Urinary Dickkopf-3 (DKK3) Is Associated with Greater eGFR Loss in Patients with Resistant Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Pressure Measurement
2.3. Assessment of uDKK3 and Renal Function
2.4. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Development of Blood Pressure and Renal Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muxfeldt, E.S.; Bloch, K.V.; Nogueira, A.R.; Salles, G.F. Twenty-four hour ambulatory blood pressure monitoring pattern of resistant hypertension. Blood Press. Monit. 2003, 8, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.L.; Powers, J.D.; Magid, D.J.; Tavel, H.M.; Masoudi, F.A.; Margolis, K.L.; O’Connor, P.J.; Selby, J.V.; Ho, P.M. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012, 125, 1635–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nicola, L.; Gabbai, F.B.; Agarwal, R.; Chiodini, P.; Borrelli, S.; Bellizzi, V.; Nappi, F.; Conte, G.; Minutolo, R. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J. Am. Coll. Cardiol. 2013, 61, 2461–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Elley, C.R.; et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA 2016, 315, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.C.; Neilson, E.G. Toward a unified theory of renal progression. Annu. Rev. Med. 2006, 57, 365–380. [Google Scholar] [CrossRef]
- Farris, A.B.; Colvin, R.B. Renal interstitial fibrosis: Mechanisms and evaluation. Curr. Opin. Nephrol. Hypertens. 2012, 21, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- He, T.; Zhang, Z.; Staessen, J.A.; Mischak, H.; Latosinska, A.; Beige, J. Proteomic Biomarkers in the Cardiorenal Syndrome: Toward Deciphering Molecular Pathophysiology. Am. J. Hypertens. 2021, 34, 669–679. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Federico, G.; Meister, M.; Mathow, D.; Heine, G.H.; Moldenhauer, G.; Popovic, Z.V.; Nordstrom, V.; Kopp-Schneider, A.; Hielscher, T.; Nelson, P.J.; et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 2016, 1, e84916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.W.; Yiu, W.H.; Wu, H.J.; Li, R.X.; Liu, Y.; Chan, K.W.; Leung, J.C.; Chan, L.Y.; Lai, K.N.; Tang, S.C. Downregulation of renal tubular Wnt/beta-catenin signaling by Dickkopf-3 induces tubular cell death in proteinuric nephropathy. Cell Death Dis. 2016, 7, e2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamariti, E.; Zhai, C.; Yu, B.; Qiao, L.; Wang, Z.; Potter, C.M.F.; Wong, M.M.; Simpson, R.M.L.; Zhang, Z.; Wang, X.; et al. DKK3 (Dickkopf 3) Alters Atherosclerotic Plaque Phenotype Involving Vascular Progenitor and Fibroblast Differentiation into Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Lipphardt, M.; Dihazi, H.; Jeon, N.L.; Dadafarin, S.; Ratliff, B.B.; Rowe, D.W.; Muller, G.A.; Goligorsky, M.S. Dickkopf-3 in aberrant endothelial secretome triggers renal fibroblast activation and endothelial-mesenchymal transition. Nephrol. Dial. Transplant. 2019, 34, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Hu, J.; Chen, Y.; Shen, W.; Ke, B. Dickkopf-3: Current Knowledge in Kidney Diseases. Front. Physiol. 2020, 11, 533344. [Google Scholar] [CrossRef]
- Zewinger, S.; Rauen, T.; Rudnicki, M.; Federico, G.; Wagner, M.; Triem, S.; Schunk, S.J.; Petrakis, I.; Schmit, D.; Wagenpfeil, S.; et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J. Am. Soc. Nephrol. 2018, 29, 2722–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallbach, M.; Lehnig, L.Y.; Schroer, C.; Luders, S.; Bohning, E.; Muller, G.A.; Wachter, R.; Koziolek, M.J. Effects of Baroreflex Activation Therapy on Ambulatory Blood Pressure in Patients with Resistant Hypertension. Hypertension 2016, 67, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Wallbach, M.; Lehnig, L.Y.; Schroer, C.; Hasenfuss, G.; Muller, G.A.; Wachter, R.; Koziolek, M.J. Impact of baroreflex activation therapy on renal function—A pilot study. Am. J. Nephrol. 2014, 40, 371–380. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Katz, R.; Kestenbaum, B.; Siscovick, D.; Fried, L.; Newman, A.; Rifkin, D.; Sarnak, M.J. Rapid decline of kidney function increases cardiovascular risk in the elderly. J. Am. Soc. Nephrol. 2009, 20, 2625–2630. [Google Scholar] [CrossRef] [Green Version]
- Schunk, S.J.; Beisswenger, C.; Ritzmann, F.; Herr, C.; Wagner, M.; Triem, S.; Hutter, G.; Schmit, D.; Zewinger, S.; Sarakpi, T.; et al. Measurement of urinary Dickkopf-3 uncovered silent progressive kidney injury in patients with chronic obstructive pulmonary disease. Kidney Int. 2021, 100, 1081–1091. [Google Scholar] [CrossRef]
- Neuhaus, J.; Bauer, F.; Fitzner, C.; Hilgers, R.D.; Seibert, F.; Babel, N.; Doevelaar, A.; Eitner, F.; Floege, J.; Rauen, T.; et al. Urinary Biomarkers in the Prediction of Prognosis and Treatment Response in IgA Nephropathy. Kidney Blood Press. Res. 2018, 43, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Zoccali, C. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol. Dial. Transplant. 2017, 32, ii194–ii199. [Google Scholar] [CrossRef] [Green Version]
- Schunk, S.J.; Zarbock, A.; Meersch, M.; Kullmar, M.; Kellum, J.A.; Schmit, D.; Wagner, M.; Triem, S.; Wagenpfeil, S.; Grone, H.J.; et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: An observational cohort study. Lancet 2019, 394, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Roscigno, G.; Quintavalle, C.; Biondi-Zoccai, G.; De Micco, F.; Frati, G.; Affinito, A.; Nuzzo, S.; Condorelli, G.; Briguori, C. Urinary Dickkopf-3 and Contrast-Associated Kidney Damage. J. Am. Coll. Cardiol. 2021, 77, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Seibert, F.S.; Heringhaus, A.; Pagonas, N.; Rohn, B.; Bauer, F.; Trappe, H.J.; Landmesser, U.; Babel, N.; Westhoff, T.H. Dickkopf-3 in the prediction of contrast media induced acute kidney injury. J. Nephrol. 2021, 34, 821–828. [Google Scholar] [CrossRef]
- Zhang, K.; Watanabe, M.; Kashiwakura, Y.; Li, S.A.; Edamura, K.; Huang, P.; Yamaguchi, K.; Nasu, Y.; Kobayashi, Y.; Sakaguchi, M.; et al. Expression pattern of REIC/Dkk-3 in various cell types and the implications of the soluble form in prostatic acinar development. Int. J. Oncol. 2010, 37, 1495–1501. [Google Scholar]

| Parameter | All Patients (n = 31) | Patients with Baseline uDKK ≥ 400 pg/mg Creatinine (n = 11) | Patients with Baseline uDKK < 400 pg/mg Creatinine (n = 20) | p-Value |
|---|---|---|---|---|
| Age (years) | 58 ± 13 | 62 ± 13 | 56 ± 13 | 0.12 |
| Gender (female/male) | 13/18 | 5/6 | 8/12 | 0.79 |
| Mean BMI (kg/m2) | 33 ± 6 | 34.9 ± 6.3 | 32.6 ± 6.0 | 0.47 |
| Diabetes mellitus | 16 (52%) | 6 (55%) | 10 (50%) | 0.83 |
| History of smoking | 20 (65%) | 8 (73%) | 12 (60%) | 0.56 |
| Mean office BP (mmHg) | 171 ± 23/90 ± 19 | 170 ± 29/92 ± 21 | 166 ± 18/89 ± 18 | 0.14/0.66 |
| Mean ABP (mmHg) | 148 ± 13/81 ± 11 | 151 ± 11/80 ± 7 | 147 ± 13/81 ± 12 | 0.43/0.92 |
| Mean number of antihypertensive drugs | 6.6 ± 1.5 | 5.9 ± 1.7 | 7.1 ± 1.3 | 0.0464 * |
| Median uDKK3 level (pg/mg creatinine) | 303 (150–865) | 1637 (745–3960) | 162 (116–292) | <0.0001 * |
| Mean eGFR (mL/min/1.73 m2) | 72 ± 29 | 58 ± 35 | 80 ± 23 | 0.0429 * |
| Median UACR (mg/g creatinine) | 28 (14–643) | 1117 (38–2901) | 15 (10–39) | 0.0004 * |
| CKD stage > 3 | 4 (13%) | 4 (36%) | 0 (0%) | 0.0039 * |
| Parameter | Patients with Baseline uDKK ≥ 400 pg/mg Creatinine (n = 11) | Patients with Baseline uDKK < 400 pg/mg Creatinine (n = 20) | p-Value |
|---|---|---|---|
| Delta systolic office BP (mmHg) | −29.4 ± 24.0 | −18.8 ± 25.6 | 0.28 |
| Delta diastolic office BP (mmHg) | −6.3 ± 17.1 | −5.2 ± 15.1 | 0.87 |
| Delta systolic 24 h ABP (mmHg) | −11.0 ± 23.7 | −3.7 ± 21.3 | 0.43 |
| Delta diastolic 24 h ABP (mmHg) | −6.1 ± 11.2 | −2.1 ± 14.0 | 0.36 |
| Correlation | Baseline uDKK3 Level | Baseline eGFR | Baseline UACR | |||
|---|---|---|---|---|---|---|
| Spearman’s r | p-Value | Pearson’s r | p-Value | Spearman’s r | p-Value | |
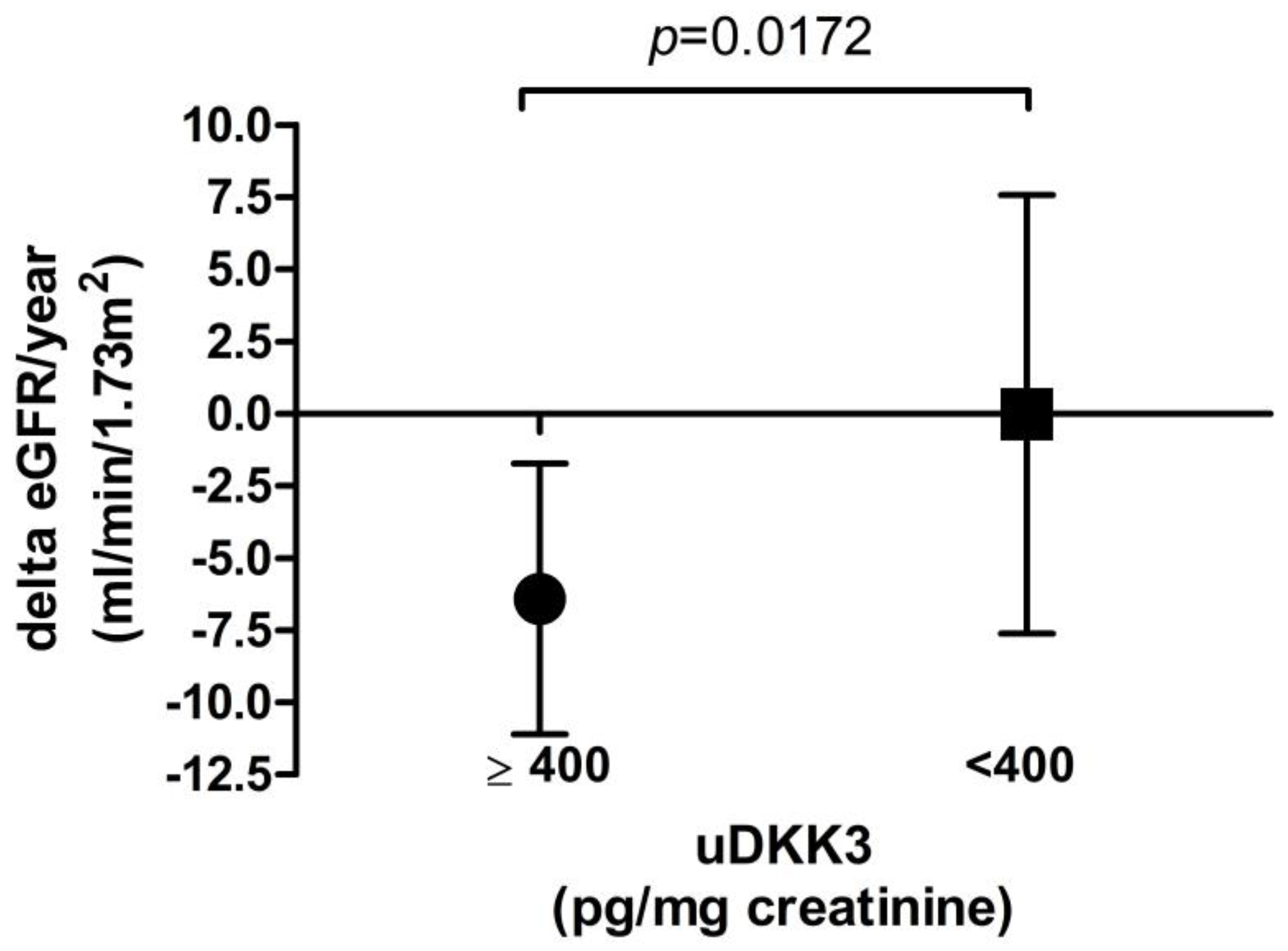
| Delta eGFR (mL/min/1.73 m2) at latest follow-up | −0.3714 | 0.0397 * | 0.1524 | 0.4129 | −0.2590 | 0.1669 |
| Delta eGFR (mL/min/1.73 m2) per year | −0.3750 | 0.0376 * | 0.1296 | 0.4872 | −0.2659 | 0.1555 |
| Percentage change in eGFR at latest follow-up | −0.4791 | 0.0064 * | 0.4714 | 0.0074 * | −0.4014 | 0.0279 * |
| Percentage change in eGFR per year | −0.4682 | 0.0079 * | 0.4110 | 0.0216 * | −0.3866 | 0.0348 * |
| Baseline | Latest Follow-Up | |||||
|---|---|---|---|---|---|---|
| eGFR Categories (mL/min/1.73 m2) | Patients with Baseline uDKK ≥ 400 pg/mg Creatinine (n = 11) | Patients with Baseline uDKK < 400 pg/mg Creatinine (n = 20) | p-Value ° | Patients with Baseline uDKK ≥ 400 pg/mg Creatinine (n = 11) | Patients with Baseline uDKK < 400 pg/mg Creatinine (n = 20) | p-Value + |
| >90 | 2 (18%) | 7 (35%) | 0.0304 * | 1 (9%) | 8 (40%) | 0.0808 |
| 60–89 | 4 (36%) | 8 (40%) | 3 (27%) | 6 (30%) | ||
| 30–59 | 1 (9%) | 5 (25%) | 2 (18%) | 5 (25%) | ||
| 15–29 | 4 (36%) | 0 (0%) | 3 (27%) | 1 (5%) | ||
| <15 | 0 (0%) | 0 (0%) | 2 (18%) | 0 (0%) | ||
| Decrease in eGFR category | - | - | 6 (55%) | 4 (20%) | 0.049 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, A.-K.C.; Pieper, D.; Dihazi, H.; Dihazi, G.H.; Lüders, S.; Koziolek, M.J.; Wallbach, M. Urinary Dickkopf-3 (DKK3) Is Associated with Greater eGFR Loss in Patients with Resistant Hypertension. J. Clin. Med. 2023, 12, 1034. https://doi.org/10.3390/jcm12031034
Schäfer A-KC, Pieper D, Dihazi H, Dihazi GH, Lüders S, Koziolek MJ, Wallbach M. Urinary Dickkopf-3 (DKK3) Is Associated with Greater eGFR Loss in Patients with Resistant Hypertension. Journal of Clinical Medicine. 2023; 12(3):1034. https://doi.org/10.3390/jcm12031034
Chicago/Turabian StyleSchäfer, Ann-Kathrin C., Dennis Pieper, Hassan Dihazi, Gry H. Dihazi, Stephan Lüders, Michael J. Koziolek, and Manuel Wallbach. 2023. "Urinary Dickkopf-3 (DKK3) Is Associated with Greater eGFR Loss in Patients with Resistant Hypertension" Journal of Clinical Medicine 12, no. 3: 1034. https://doi.org/10.3390/jcm12031034
APA StyleSchäfer, A.-K. C., Pieper, D., Dihazi, H., Dihazi, G. H., Lüders, S., Koziolek, M. J., & Wallbach, M. (2023). Urinary Dickkopf-3 (DKK3) Is Associated with Greater eGFR Loss in Patients with Resistant Hypertension. Journal of Clinical Medicine, 12(3), 1034. https://doi.org/10.3390/jcm12031034






