Diagnostics of Sacroiliac Joint Differentials to Axial Spondyloarthritis Changes by Magnetic Resonance Imaging
Abstract
1. Introduction
2. SIJ Appearance in Childhood
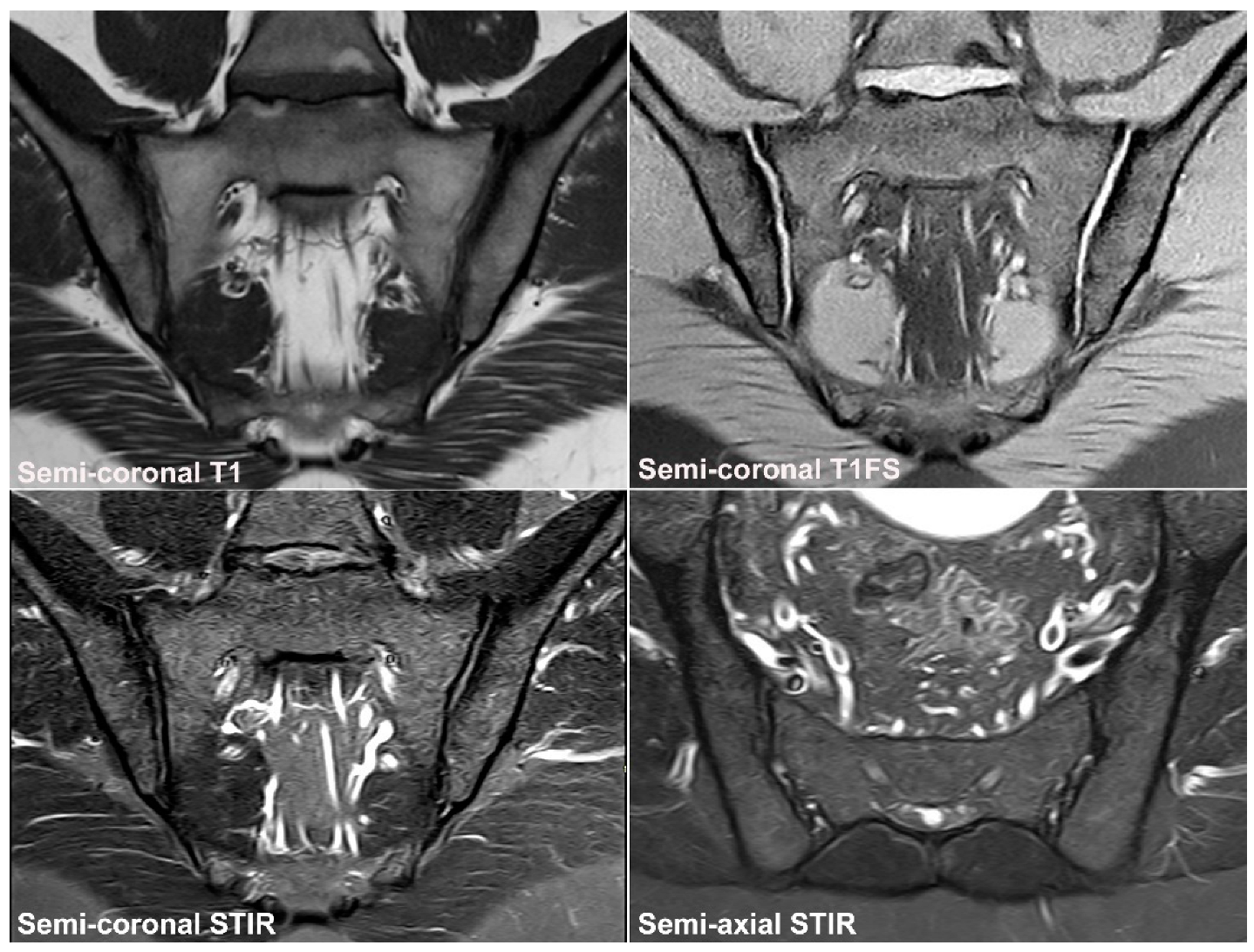
3. Normal SIJ Anatomy in Adults and Technical MRI Aspects
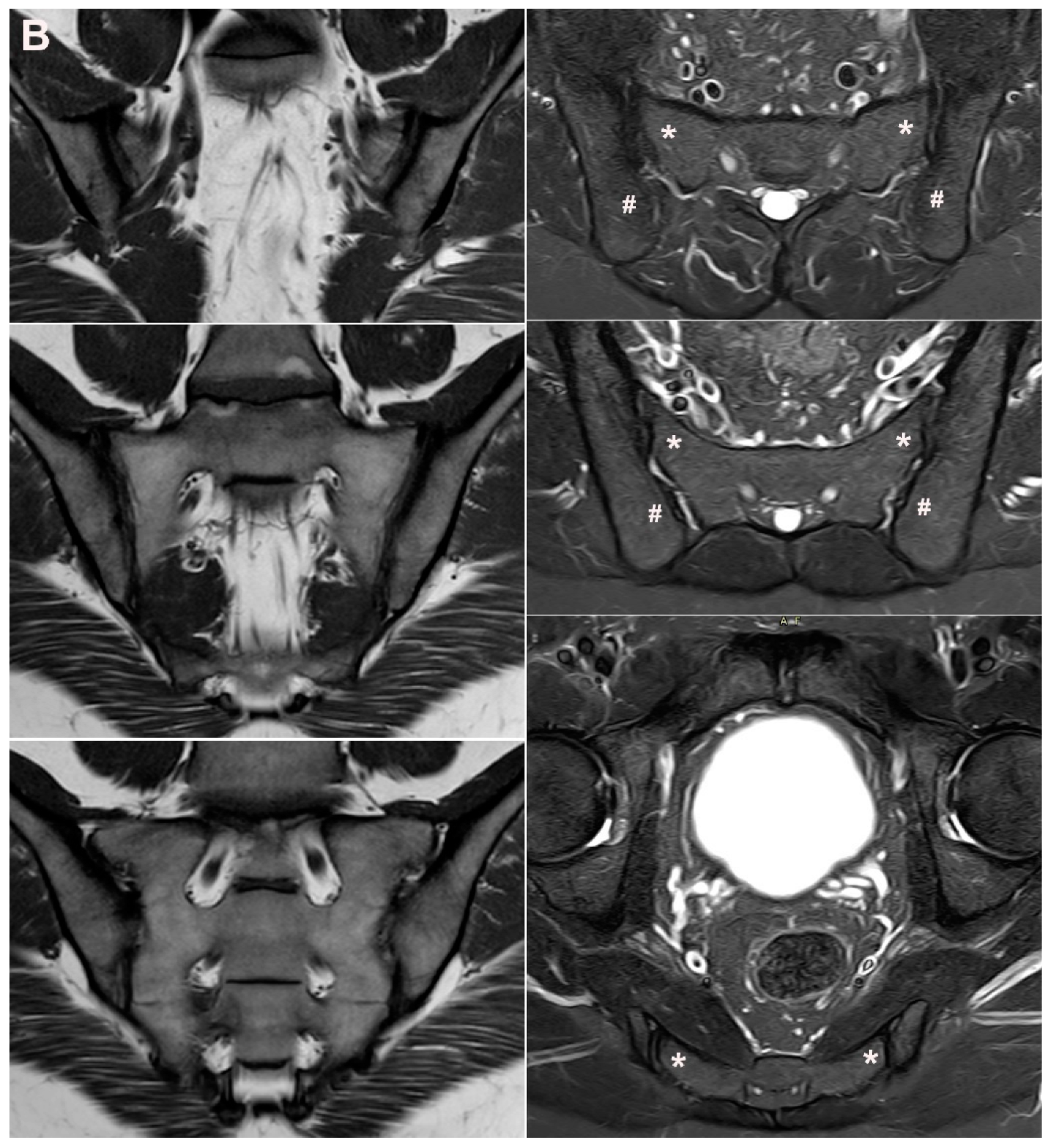
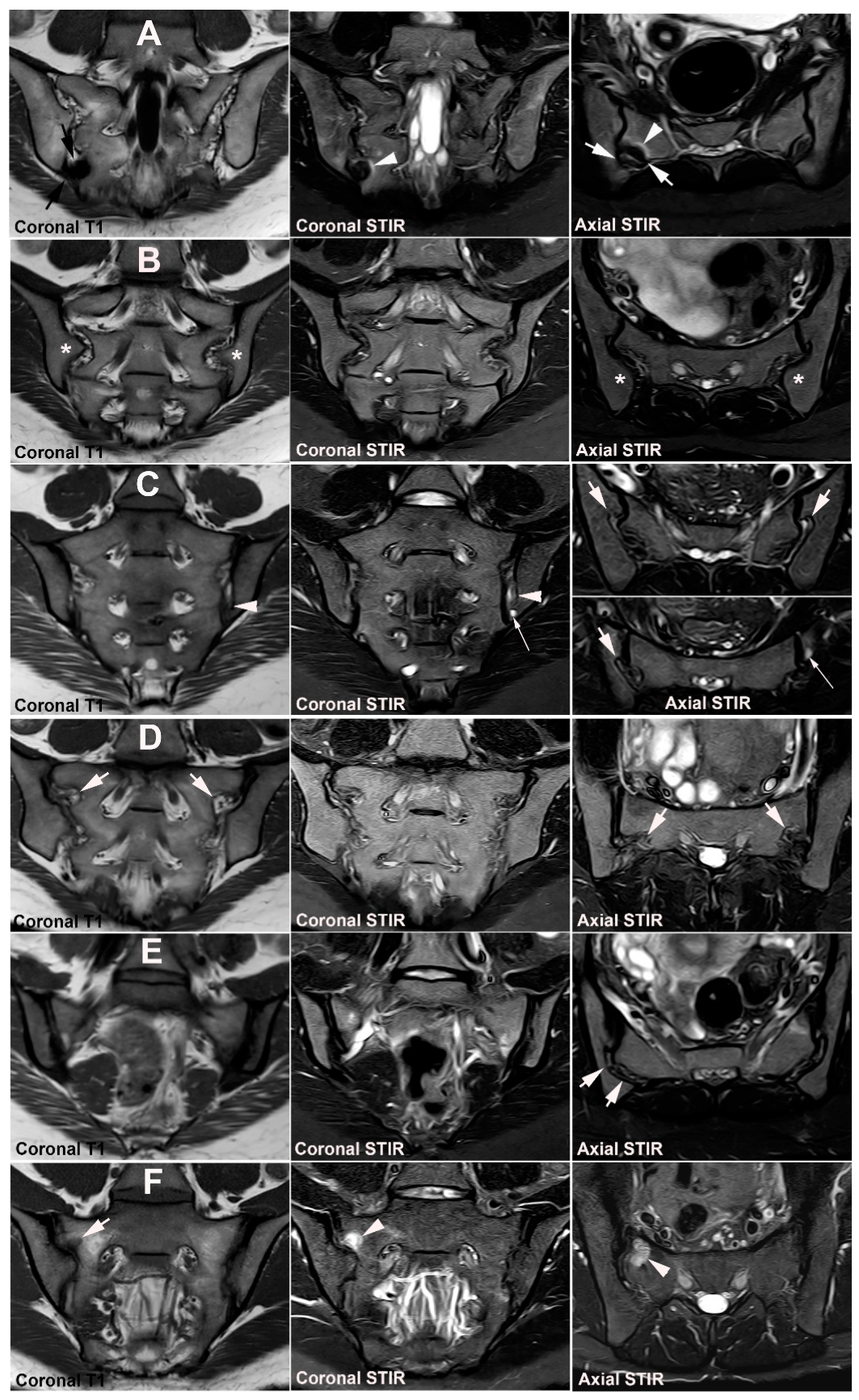
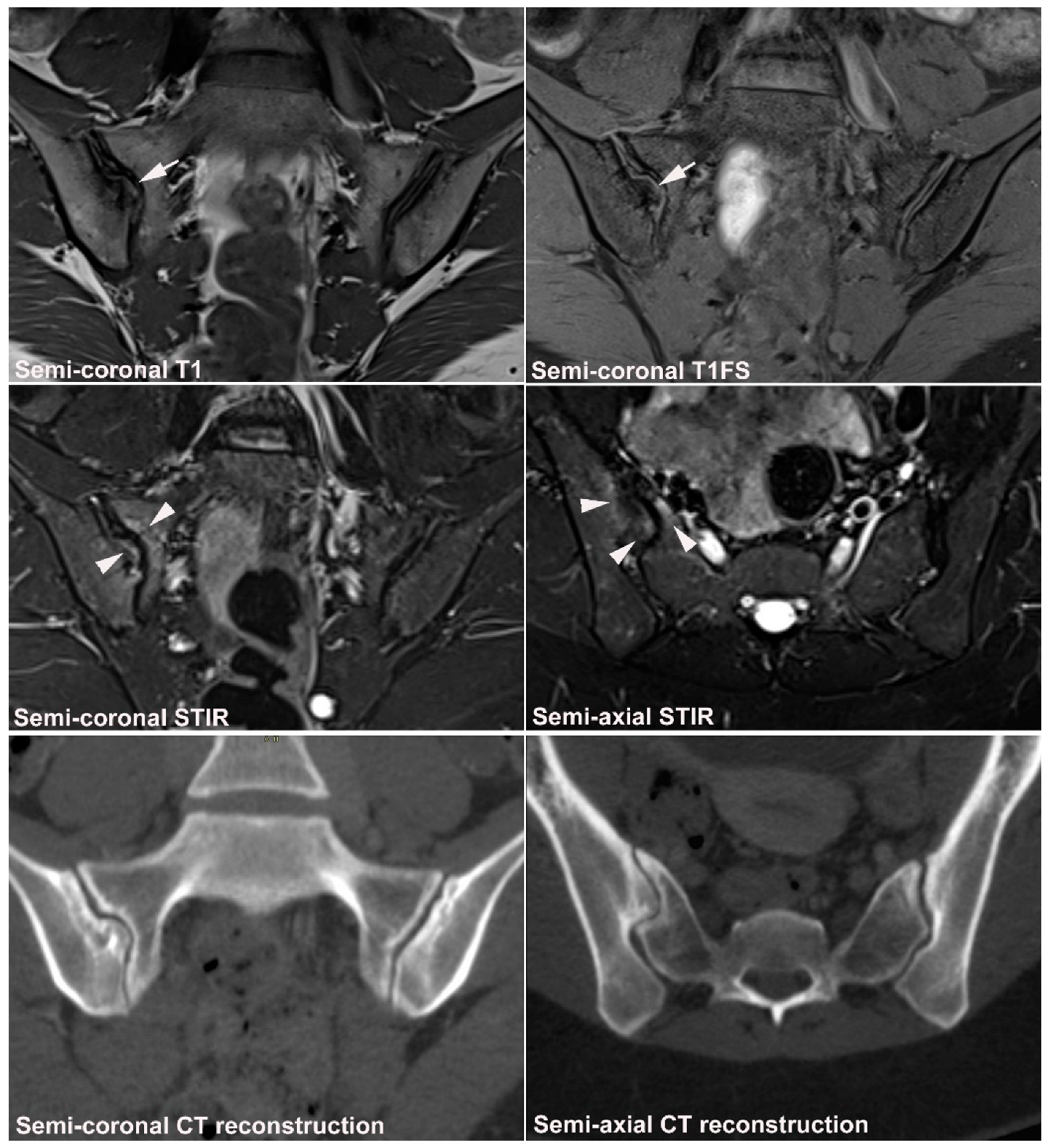
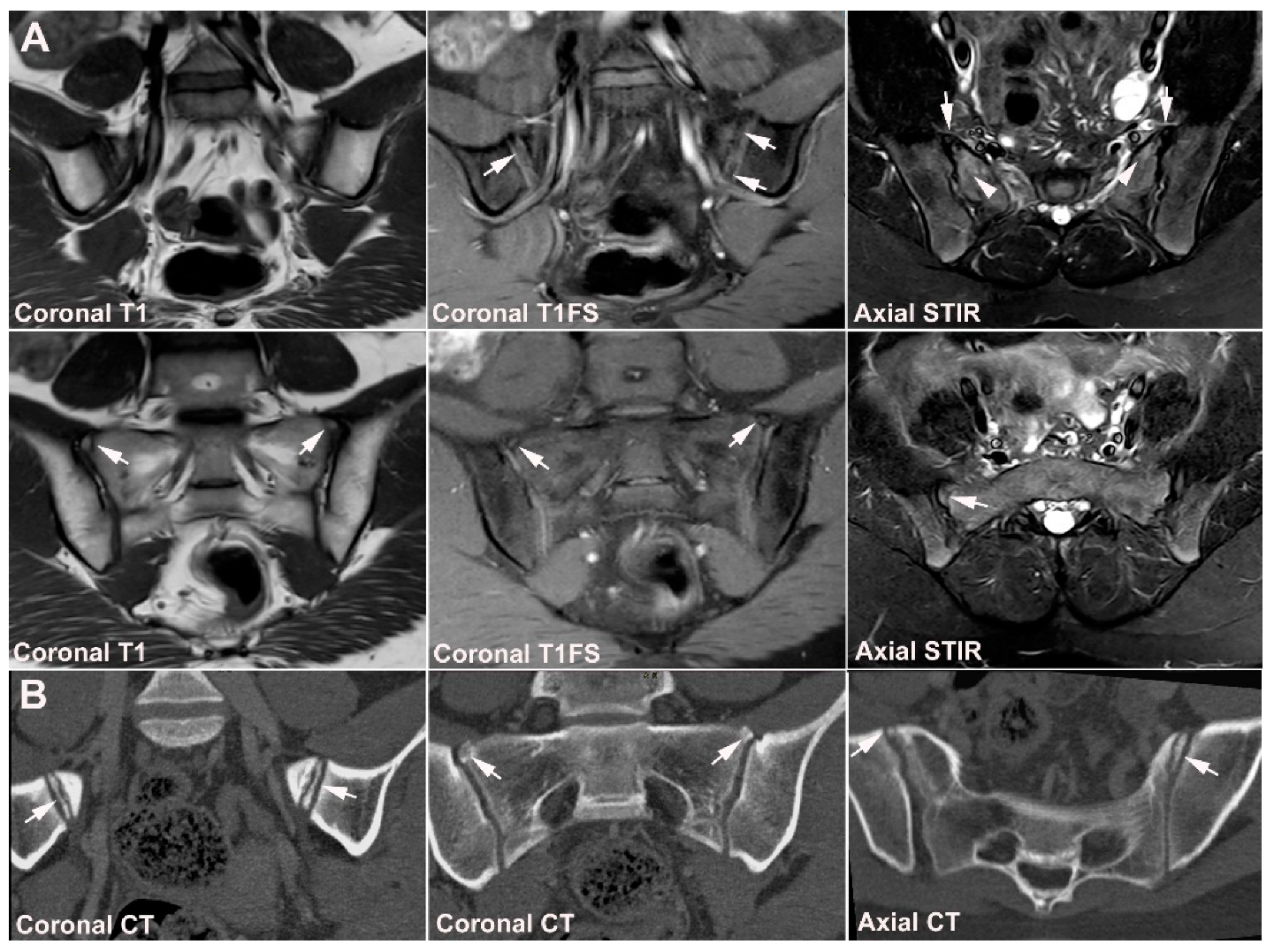
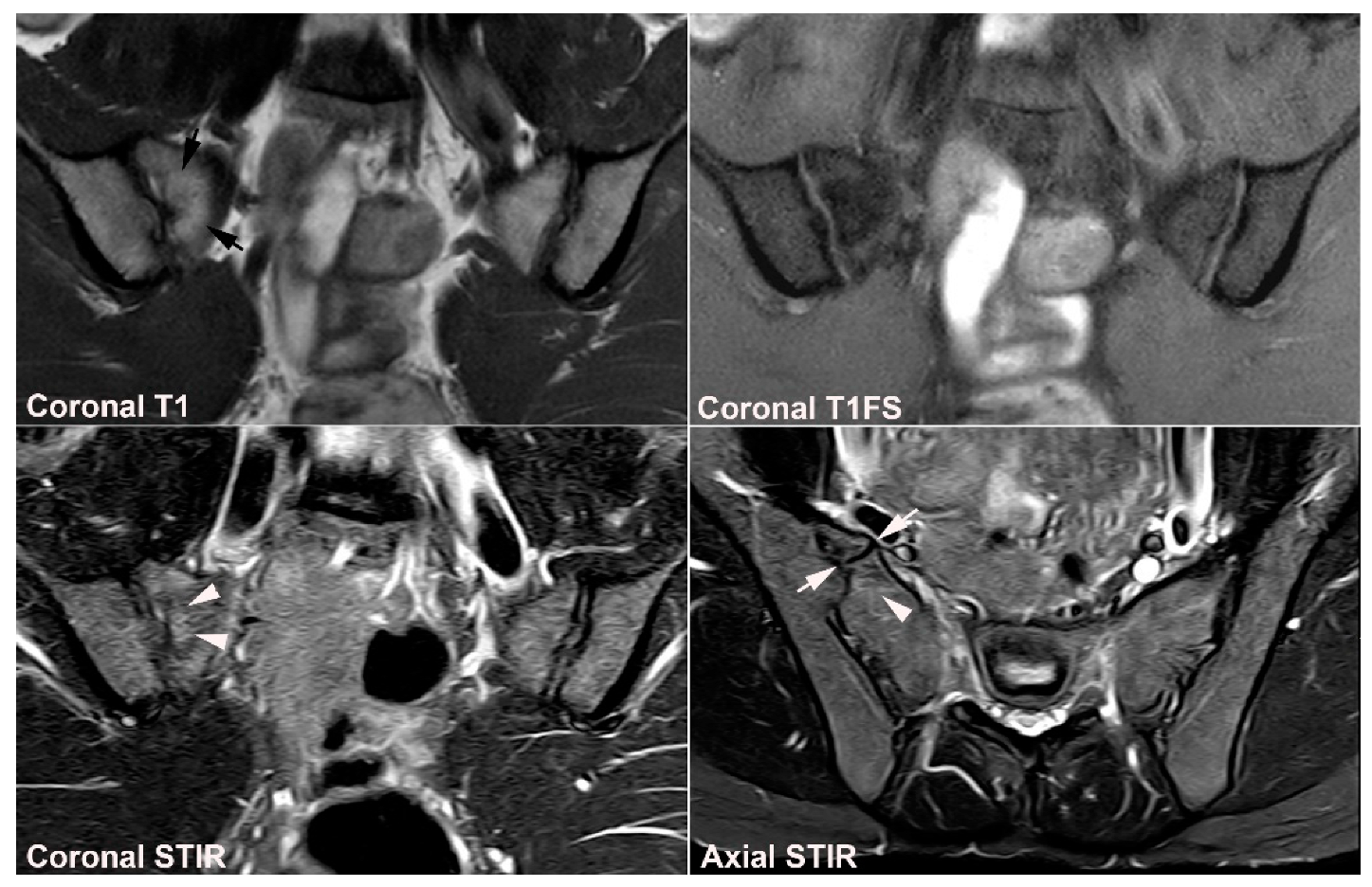
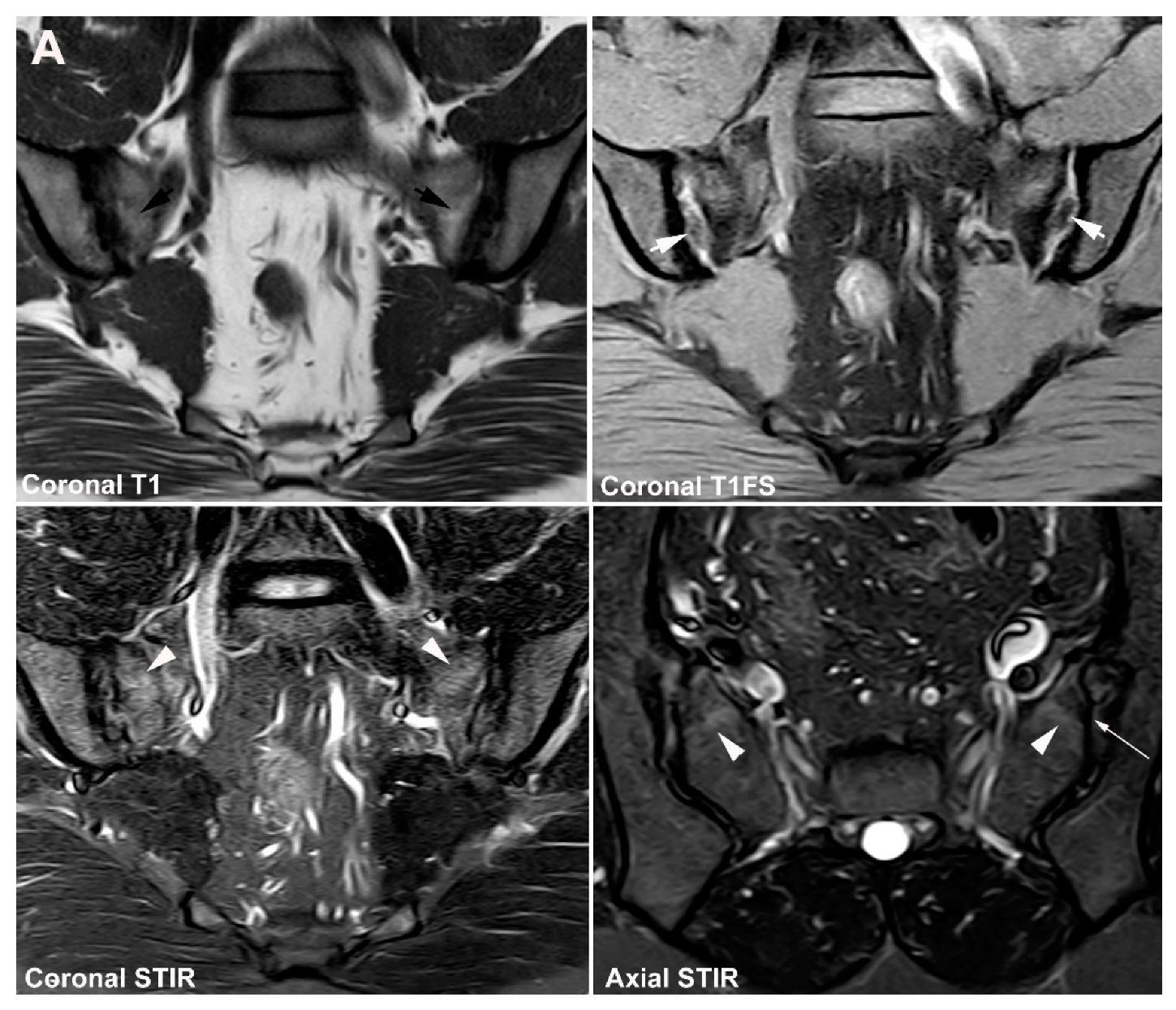
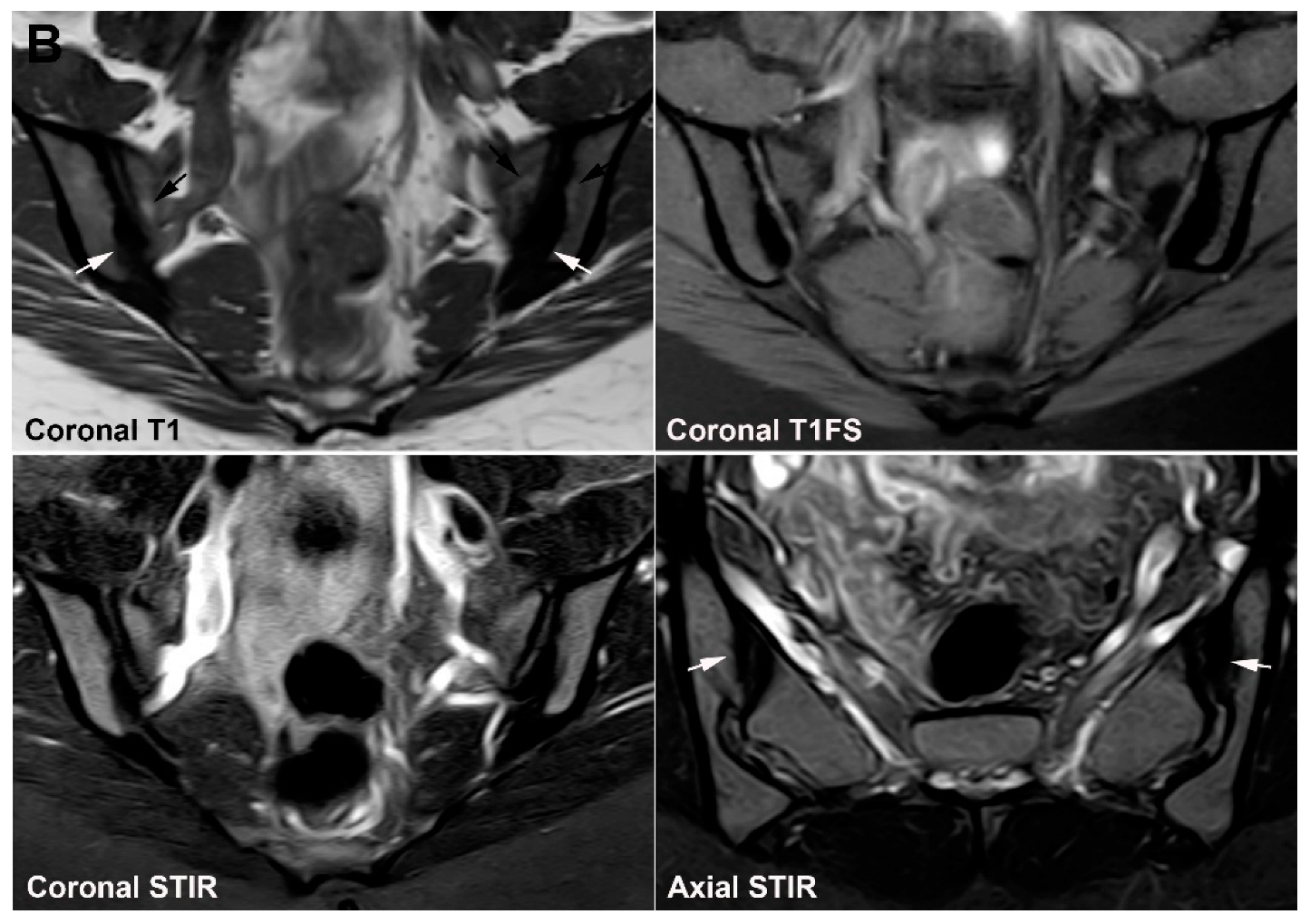
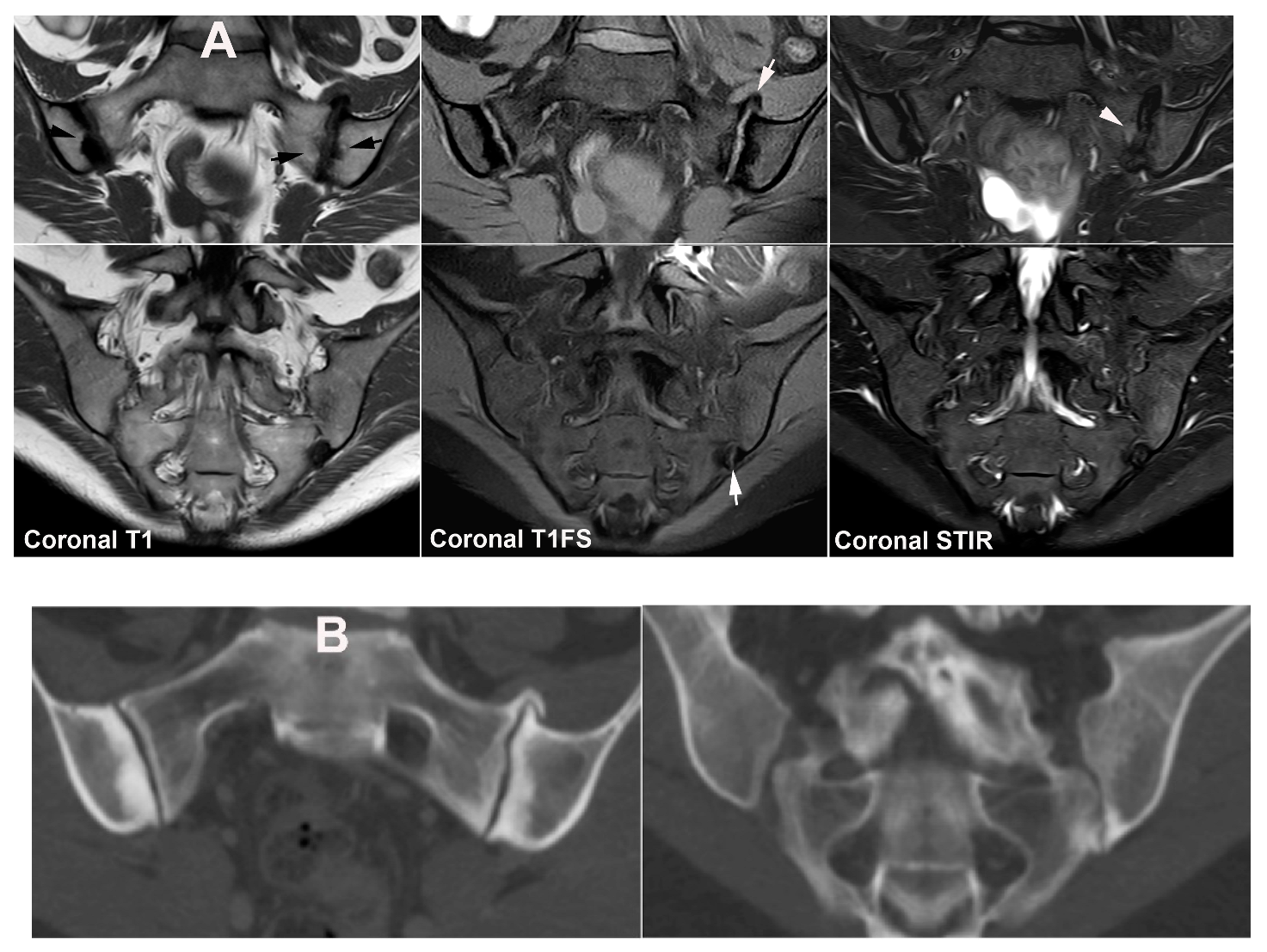
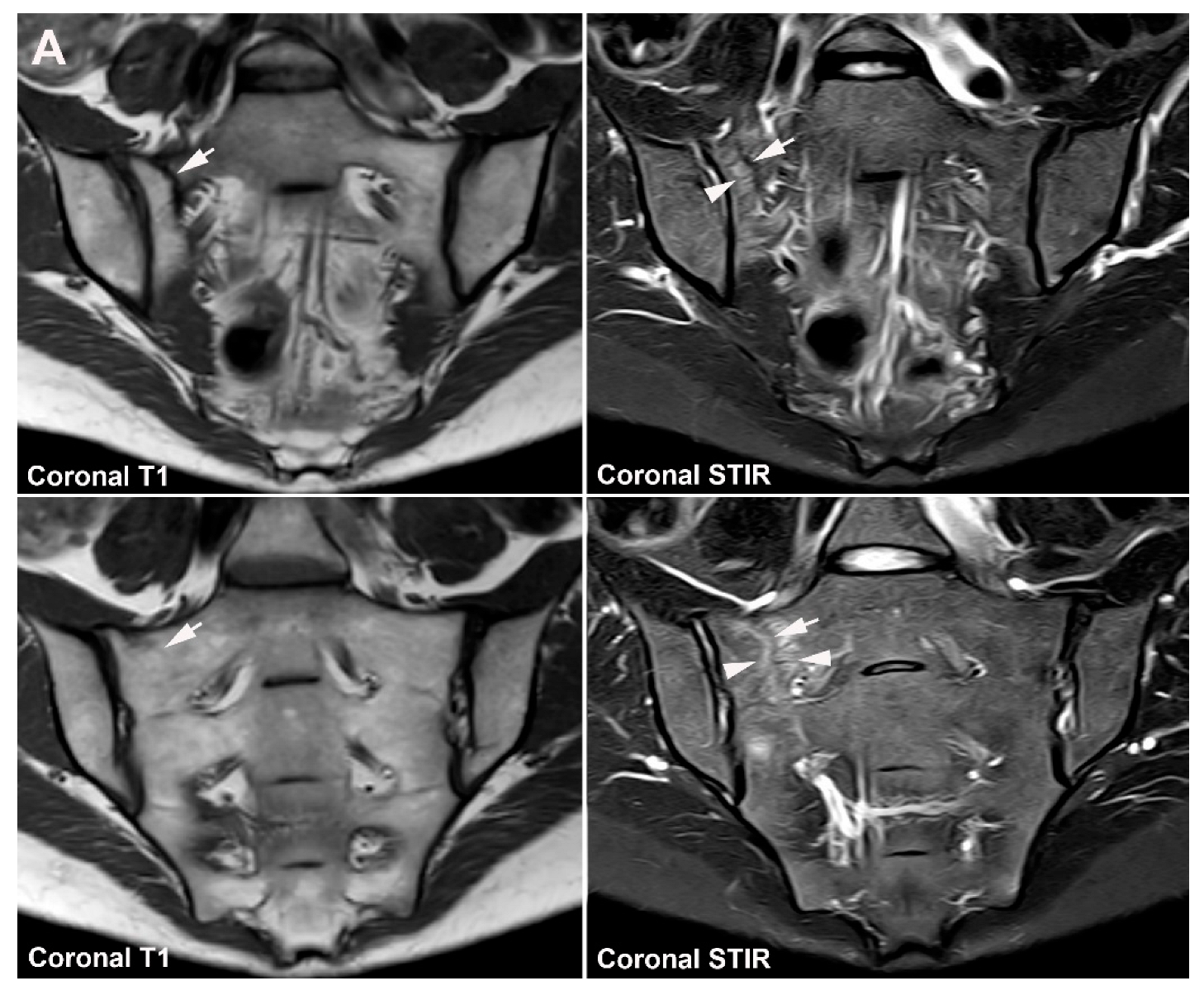
4. Anatomical Variations
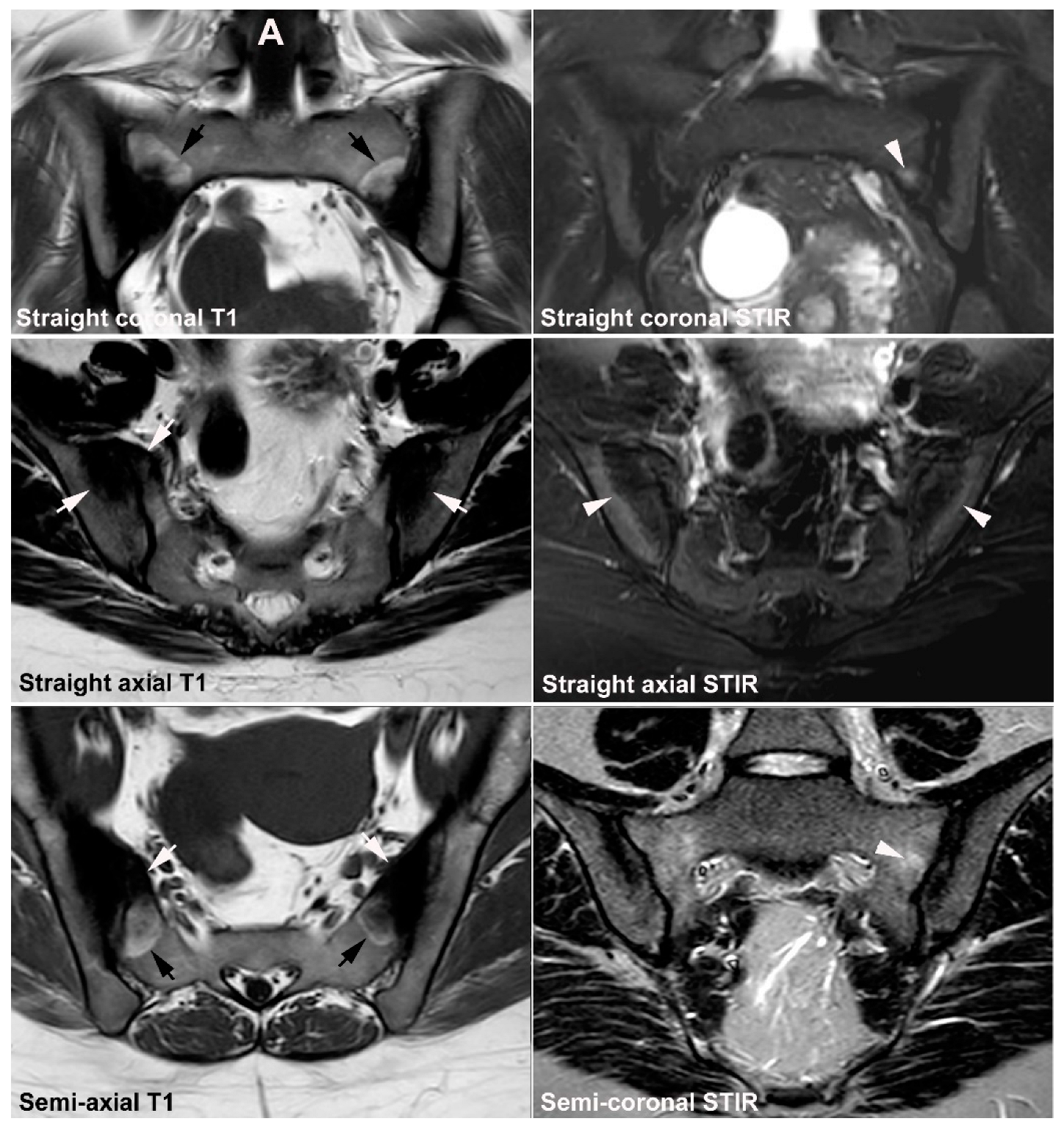
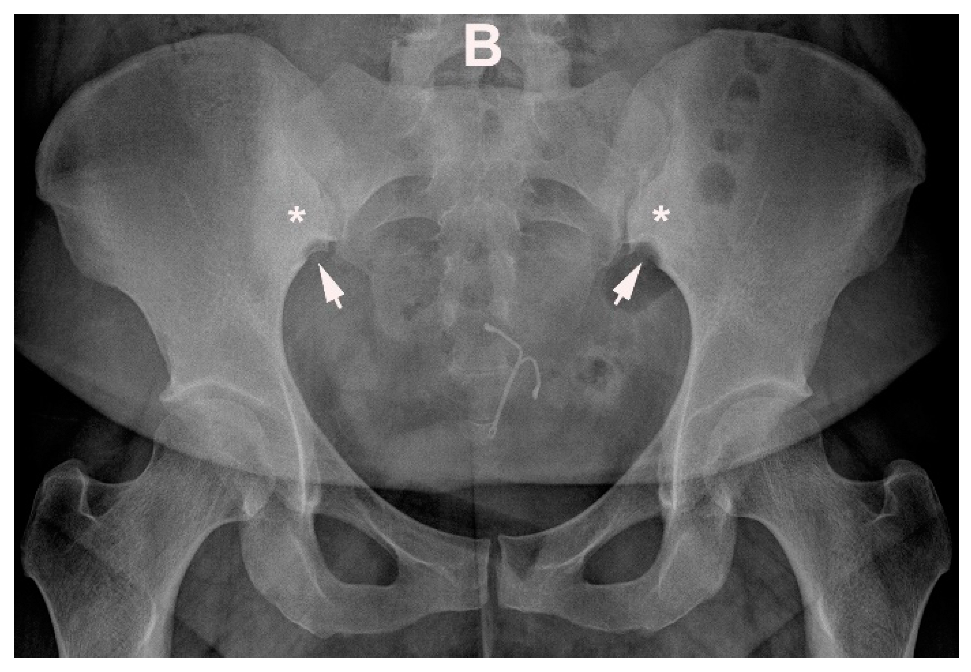
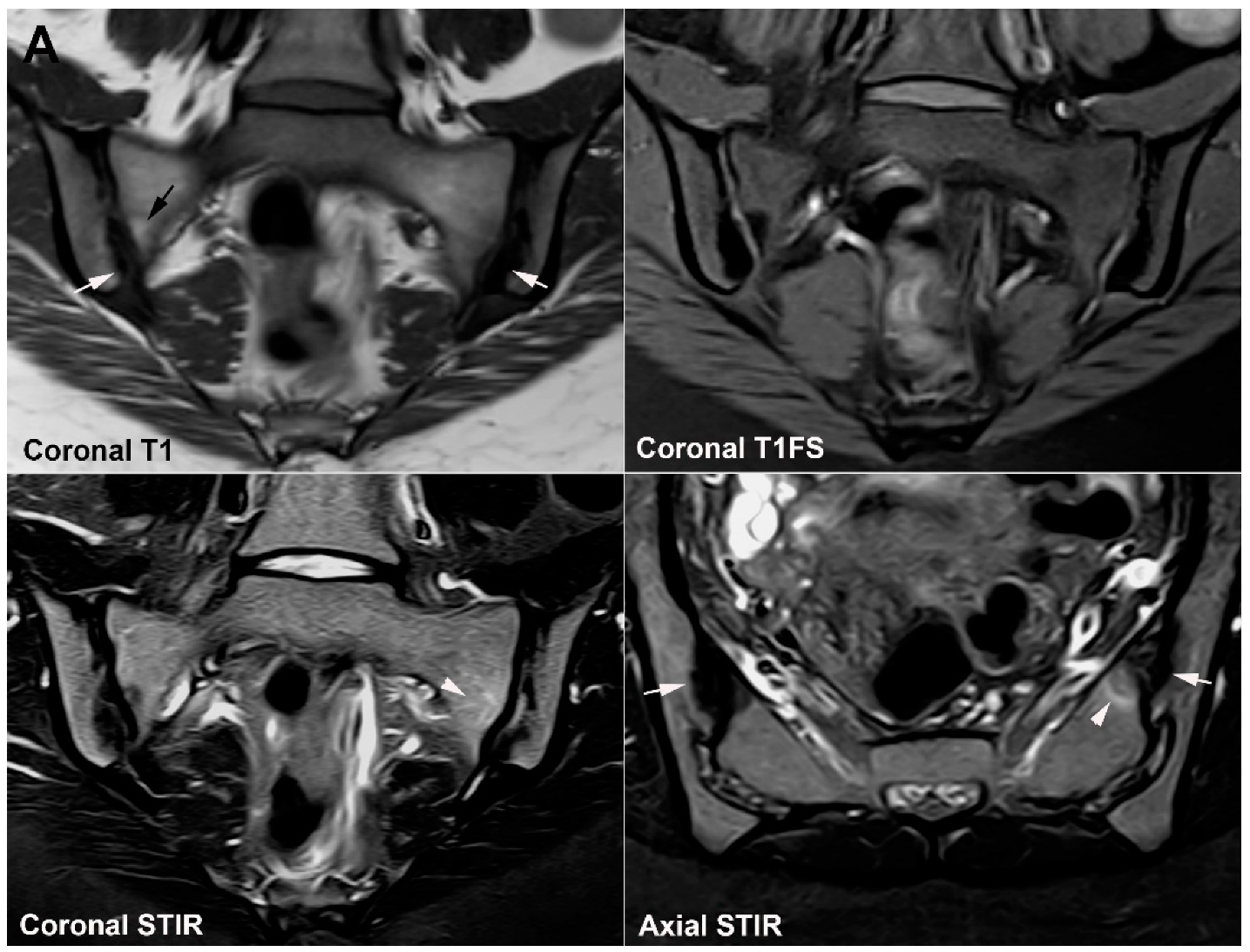
4.1. Well Described SIJ Variants
4.2. Persistence of Unfused Nuclei in Adulthood
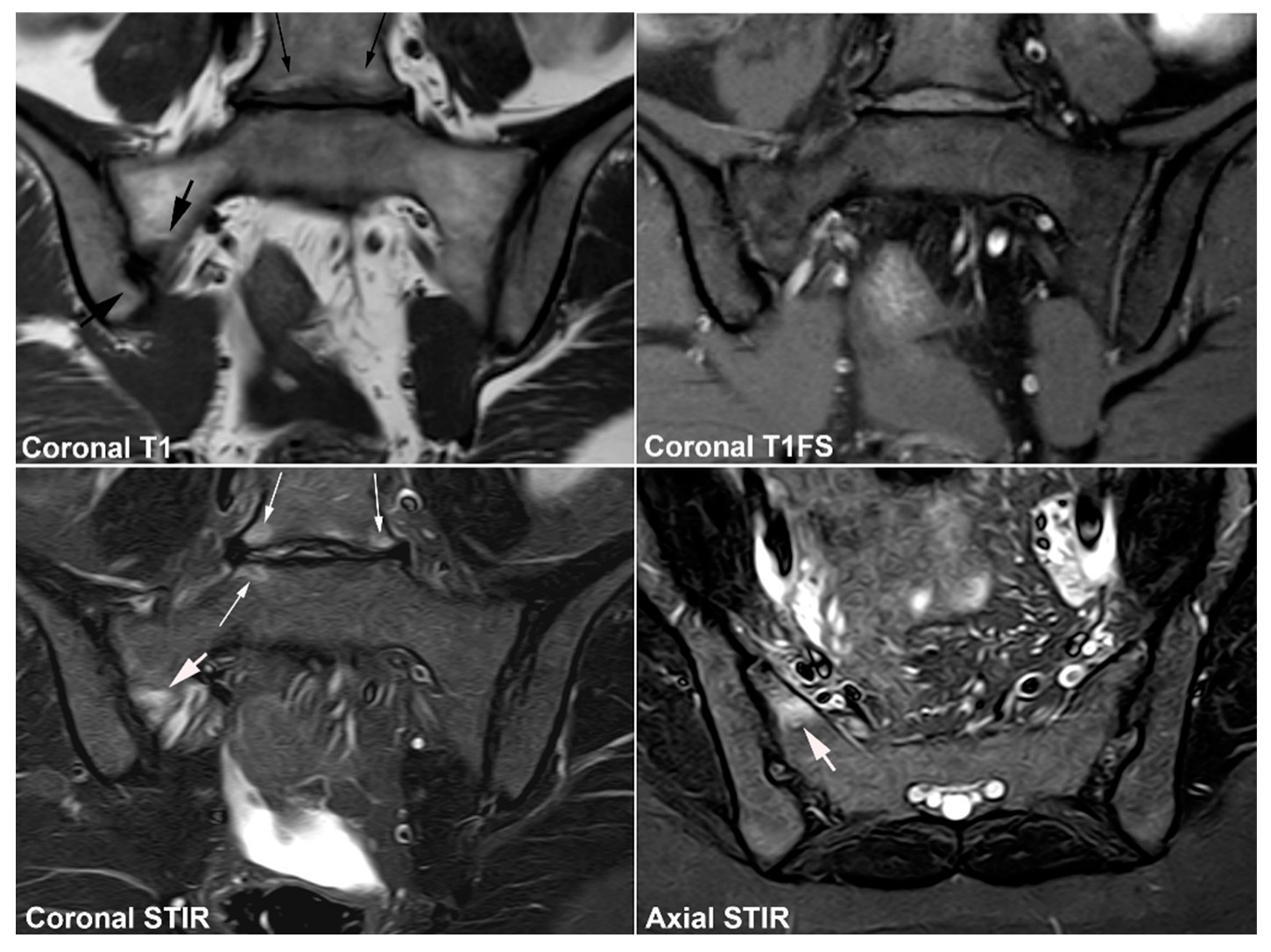
4.3. Lumbosacral Transitional Vertebrae
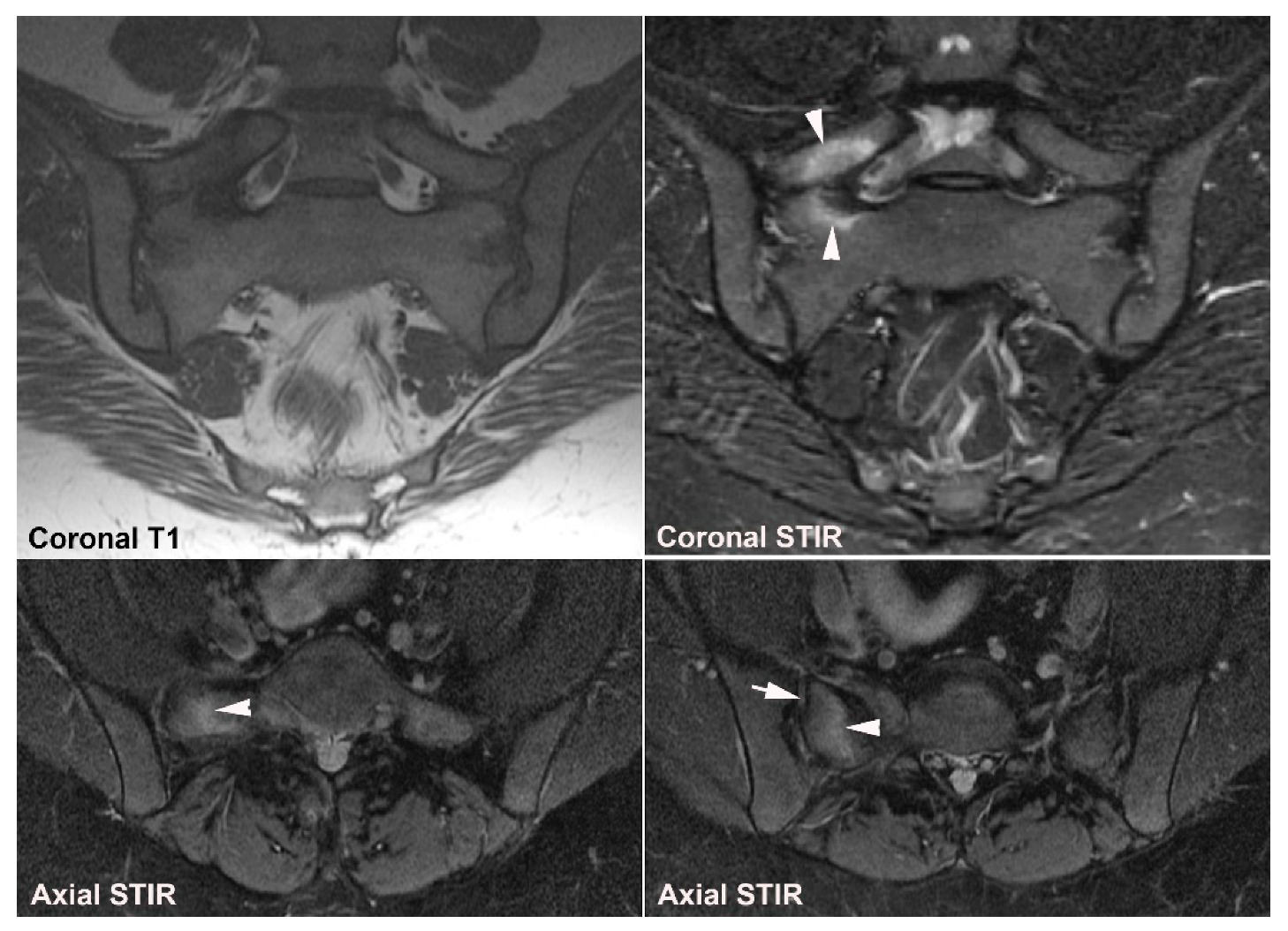
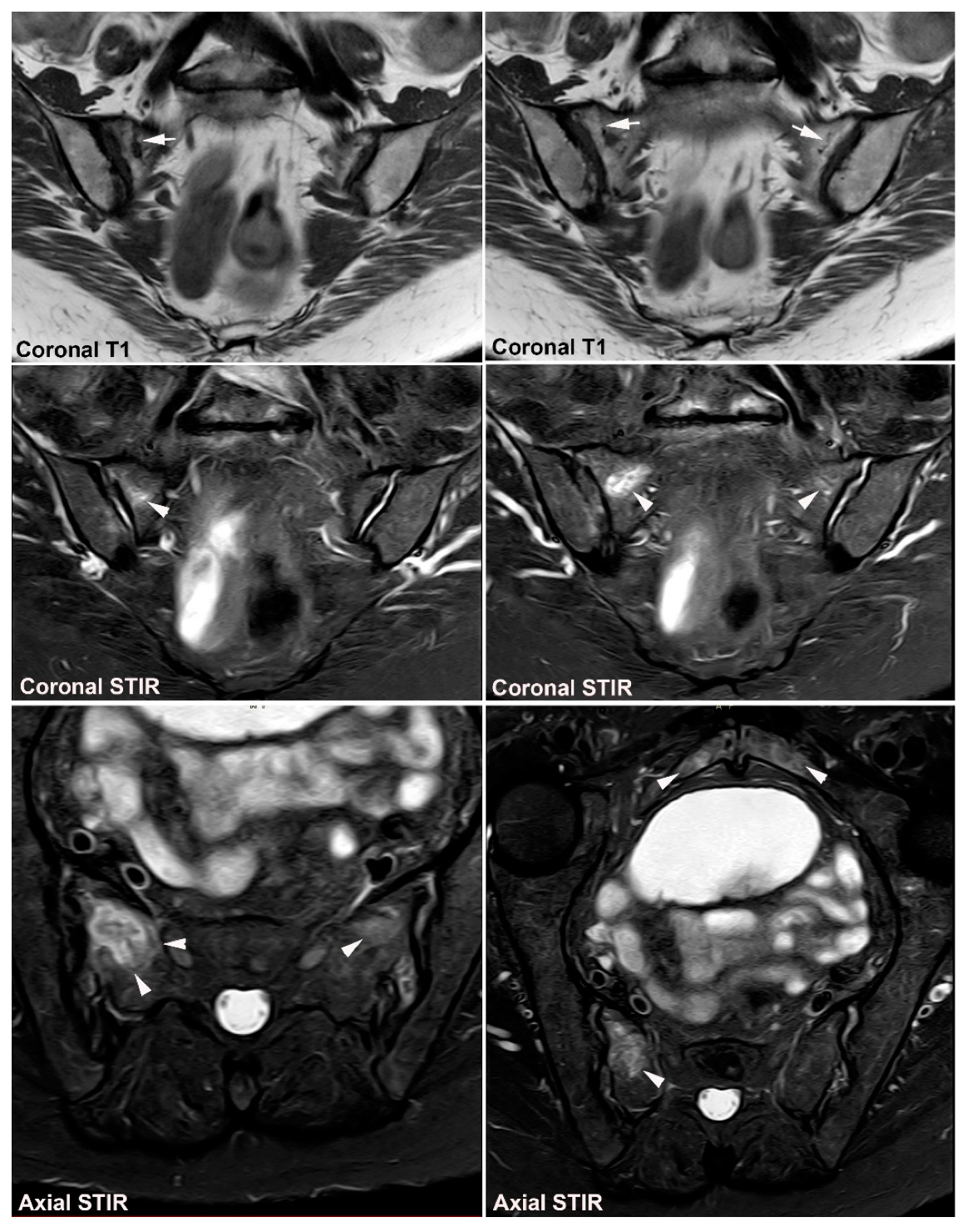
5. Osteitis Condensans ilii and Pregnancy-Related Changes
5.1. Osteitis Condensans ilii (OCI)
5.2. Pregnancy-Related Changes
6. Other Strain-Related SIJ Changes
7. Degenerative SIJ Changes/Osteoarthritis
8. Diffuse Idiopathic Skeletal Hyperostosis (DISH)
9. Infectious Sacroiliitis
10. Fractures
10.1. Sacral Stress Fracture
10.2. Sacral Insufficiency Fracture
11. Inflammatory Conditions
11.1. Other Types of Arthritis, Including Gout and Pseudo-Gout
11.2. Chronic Non-Bacterial Osteitis (CNO)
12. Hyperparathyroidism and Other Disorders of Mineral Metabolism
13. Tumors
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of Diagnostic Criteria for Ankylosing Spondylitis. A Proposal for Modification of the New York Criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Sieper, J.; van der Heijde, D. Review: Nonradiographic Axial Spondyloarthritis: New Definition of an Old Disease? Arthritis Rheum. 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Lambert, R.G.W.; Østergaard, M.; Pedersen, S.J.; Machado, P.M.; Weber, U.; Bennett, A.N.; Braun, J.; Burgos-Vargas, R.; de Hooge, M.; et al. MRI Lesions in the Sacroiliac Joints of Patients with Spondyloarthritis: An Update of Definitions and Validation by the ASAS MRI Working Group. Ann. Rheum. Dis. 2019, 78, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Jurik, A.G.; Hermann, K.G.A.; Landewé, R.; van der Heijde, D.; Baraliakos, X.; Marzo-Ortega, H.; Østergaard, M.; Braun, J.; Sieper, J. Defining Active Sacroiliitis on Magnetic Resonance Imaging (MRI) for Classification of Axial Spondyloarthritis: A Consensual Approach by the ASAS/OMERACT MRI Group. Ann. Rheum. Dis. 2009, 68, 1520–1527. [Google Scholar] [CrossRef]
- Lambert, R.G.; Bakker, P.A.; van der Heijde, D.; Weber, U.; Rudwaleit, M.; Hermann, K.G.; Sieper, J.; Baraliakos, X.; Bennett, A.; Braun, J.; et al. Defining Active Sacroiliitis on MRI for Classification of Axial Spondyloarthritis: Update by the ASAS MRI Working Group. Ann. Rheum. Dis. 2016, 75, 1958–1963. [Google Scholar] [CrossRef]
- Wallis, D.; Haroon, N.; Ayearst, R.; Carty, A.; Inman, R.D. Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis: Part of a Common Spectrum or Distinct Diseases? J. Rheumatol. 2013, 40, 2038–2041. [Google Scholar] [CrossRef]
- Eshed, I.; Miloh-Raz, H.; Dulitzki, M.; Lidar, Z.; Aharoni, D.; Liberman, B.; Lidar, M. Peripartum Changes of the Sacroiliac Joints on MRI: Increasing Mechanical Load Correlating with Signs of Edema and Inflammation Kindling Spondyloarthropathy in the Genetically Prone. Clin. Rheumatol. 2015, 34, 1419–1426. [Google Scholar] [CrossRef]
- Seven, S.; Østergaard, M.; Morsel-Carlsen, L.; Sørensen, I.J.; Bonde, B.; Thamsborg, G.; Lykkegaard, J.J.; Hendricks, O.; Jørgensen, N.R.; Pedersen, S.J. Magnetic Resonance Imaging of Lesions in the Sacroiliac Joints for Differentiation of Patients with Axial Spondyloarthritis from Control Subjects with or without Pelvic or Buttock Pain: A Prospective, Cross-Sectional Study of 204 Participants. Arthritis Rheumatol. 2019, 71, 2034–2046. [Google Scholar] [CrossRef]
- Renson, T.; Renson, T.; Depicker, A.; de Craemer, A.S.; de Craemer, A.S.; Deroo, L.; Deroo, L.; Varkas, G.; Varkas, G.; de Hooge, M.; et al. High Prevalence of Spondyloarthritis-like MRI Lesions in Postpartum Women: A Prospective Analysis in Relation to Maternal, Child and Birth Characteristics. Ann. Rheum. Dis. 2020, 79, 929–934. [Google Scholar] [CrossRef]
- De Winter, J.; De Hooge, M.; Van De Sande, M.; De Jong, H.; Van Hoeven, L.; De Koning, A.; Berg, I.J.; Ramonda, R.; Baeten, D.; Van Der Heijde, D.; et al. Magnetic Resonance Imaging of the Sacroiliac Joints Indicating Sacroiliitis According to the Assessment of SpondyloArthritis International Society Definition in Healthy Individuals, Runners, and Women with Postpartum Back Pain. Arthritis Rheumatol. 2018, 70, 1042–1048. [Google Scholar] [CrossRef]
- Varkas, G.; de Hooge, M.; Renson, T.; de Mits, S.; Carron, P.; Jacques, P.; Moris, M.; Souverijns, G.; Jans, L.; Elewaut, D.; et al. Effect of Mechanical Stress on Magnetic Resonance Imaging of the Sacroiliac Joints: Assessment of Military Recruits by Magnetic Resonance Imaging Study. Rheumatology 2018, 57, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Arnbak, B.; Jensen, T.S.; Egund, N.; Zejden, A.; Horslev-Petersen, K.; Manniche, C.; Jurik, A.G. Prevalence of Degenerative and Spondyloarthritis-Related Magnetic Resonance Imaging Findings in the Spine and Sacroiliac Joints in Patients with Persistent Low Back Pain. Eur. Radiol. 2016, 26, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Kiil, R.M.; Mistegaard, C.E.; Loft, A.G.; Zejden, A.; Hendricks, O.; Jurik, A.G. Differences in Topographical Location of Sacroiliac Joint MRI Lesions in Patients with Early Axial Spondyloarthritis and Mechanical Back Pain. Arthritis Res. Ther. 2022, 24, 75. [Google Scholar] [CrossRef]
- Agten, C.A.; Zubler, V.; Zanetti, M.; Binkert, C.A.; Kolokythas, O.; Prentl, E.; Buck, F.M.; Pfirrmann, C.W.A. Postpartum Bone Marrow Edema at the Sacroiliac Joints May Mimic Sacroiliitis of Axial Spondyloarthritis on MRI. AJR Am. J. Roentgenol. 2018, 211, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Weber, U.; Jurik, A.G.; Zejden, A.; Larsen, E.; Jorgensen, S.H.; Rufibach, K.; Schioldan, C.; Schmidt-Olsen, S. Frequency and Anatomic Distribution of Magnetic Resonance Imaging Features in the Sacroiliac Joints of Young Athletes: Exploring “Background Noise” toward a Data-Driven Definition of Sacroiliitis in Early Spondyloarthritis. Arthritis Rheumatol. 2018, 70, 736–745. [Google Scholar] [CrossRef]
- Renson, T.; de Hooge, M.; de Craemer, A.S.; Deroo, L.; Lukasik, Z.; Carron, P.; Herregods, N.; Jans, L.; Colman, R.; van den Bosch, F.; et al. Progressive Increase in Sacroiliac Joint and Spinal Lesions Detected on Magnetic Resonance Imaging in Healthy Individuals in Relation to Age. Arthritis Rheumatol. 2022, 74, 1506–1514. [Google Scholar] [CrossRef]
- Eshed, I.; Lidar, M. MRI Findings of the Sacroiliac Joints in Patients with Low Back Pain: Alternative Diagnosis to Inflammatory Sacroiliitis. Isr. Med. Assoc. J. 2017, 19, 666–669. [Google Scholar]
- Jans, L.; van Praet, L.; Elewaut, D.; van den Bosch, F.; Carron, P.; Jaremko, J.L.; Behaeghe, M.; Denis, A.; Huysse, W.; Lambrecht, V.; et al. MRI of the SI Joints Commonly Shows Non-Inflammatory Disease in Patients Clinically Suspected of Sacroiliitis. Eur. J. Radiol. 2014, 83, 179–184. [Google Scholar] [CrossRef]
- Kiil, R.M.; Mistegaard, C.E.; Jurik, A.G.; Christiansen, A.A.; Hendricks, O.; Schiøttz-Christensen, B.; Loft, A.G. Diagnosing Axial Spondyloarthritis by Multidiciplinary Team Conference at 3.5 Years’ Follow-up in a Cohort of Patients with Disease Features According to the ASAS Criteria. Scand. J. Rheumatol. 2022, 51, 291–299. [Google Scholar] [CrossRef]
- Laor, T.; Jaramillo, D. MR Imaging Insights into Skeletal Maturation: What Is Normal? Radiology 2009, 250, 28–38. [Google Scholar] [CrossRef]
- Storey, T.; Borgia, R.E.; Merritt, P.; Woolnough, L. Normal Magnetic Resonance Imaging Appearance of Marrow Adjacent to the Sacroiliac Joints in Children During Development. J. Comput. Assist. Tomogr. 2022, 46, 91–96. [Google Scholar] [CrossRef]
- Herregods, N.; Jans, L.B.O.; Chen, M.; Paschke, J.; de Buyser, S.L.; Renson, T.; Dehoorne, J.; Joos, R.; Lambert, R.G.W.; Jaremko, J.L. Normal Subchondral High T2 Signal on MRI Mimicking Sacroiliitis in Children: Frequency, Age Distribution, and Relationship to Skeletal Maturity. Eur. Radiol. 2021, 31, 3498–3507. [Google Scholar] [CrossRef]
- Zejden, A.; Jurik, A.G. Anatomy of the Sacroiliac Joints in Children and Adolescents by Computed Tomography. Pediatr. Rheumatol. Online J. 2017, 15, 82. [Google Scholar] [CrossRef]
- Bollow, M.; Braun, J.; Kannenberg, J.; Biedermann, T.; Schauer-Petrowskaja, C.; Paris, S.; Mutze, S.; Hamm, B. Normal Morphology of Sacroiliac Joints in Children: Magnetic Resonance Studies Related to Age and Sex. Skelet. Radiol. 1997, 26, 697–704. [Google Scholar] [CrossRef]
- Demir, M.; Mavi, A.; Gumusburun, E.; Bayram, M.; Gursoy, S.; Nishio, H. Anatomical Variations with Joint Space Measurements on CT. Kobe J. Med. Sci. 2007, 53, 209–217. [Google Scholar]
- Prassopoulos, P.K.; Faflia, C.P.; Voloudaki, A.E.; Gourtsoyiannis, N.C. Sacroiliac Joints: Anatomical Variants on CT. J. Comput. Assist. Tomogr. 1999, 23, 323–327. [Google Scholar] [CrossRef]
- Herregods, N.; Jans, L.B.O.; Paschke, J.; de Buyser, S.L.; Renson, T.; Dehoorne, J.; Joos, R.; Lambert, R.G.W.; Jaremko, J.L. Magnetic Resonance Imaging Findings in the Normal Pediatric Sacroiliac Joint Space That Can Simulate Disease. Pediatr. Radiol. 2021, 51, 2530–2538. [Google Scholar] [CrossRef]
- Herregods, N.; Lambert, R.G.W.; Schiettecatte, E.; Dehoorne, J.; Renson, T.; Laloo, F.; van den Berghe, T.; Jans, L.B.O.; Jaremko, J.L. Blurring and Irregularity of the Subchondral Cortex in Pediatric Sacroiliac Joints on T1 Images: Incidence of Normal Findings That Can Mimic Erosions. Arthritis Care Res. 2021, 75, 190–197. [Google Scholar] [CrossRef]
- Vleeming, A.; Schuenke, M.D.; Masi, A.T.; Carreiro, J.E.; Danneels, L.; Willard, F.H. The Sacroiliac Joint: An Overview of Its Anatomy, Function and Potential Clinical Implications. J. Anat. 2012, 221, 537–567. [Google Scholar] [CrossRef]
- Egund, N.; Jurik, A.G. Anatomy and Histology of the Sacroiliac Joints. Semin. Musculoskelet. Radiol. 2014, 18, 332–339. [Google Scholar] [CrossRef]
- Faflia, C.P.; Prassopoulos, P.K.; Daskalogiannaki, M.E.; Gourtsoyiannis, N.C. Variation in the Appearance of the Normal Sacroiliac Joint on Pelvic CT. Clin. Radiol. 1998, 53, 742–746. [Google Scholar] [CrossRef]
- Puhakka, K.B.; Melsen, F.; Jurik, A.G.; Boel, L.W.; Vesterby, A.; Egund, N. MR Imaging of the Normal Sacroiliac Joint with Correlation to Histology. Skelet. Radiol. 2004, 33, 15–28. [Google Scholar] [CrossRef]
- Weber, U.; Jurik, A.G.; Zejden, A.; Larsen, E.; Jørgensen, S.H.; Rufibach, K.; Schioldan, C.; Schmidt-Olsen, S. MRI of the Sacroiliac Joints in Athletes: Recognition of Non-Specific Bone Marrow Oedema by Semi-Axial Added to Standard Semi-Coronal Scans. Rheumatology 2020, 59, 1381–1390. [Google Scholar] [CrossRef]
- Diekhoff, T.; Lambert, R.; Hermann, K.G. MRI in Axial Spondyloarthritis: Understanding an ‘ASAS-Positive MRI’ and the ASAS Classification Criteria. Skelet. Radiol. 2022, 51, 1721–1730. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Diekhoff, T.; Baraliakos, X.; Hermann, K.G.A.; Sieper, J. Diagnostic Evaluation of the Sacroiliac Joints for Axial Spondyloarthritis: Should MRI Replace Radiography? Ann. Rheum. Dis. 2022, 81, 1486–1490. [Google Scholar] [CrossRef]
- Lambert, R.; Baraliakos, X.; Bernard, S.; Carrino, J.; Diekhoff, T.; Eshed, I.; Hermann, K.G.; Herregods, N.; Jaremko, J.L.; Jans, L.; et al. Development of International Consensus on a Standardized Image Acquisition Protocol for Diagnostic Evaluation of the Sacroiliac Joints by MRI—An ASAS-SPARTAN Collaboration. Ann. Rheum. Dis. 2022, 81, 802–803. [Google Scholar] [CrossRef]
- Wittram, C.; Whitehouse, G.H. Normal Variation in the Magnetic Resonance Imaging Appearances of the Sacroiliac Joints: Pitfalls in the Diagnosis of Sacroiliitis. Clin. Radiol. 1995, 50, 371–376. [Google Scholar] [CrossRef]
- Ziegeler, K.; Eshkal, H.; Schorr, C.; Sieper, J.; Diekhoff, T.; Makowski, M.R.; Hamm, B.; Hermann, K.G.A. Age- and Sex-Dependent Frequency of Fat Metaplasia and Other Structural Changes of the Sacroiliac Joints in Patients without Axial Spondyloarthritis: A Retrospective, Cross-Sectional MRI Study. J. Rheumatol. 2018, 45, 915–921. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Lambert, R.G.; Baraliakos, X.; Weber, U.; MacHado, P.M.; Pedersen, S.J.; de Hooge, M.; Sieper, J.; Wichuk, S.; Poddubnyy, D.; et al. Data-Driven Definitions for Active and Structural MRI Lesions in the Sacroiliac Joint in Spondyloarthritis and Their Predictive Utility. Rheumatology 2021, 60, 4778–4789. [Google Scholar] [CrossRef]
- Kiil, R.M.; Jurik, A.G.; Zejden, A. Anatomical Variation at the Sacroiliac Joints in Young Adults: Estimated Prevalence by CT and Concomitant Diagnostics by MRI. Skelet. Radiol. 2022, 51, 595–605. [Google Scholar] [CrossRef]
- Tok Umay, S.; Korkmaz, M. Frequency of Anatomical Variation of the Sacroiliac Joint in Asymptomatic Young Adults and Its Relationship with Sacroiliac Joint Degeneration. Clin. Anat. 2020, 33, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, K.; Kreutzinger, V.; Proft, F.; Poddubnyy, D.; Hermann, K.G.A.; Diekhoff, T. Joint Anatomy in Axial Spondyloarthritis: Strong Associations between Sacroiliac Joint Form Variation and Symptomatic Disease. Rheumatology 2021, 61, 388–393. [Google Scholar] [CrossRef]
- Teran-Garza, R.; Verdines-Perez, A.M.; Tamez-Garza, C.; Pinales-Razo, R.; Vilchez-Cavazos, J.F.; Gutierrez-de la O, J.; Quiroga-Garza, A.; Elizondo-Omaña, R.E.; Guzman-Lopez, S. Anatomical Variations of the Sacro-Iliac Joint: A Computed Tomography Study. Surg. Radiol. Anat. 2021, 43, 819–825. [Google Scholar] [CrossRef]
- Ehara, S.; El-Khoury, G.Y.; Bergman, R.A. The Accessory Sacroiliac Joint: A Common Anatomic Variant. Am. J. Roentgenol. 1988, 150, 857–859. [Google Scholar] [CrossRef]
- El Rafei, M.; Badr, S.; Lefebvre, G.; Machuron, F.; Capon, B.; Flipo, R.M.; Cotten, A. Sacroiliac Joints: Anatomical Variations on MR Images. Eur. Radiol. 2018, 28, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.D.; Ballard, K.E. The Frequency of Accessory Sacroiliac Joints. Clin. Anat. 2009, 22, 876–877. [Google Scholar] [CrossRef]
- Hadley, L.A. Accessory Sacroiliac Articulations with Arthritic Changes. Radiology 1950, 55, 403–409. [Google Scholar] [CrossRef]
- Slobodin, G.; Lidar, M.; Eshed, I. Clinical and Imaging Mimickers of Axial Spondyloarthritis. Semin. Arthritis Rheum. 2017, 47, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, E.; Aubry, S.; Runge, M. Unilateral Accessory Sacroiliac Joint with Bone Marrow Edema Mimicking Sacroiliitis. J. Rheumatol. 2018, 45, 1327–1328. [Google Scholar] [CrossRef]
- Ziegeler, K.; Kreutzinger, V.; Diekhoff, T.; Roehle, R.; Poddubnyy, D.; Pumberger, M.; Hamm, B.; Hermann, K.G.A. Impact of Age, Sex, and Joint Form on Degenerative Lesions of the Sacroiliac Joints on CT in the Normal Population. Sci. Rep. 2021, 11, 5903. [Google Scholar] [CrossRef]
- Castellvi, A.E.; Goldstein, L.A.; Chan, D.P.K. Lumbosacral Transitional Vertebrae and Their Relationship with Lumbar Extradural Defects. Spine 1984, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, F.; ter Horst, S.; Bloem, J.L.; van den Berg, R.; de Hooge, M.; van Gaalen, F.; Dagfinrud, H.; van Oosterhout, M.; Landewé, R.; van der Heijde, D.; et al. Prevalence and Clinical Significance of Lumbosacral Transitional Vertebra (LSTV) in a Young Back Pain Population with Suspected Axial Spondyloarthritis: Results of the SPondyloArthritis Caught Early (SPACE) Cohort. Skelet. Radiol. 2017, 46, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R. Osteitis Condensans Ilii. Rheumatol. Int. 2010, 30, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Numaguchi, Y. Osteitis Condensans Ilii, Including Its Resolution. Radiology 1971, 98, 1–8. [Google Scholar] [CrossRef]
- Borlandelli, E.; Ciaffi, J.; Festuccia, G.; Facchini, G.; Miceli, M.; Brusi, V.; Mancarella, L.; Lisi, L.; di Martino, A.; Faldini, C.; et al. Osteitis Condensans Ilii: Prevalence and Characteristics of a Neglected Mimic of Sacroiliitis. Clin. Rheumatol. 2022, 41, 483–490. [Google Scholar] [CrossRef]
- Klang, E.; Lidar, M.; Lidar, Z.; Aharoni, D.; Eshed, I. Prevalence and Awareness of Sacroiliac Joint Alterations on Lumbar Spine CT in Low Back Pain Patients Younger than 40 Years. Acta Radiol. 2017, 58, 449–455. [Google Scholar] [CrossRef]
- Ma, L.; Gao, Z.; Zhong, Y.; Meng, Q. Osteitis Condensans Ilii May Demonstrate Bone Marrow Edema on Sacroiliac Joint Magnetic Resonance Imaging. Int. J. Rheum. Dis. 2018, 21, 299–307. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Weineck, H.; Diekhoff, T.; Redeker, I.; Gobejishvili, N.; Llop, M.; Rodriguez, V.R.; Proft, F.; Protopopov, M.; Haibel, H.; et al. Clinical and Imaging Characteristics of Osteitis Condensans Ilii as Compared with Axial Spondyloarthritis. Rheumatology 2020, 59, 3798–3806. [Google Scholar] [CrossRef]
- Kiil, R.M.; Weber, U.; Loft, A.G.; Maimburg, R.D.; Jurik, A.G. Evolution of MRI Lesions at the Sacroiliac Joints during and after Pregnancy by Serial MRI from Gestational Week 20 to 12 Months Postpartum. Arthritis Rheumatol. 2023. accepted. [Google Scholar] [CrossRef]
- Diekhoff, T.; Eshed, I.; Giraudo, C.; Hermann, K.G.; de Hooge, M.; Jans, L.; Jurik, A.G.; Lambert, R.G.; Machado, P.M.; Maksymowych, W.P.; et al. ASAS Recommendations for Requesting and Reporting Imaging Examinations in Patients with Suspected Axial Spondyloarthritis. Ann. Rheum. Dis. 2022, 81, 97. [Google Scholar] [CrossRef]
- Jans, L.; van Langenhove, C.; van Praet, L.; Carron, P.; Elewaut, D.; van den Bosch, F.; Lambrecht, V.; Jaremko, J.L.; Verstraete, K. Diagnostic Value of Pelvic Enthesitis on MRI of the Sacroiliac Joints in Spondyloarthritis. Eur. Radiol. 2014, 24, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Eno, J.J.T.; Bellino, M.J.; Bishop, J.A.; Boone, C.R. The Prevalence of Sacroiliac Joint Degeneration in Asymptomatic Adults. J. Bone Jt. Surg. Am. 2015, 97, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Muellner, M.; Kreutzinger, V.; Becker, L.; Diekhoff, T.; Pumberger, M.; Schömig, F.; Heyland, M.; Ziegeler, K. Unexpected Sex Differences in the Relationship of Sacroiliac Joint and Lumbar Spine Degeneration. Diagnostics 2022, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Weinfeld, R.M.; Olson, P.N.; Maki, D.D.; Griffiths, H.J. The Prevalence of Diffuse Idiopathic Skeletal Hyperostosis (DISH) in Two Large American Midwest Metropolitan Hospital Populations. Skelet. Radiol. 1997, 26, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Pappone, N.; Baraliakos, X.; Eshed, I.; Sarzi-Puttini, P.; Atzeni, F.; Bieber, A.; Novofastovski, I.; Kiefer, D.; Verlaan, J.J.; et al. Diffuse Idiopathic Skeletal Hyperostosis (DISH) and a Possible Inflammatory Component. Curr. Rheumatol. Rep. 2021, 23, 6. [Google Scholar] [CrossRef]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A.; Mader, R.; Khan, M.A. Diffuse Idiopathic Skeletal Hyperostosis: Differentiation from Ankylosing Spondylitis. Curr. Rheumatol. Rep. 2009, 11, 321–328. [Google Scholar] [CrossRef]
- Baraliakos, X.; Listing, J.; Buschmann, J.; von der Recke, A.; Braun, J. A Comparison of New Bone Formation in Patients with Ankylosing Spondylitis and Patients with Diffuse Idiopathic Skeletal Hyperostosis: A Retrospective Cohort Study over Six Years. Arthritis Rheum. 2012, 64, 1127–1133. [Google Scholar] [CrossRef]
- Leibushor, N.; Slonimsky, E.; Aharoni, D.; Lidar, M.; Eshed, I. CT Abnormalities in the Sacroiliac Joints of Patients with Diffuse Idiopathic Skeletal Hyperostosis. Am. J. Roentgenol. 2017, 208, 834–837. [Google Scholar] [CrossRef]
- Latourte, A.; Charlon, S.; Etcheto, A.; Feydy, A.; Allanore, Y.; Dougados, M.; Molto, A. Imaging Findings Suggestive of Axial Spondyloarthritis in Diffuse Idiopathic Skeletal Hyperostosis. Arthritis Care Res. 2018, 70, 145–152. [Google Scholar] [CrossRef]
- Hermet, M.; Minichiello, E.; Flipo, R.M.; Dubost, J.J.; Allanore, Y.; Ziza, J.M.; Gaudin, P.; Thomas, T.; Dernis, E.; Glace, B.; et al. Infectious Sacroiliitis: A Retrospective, Multicentre Study of 39 Adults. BMC Infect. Dis. 2012, 12, 305. [Google Scholar] [CrossRef]
- Kang, Y.; Hong, S.H.; Kim, J.Y.; Yoo, H.J.; Choi, J.Y.; Yi, M.; Kang, H.S. Unilateral Sacroiliitis: Differential Diagnosis between Infectious Sacroiliitis and Spondyloarthritis Based on MRI Findings. AJR Am. J. Roentgenol. 2015, 205, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Stürzenbecher, A.; Braun, J.; Paris, S.; Biedermann, T.; Hamm, B.; Bollow, M. MR Imaging of Septic Sacroiliitis. Skelet. Radiol. 2000, 29, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Dubash, S.; Pease, C.; Aslam, A.; Coady, D.; McGonagle, D.; Marzo-Ortega, H. Acute Unilateral Sacroiliitis Mimicking Infection on Magnetic Resonance Imaging with Response to Nonsteroidal Antiinflammatory Drugs: A Distinct Presentation of Spondyloarthritis? J. Rheumatol. 2018, 45, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.J.; Curtis, C. Stress Fractures in the Spine and Sacrum. Clin. Sports Med. 2006, 25, 75–88. [Google Scholar] [CrossRef]
- Yan, C.X.B.; Vautour, L.; Martin, M.H. Postpartum Sacral Insufficiency Fractures. Skelet. Radiol. 2016, 45, 413–417. [Google Scholar] [CrossRef]
- Wurdinger, S.; Humbsch, K.; Rgen, J.; Reichenbach, R.; Peiker, G.; Seewald, H.-J.; Kaiser, W.A. MRI of the Pelvic Ring Joints Postpartum: Normal and Pathological Findings. J. Magn. Reson. Imaging 2002, 15, 324–329. [Google Scholar] [CrossRef]
- Kinoshita, H.; Miyakoshi, N.; Kobayashi, T.; Abe, T.; Kikuchi, K.; Shimada, Y. Comparison of Patients with Diagnosed and Suspected Sacral Insufficiency Fractures. J. Orthop. Sci. 2019, 24, 702–707. [Google Scholar] [CrossRef]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P.V. Sacral Insufficiency Fractures: Current Concepts of Management. Osteoporos. Int. 2006, 17, 1716–1725. [Google Scholar] [CrossRef]
- Cabarrus, M.C.; Ambekar, A.; Lu, Y.; Link, T.M. MRI and CT of Insufficiency Fractures of the Pelvis and the Proximal Femur. AJR Am. J. Roentgenol. 2008, 191, 995–1001. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, L.; Dong, T.; Mai, H.; Lu, B.; Huang, L.; Li, J. Clinical and MRI Features of Sacral Insufficiency Fractures after Radiotherapy in Patients with Cervical Cancer. BMC Womens Health 2022, 22, 166. [Google Scholar] [CrossRef]
- Konatalapalli, R.M.; DeMarco, P.J.; Jelinek, J.S.; Murphey, M.; Gibson, M.; Jennings, B.; Weinstein, A. Gout in the Axial Skeleton. J. Rheumatol. 2009, 36, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.N.; Omoumi, P.; Wieers, G.; Maldague, B.; Malghem, J.; Lecouvet, F.E.; vande Berg, B.C. Spinal and Sacroiliac Gouty Arthritis: Report of a Case and Review of the Literature. Acta Radiol. Short Rep. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Li, Y.; Zhao, Z.; Feng, L.; Zhu, J.; Zhang, J.; Huang, F. Gout Mimicking Spondyloarthritis: Case Report and Literature Review. J. Pain Res. 2017, 10, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Namas, R.; Hegazin, S.B.; Memisoglu, E.; Joshi, A. Lower Back Pain as a Manifestation of Acute Gouty Sacroiliitis: Utilization of Dual-Energy Computed Tomography (DECT) in Establishing a Diagnosis. Eur. J. Rheumatol. 2019, 6, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Oostveen, J.C.M.; van de Laar, M.A.F.J. Magnetic Resonance Imaging in Rheumatic Disorders of the Spine and Sacroiliac Joints. Semin. Arthritis Rheum. 2000, 30, 52–69. [Google Scholar] [CrossRef] [PubMed]
- El Maghraoui, A.; Lecoules, S.; Lechevalier, D.; Magnin, J.; Eulry, F. Acute Sacroiliitis as a Manifestation of Calcium Pyrophosphate Dihydrate Crystal Deposition Disease. Clin. Exp. Rheumatol. 1999, 17, 477–478. [Google Scholar]
- Martens, H.A.; van Bokhoven, S.C.; Stenger, A.A.M.E. Calcium Pyrophosphate Deposition Disease Induced Sacroiliitis. Rheumatology 2018, 57, 1422. [Google Scholar] [CrossRef]
- Moreno Martinez, M.J.; Moreno Ramos, M.J.; Linares Ferrando, L.F. Sacroiliitis Due to Calcium Pyrophosphate Deposition Disease. Reumatol. Clin. 2018, 14, 175–176. [Google Scholar] [CrossRef]
- Dudler, J.; Stucki, R.F.; Gerster, J.C. Aseptic Psoas Pyomyositis and Erosive Discitis in a Case of Calcium Pyrophosphate Crystal Deposition Disease. Rheumatology 2000, 39, 1290–1292. [Google Scholar] [CrossRef]
- Bartlett, C.S.; Casden, A.M.; Abdelwahab, I.F. Calcium Pyrophosphate Deposition Disease Mimicking Infection in the Lumbar Spine. Orthopedics 1999, 22, 79–81. [Google Scholar] [CrossRef]
- Jurik, A.G.; Klicman, R.F.; Simoni, P.; Robinson, P.; Teh, J. SAPHO and CRMO: The Value of Imaging. Semin. Musculoskelet. Radiol. 2018, 22, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Depasquale, R.; Kumar, N.; Lalam, R.K.; Tins, B.J.; Tyrrell, P.N.M.; Singh, J.; Cassar-Pullicino, V.N. SAPHO: What Radiologists Should Know. Clin. Radiol. 2012, 67, 195–206. [Google Scholar] [CrossRef]
- Andreasen, C.M.; Jurik, A.G.; Glerup, M.B.; Høst, C.; Mahler, B.T.; Hauge, E.M.; Herlin, T. Response to Early-Onset Pamidronate Treatment in Chronic Nonbacterial Osteomyelitis: A Retrospective Single-Center Study. J. Rheumatol. 2019, 46, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, C.; Xu, W.; Wu, X.; Sun, X.; Zhang, W.; Jing, H.; Gu, Z.; Yuan, S.; Li, L.; et al. Spinal and Sacroiliac Involvement in SAPHO Syndrome: A Single Center Study of a Cohort of 354 Patients. Semin. Arthritis Rheum. 2019, 48, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.M.; Klicman, R.F.; Herlin, T.; Hauge, E.M.; Jurik, A.G. Standardized Reporting and Quantification of Whole-Body MRI Findings in Children with Chronic Non-Bacterial Osteomyelitis Treated with Pamidronate. Pediatr. Rheumatol. Online J. 2022, 20, 85. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Skeletal Abnormalities in Hypoparathyroidism and in Primary Hyperparathyroidism. Rev. Endocr. Metab. Disord. 2021, 22, 789–802. [Google Scholar] [CrossRef]
- Murphey, M.D.; Sartoris, D.J.; Quale, J.L.; Pathria, M.N.; Martin, N.L. Musculoskeletal Manifestations of Chronic Renal Insufficiency. Radiographics 1993, 13, 357–379. [Google Scholar] [CrossRef]
- Badr, S.; Jacques, T.; Lefebvre, G.; Boulil, Y.; Diwan, R.A.; Cotten, A. Main Diagnostic Pitfalls in Reading the Sacroiliac Joints on MRI. Diagnostics 2021, 11, 2001. [Google Scholar] [CrossRef]
- Kreutzinger, V.; Diekhoff, T.; Liefeldt, L.; Poddubnyy, D.; Hermann, K.G.A.; Ziegeler, K. Asymptomatic Secondary Hyperparathyroidism Can Mimic Sacroiliitis on Computed Tomography. Sci. Rep. 2021, 11, 4323. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Rosenthal, D.I.; Mitchell, D.M.; Handa, A.; Kattapuram, S.V.; Huang, A.J. Imaging Findings of Metabolic Bone Disease. Radiographics 2016, 36, 1871–1887. [Google Scholar] [CrossRef]
- Goswami, R.; Ray, D.; Sharma, R.; Tomar, N.; Gupta, R.; Gupta, N.; Sreenivas, V. Presence of Spondyloarthropathy and Its Clinical Profile in Patients with Hypoparathyroidism. Clin. Endocrinol. 2008, 68, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Diel, J.; Ortiz, O.; Losada, R.A.; Price, D.B.; Hayt, M.W.; Katz, D.S. The Sacrum: Pathologic Spectrum, Multimodality Imaging, and Subspecialty Approach. Radiographics 2001, 21, 83–104. [Google Scholar] [CrossRef] [PubMed]
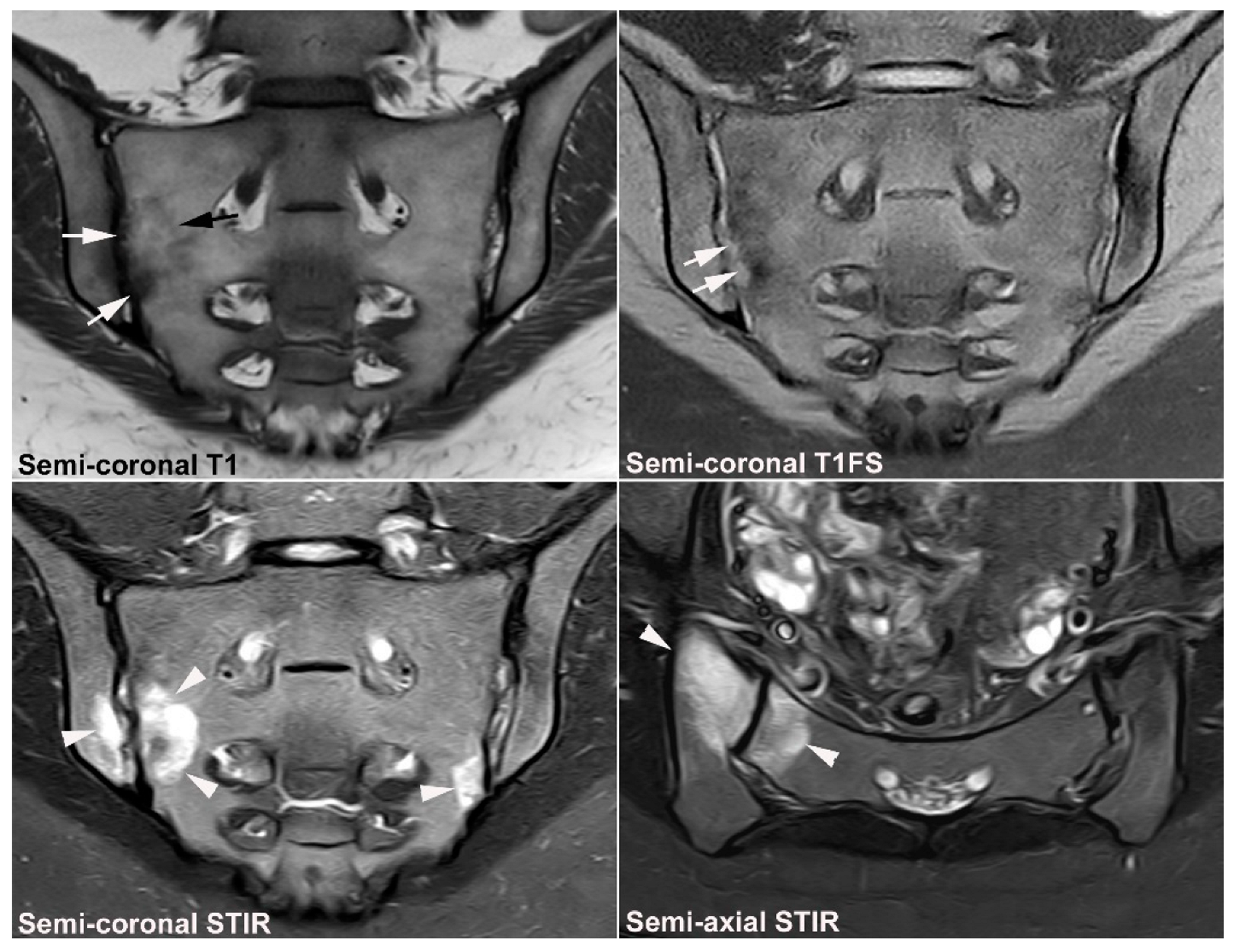
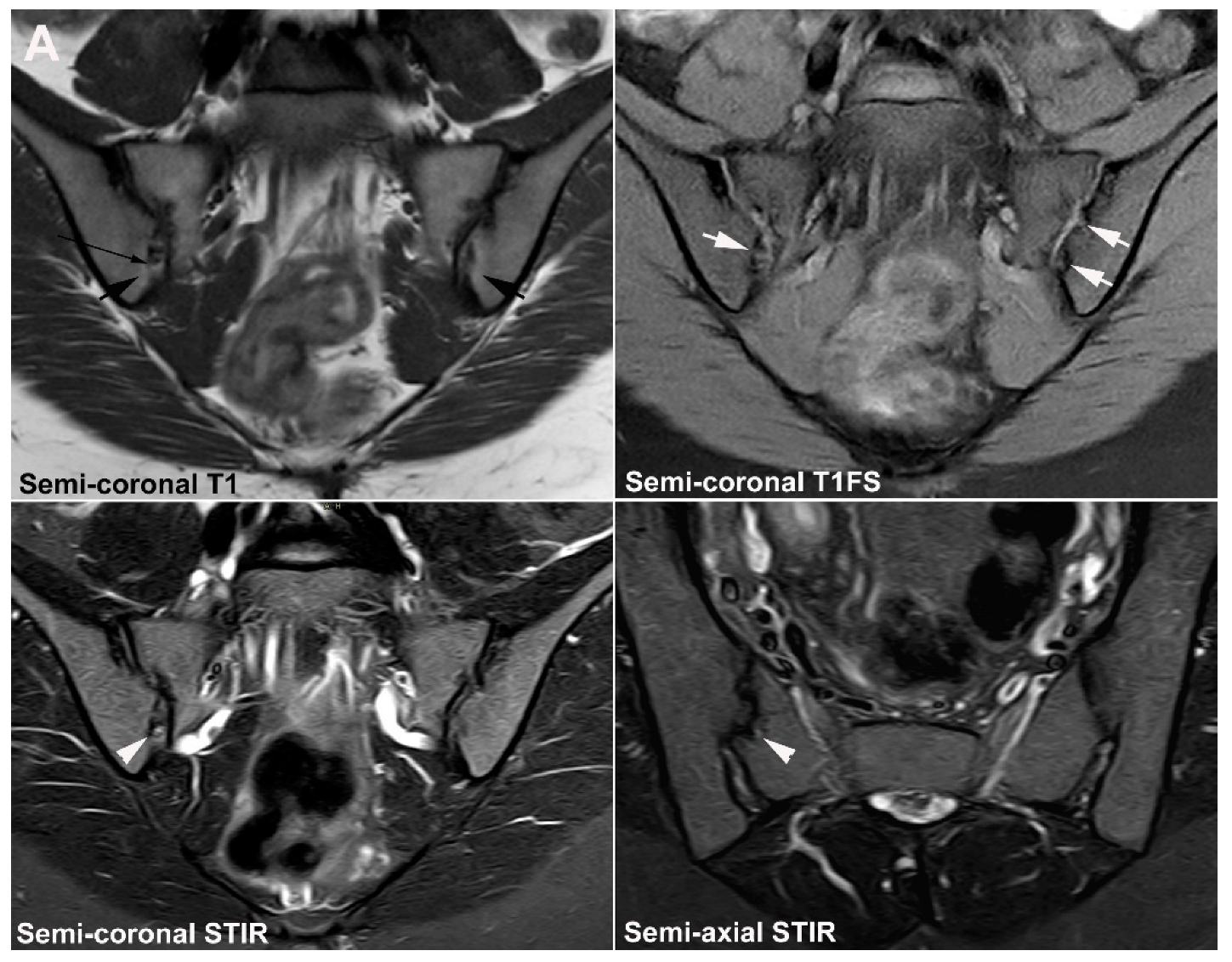
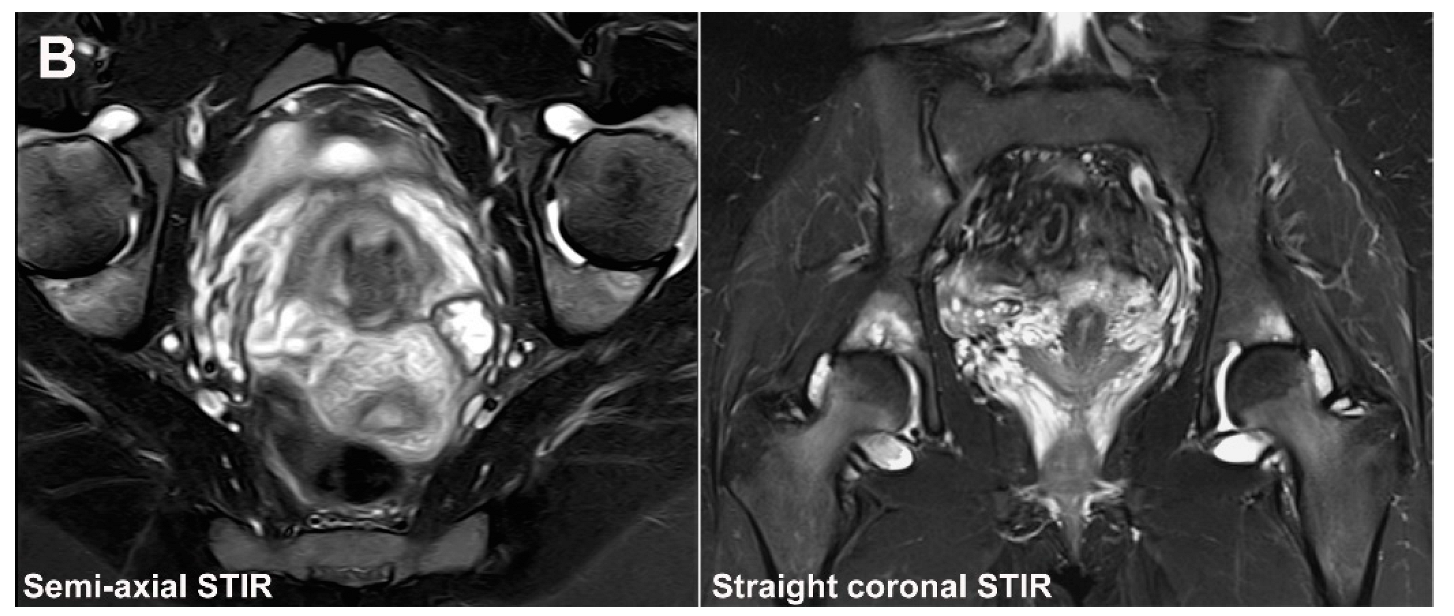

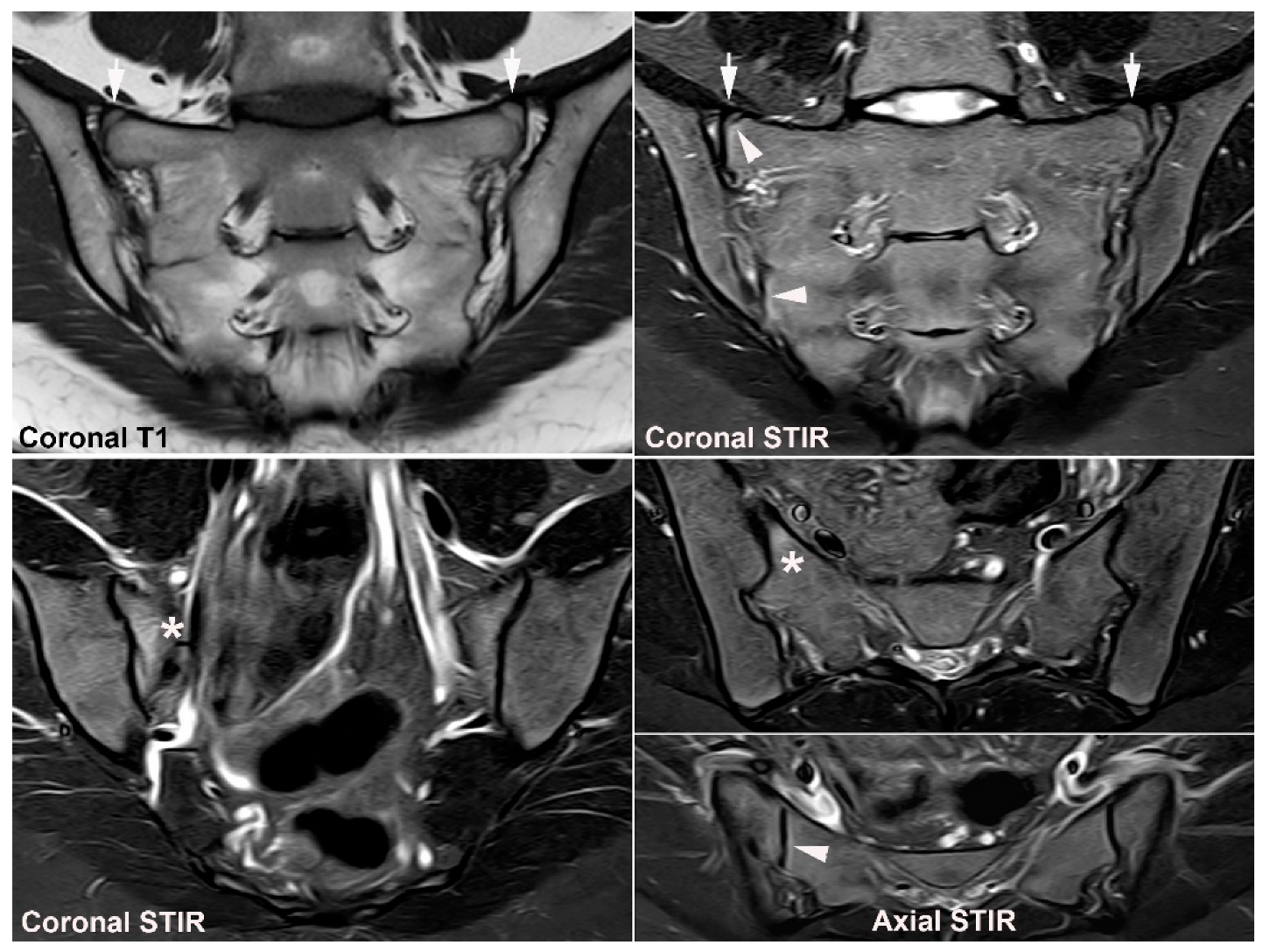












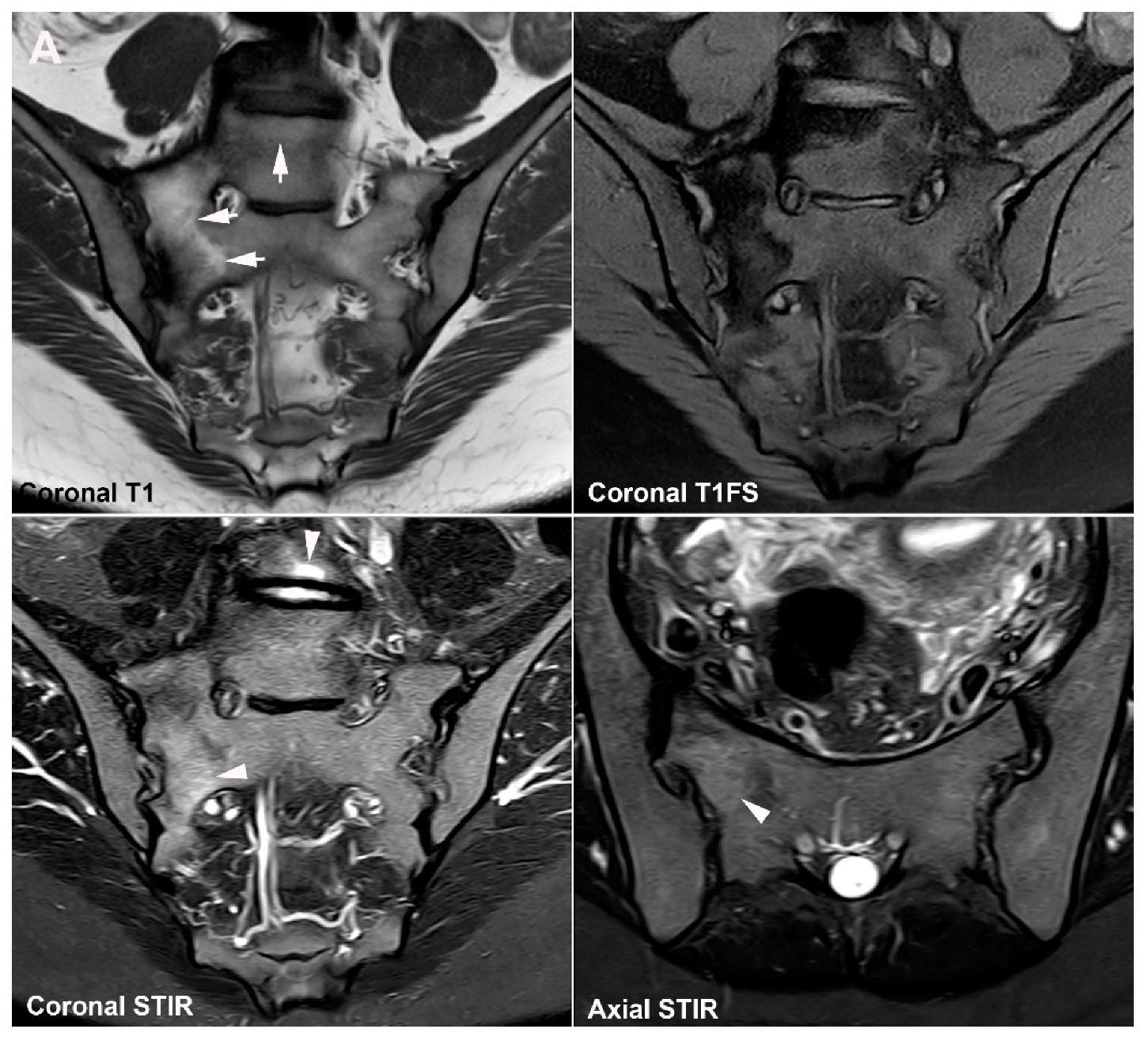

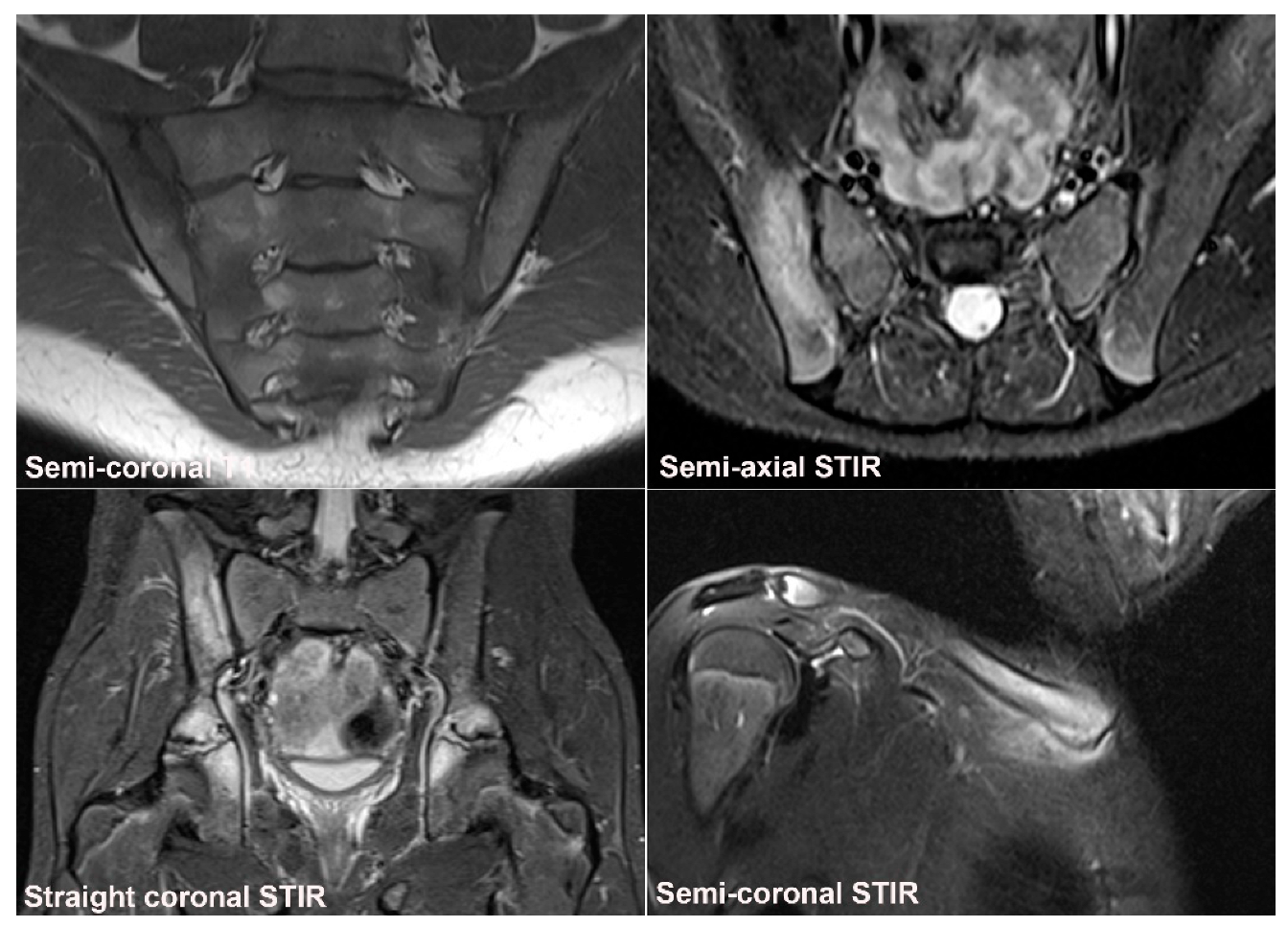
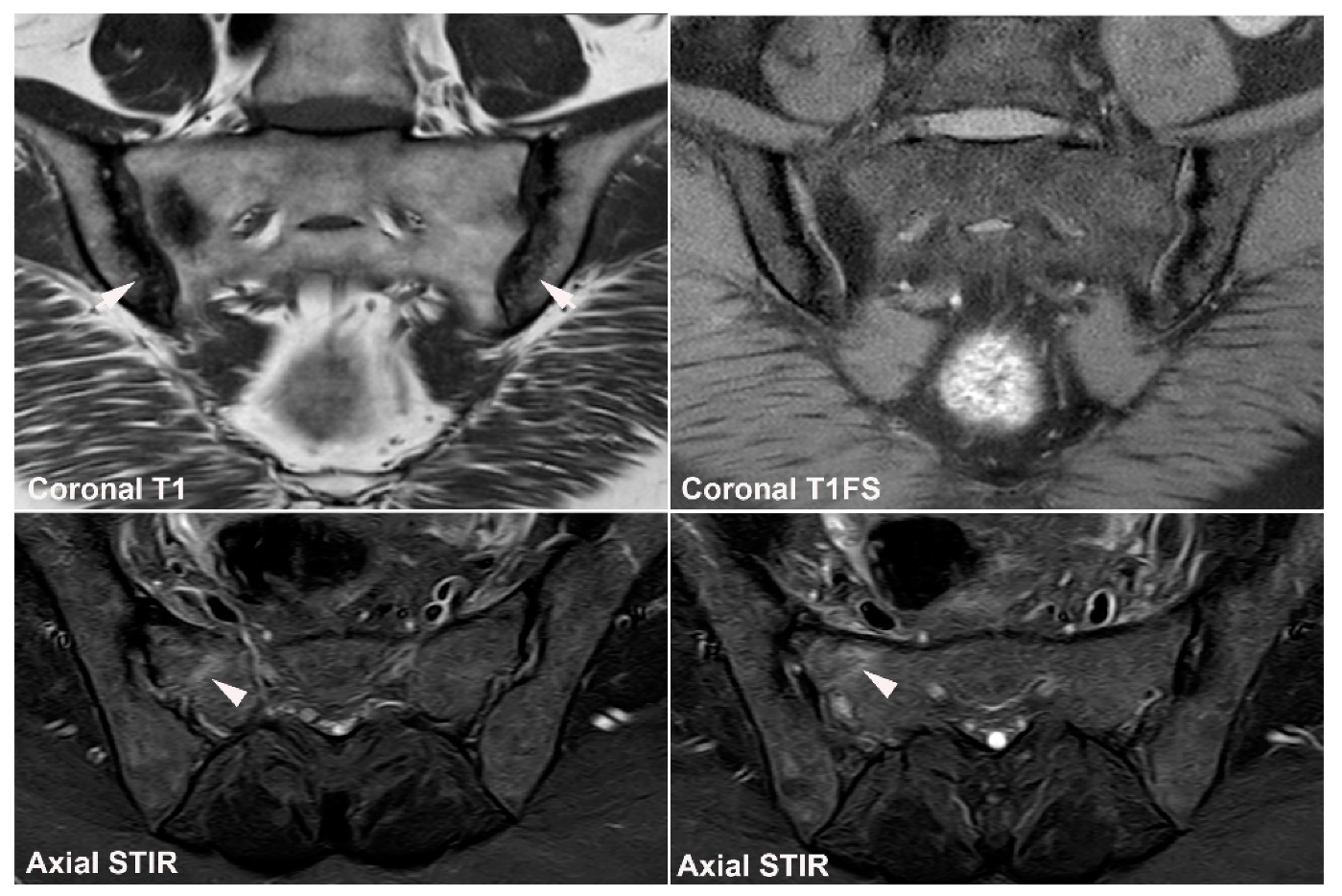
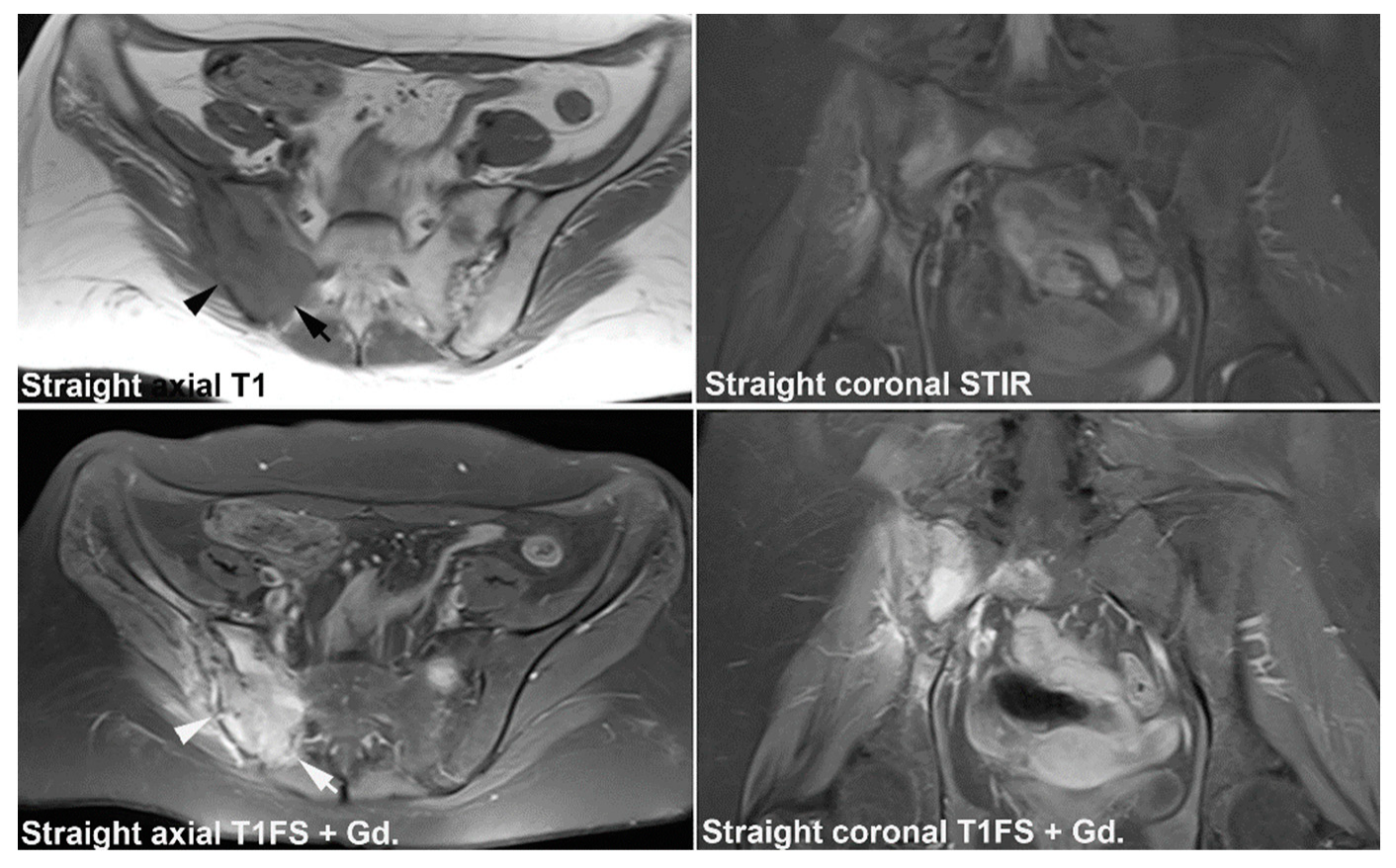
| Rheumatologist Referral, n = 691 * | Referral from Different Specialties, n = 281 ** | Combined Data, n = 972 | ||||
|---|---|---|---|---|---|---|
| Age, mean, years | 36 | 44 | ||||
| Males/females | 261/430 | 116/165 | 377/595 | |||
| n | % | n | % | n | % | |
| SpA | 249 | 36 | 71 | 25 | 320 | 33 |
| Normal SIJ findings | 285 | 41 | 123 | 44 | 408 | 42 |
| Alternative SIJ-related diagnoses | 130 | 19 | 87 | 31 | 217 | 22 |
| Anatomic variants | 41 # | 5.9 # | 15 ## | 5.3 ## | 56 | 5.7 |
| OCI | 17 | 2.5 | 25 | 8.9 | 42 | 4.3 |
| Degenerative SIJ findings | 25 | 3.6 | 12 | 4.3 | 37 | 3.8 |
| SIJ DISH findings | 24 | 3.5 | 4 | 1.5 | 28 | 2.9 |
| Septic sacroiliitis/discitis | 4 | 0.6 | 15 | 5.3 | 19 | 2.0 |
| Stress reaction/fracture | 8 | 1.2 | 2 | 0,7 | 10 | 1.0 |
| Tumor | 11 | 1.6 | 1 | 0.3 | 12 | 1.2 |
| Spine disorders | ||||||
| Degenerative spinal disease | 305 ¤ | 44.1 ¤ | 11 ¤¤ | 4 ¤¤ | 316 | 33 |
| Other spinal disorders | 10 | 1.5 | 2 | 0.7 | 12 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurik, A.G. Diagnostics of Sacroiliac Joint Differentials to Axial Spondyloarthritis Changes by Magnetic Resonance Imaging. J. Clin. Med. 2023, 12, 1039. https://doi.org/10.3390/jcm12031039
Jurik AG. Diagnostics of Sacroiliac Joint Differentials to Axial Spondyloarthritis Changes by Magnetic Resonance Imaging. Journal of Clinical Medicine. 2023; 12(3):1039. https://doi.org/10.3390/jcm12031039
Chicago/Turabian StyleJurik, Anne Grethe. 2023. "Diagnostics of Sacroiliac Joint Differentials to Axial Spondyloarthritis Changes by Magnetic Resonance Imaging" Journal of Clinical Medicine 12, no. 3: 1039. https://doi.org/10.3390/jcm12031039
APA StyleJurik, A. G. (2023). Diagnostics of Sacroiliac Joint Differentials to Axial Spondyloarthritis Changes by Magnetic Resonance Imaging. Journal of Clinical Medicine, 12(3), 1039. https://doi.org/10.3390/jcm12031039







