.5) |
| Other | 56 (16.3) |
| Not specified | 31 (9) |
| SF-36 Parameter | Overall (n = 343) | No PTSD (n = 234) | PTSD (n = 109) | p-Value |
|---|---|---|---|---|
| Physical Functioning | 85 (60–95) | 85 (70–95) | 70 (40–90) | <0.001 |
| Physical Role | 75 (0–100) | 75 (0–100) | 25 (0–100) | <0.001 |
| Emotional Role | 100 (33–100) | 100 (50–100) | 33.3 (0–100) | <0.001 |
| Bodily Pain | 77.5 (45–100) | 84 (55–100) | 67.5 (41–100) | 0.001 |
| General Health | 55 (35–72) | 60 (45–75) | 45 (20–60) | <0.001 |
| Vitality | 55 (40–70) | 60 (45–75) | 50 (30–60) | <0.001 |
| Social Functioning | 75 (50–100) | 87.5 (50–100) | 55 (37.5–75) | <0.001 |
| Mental Health | 68 (52–84) | 72 (60–88) | 56 (44–72) | <0.001 |
| Health change | 50 (25–75) | 50 (25–75) | 48 (25–75) | 0.041 |
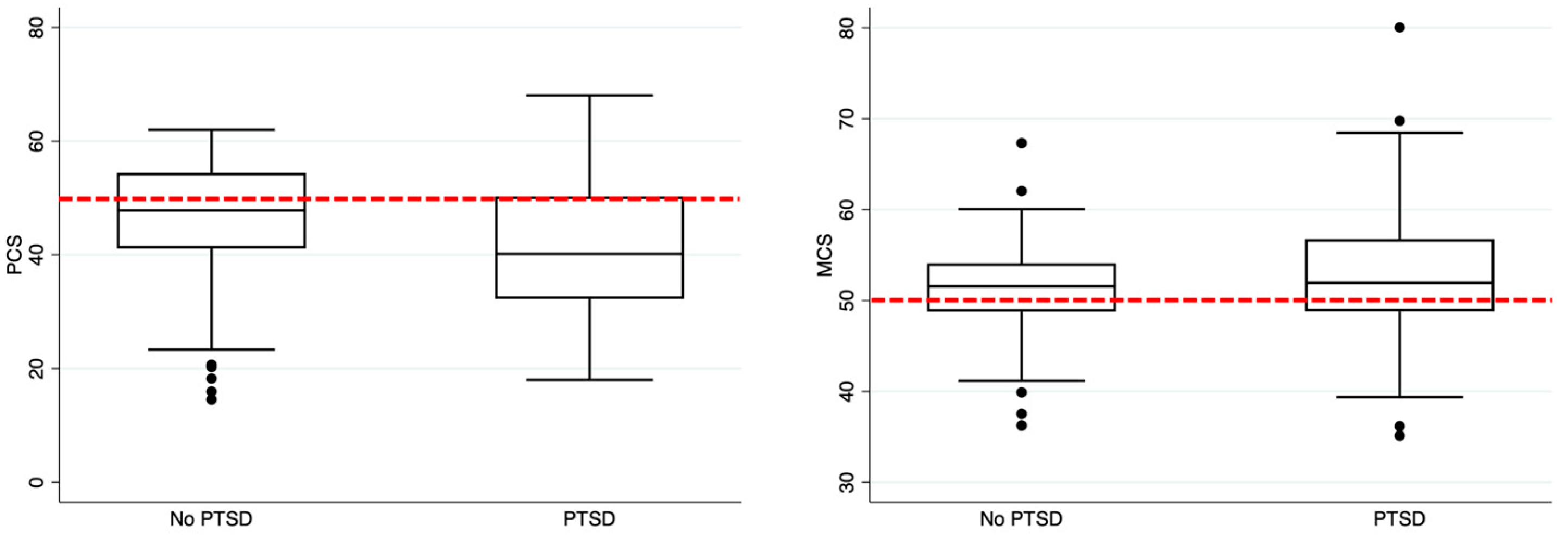
| PCS | 45.9 (36.5–53.5) | 47.8 (41.1–54.4) | 40.2 (32.2–50.3) | <0.001 |
| MCS | 51.7 (48.8–54.3) | 51.6 (48.8–54.1) | 51.9 (48.8–56.8) | 0.123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deana, C.; Vetrugno, L.; Cortegiani, A.; Mongodi, S.; Salve, G.; Mangiagalli, M.; Boscolo, A.; Pettenuzzo, T.; Miori, S.; Sanna, A.; et al. Quality of Life in COVID-Related ARDS Patients One Year after Intensive Care Discharge (Odissea Study): A Multicenter Observational Study. J. Clin. Med. 2023, 12, 1058. https://doi.org/10.3390/jcm12031058
Deana C, Vetrugno L, Cortegiani A, Mongodi S, Salve G, Mangiagalli M, Boscolo A, Pettenuzzo T, Miori S, Sanna A, et al. Quality of Life in COVID-Related ARDS Patients One Year after Intensive Care Discharge (Odissea Study): A Multicenter Observational Study. Journal of Clinical Medicine. 2023; 12(3):1058. https://doi.org/10.3390/jcm12031058
Chicago/Turabian StyleDeana, Cristian, Luigi Vetrugno, Andrea Cortegiani, Silvia Mongodi, Giulia Salve, Matteo Mangiagalli, Annalisa Boscolo, Tommaso Pettenuzzo, Sara Miori, Andrea Sanna, and et al. 2023. "Quality of Life in COVID-Related ARDS Patients One Year after Intensive Care Discharge (Odissea Study): A Multicenter Observational Study" Journal of Clinical Medicine 12, no. 3: 1058. https://doi.org/10.3390/jcm12031058
APA StyleDeana, C., Vetrugno, L., Cortegiani, A., Mongodi, S., Salve, G., Mangiagalli, M., Boscolo, A., Pettenuzzo, T., Miori, S., Sanna, A., Lassola, S., Magnoni, S., Ferrari, E., Biagioni, E., Bassi, F., Castaldo, N., Fantin, A., Longhini, F., Corradi, F., ... on behalf of the Italian Odissea Group. (2023). Quality of Life in COVID-Related ARDS Patients One Year after Intensive Care Discharge (Odissea Study): A Multicenter Observational Study. Journal of Clinical Medicine, 12(3), 1058. https://doi.org/10.3390/jcm12031058













