FABP4 Expression in Subcutaneous Adipose Tissue Is Independently Associated with Circulating Triglycerides in Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical and Biochemical Characteristics
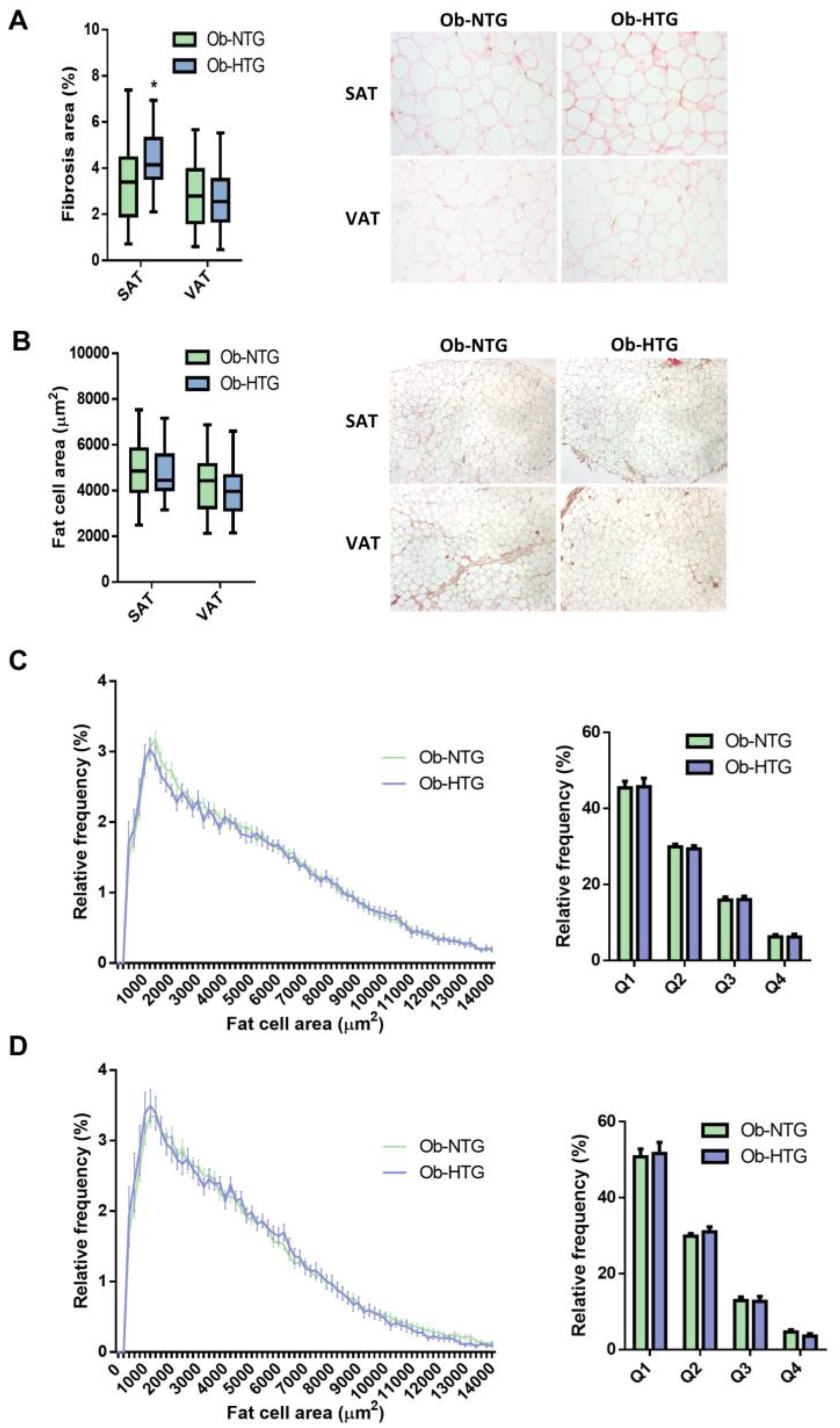
2.3. Tissue Processing and Histology
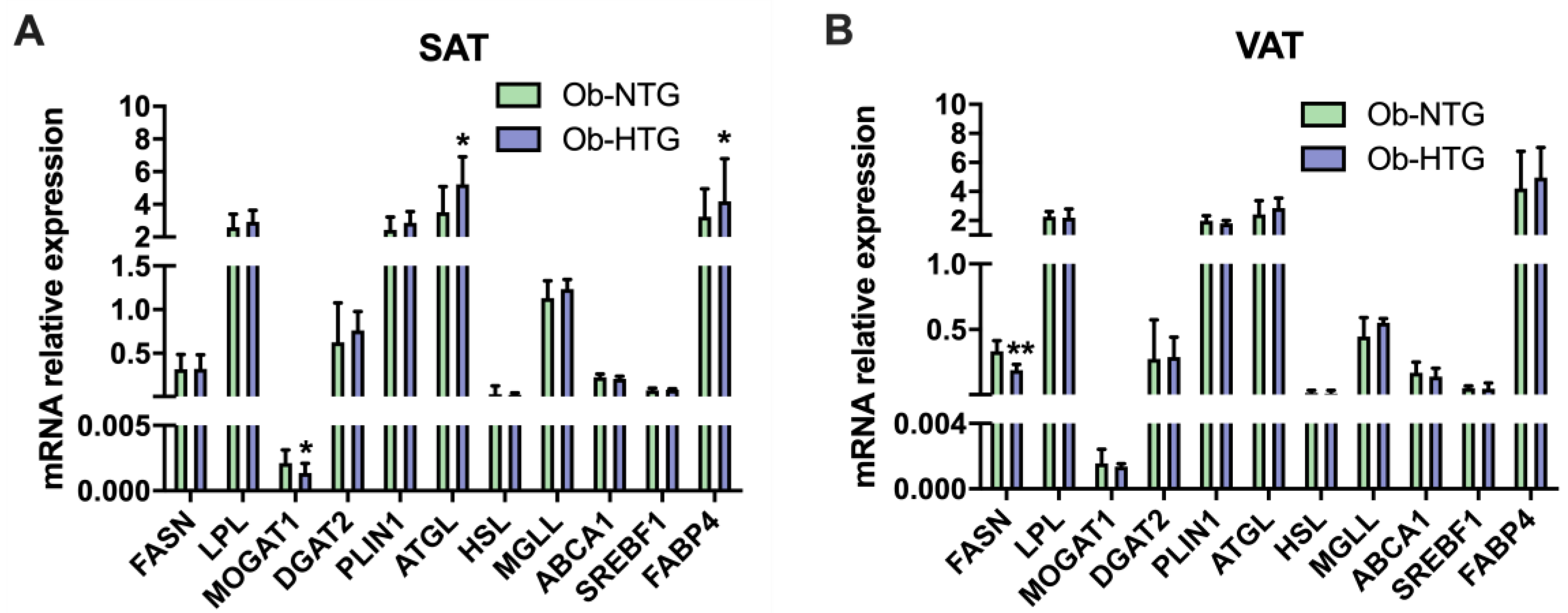
2.4. Quantitative Real-Time PCR
2.5. Statistics
3. Results
3.1. Differences in Adipose Tissue Parameters between Ob-NTG and Ob-HTG Patients
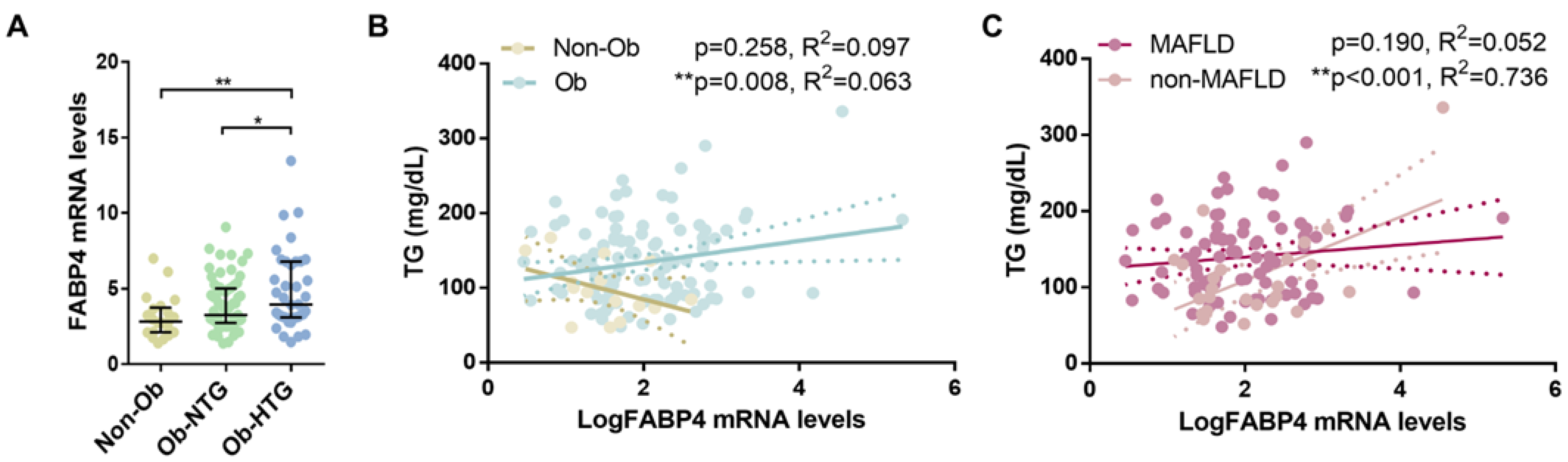
3.2. Associations between Gene Expression and Triglyceride Levels in Patients with Severe Obesity
3.3. Subcutaneous Gene Expression of FABP4 and Association with Triglyceride Levels in Subjects without Obesity and Individuals with Severe Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol. 2016, 6, 150272. [Google Scholar] [CrossRef]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R.A. Clinical review on triglycerides. Eur. Heart J. 2019, 41, 99–109c. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 Incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Sarwar, N.; Sandhu, M.S.; Ricketts, S.L.; Butterworth, A.S.; Di Angelantonio, E.; Matthijs Boekholdt, S.; Ouwehand, W.; Watkins, H.; Samani, N.J.; Saleheen, D.; et al. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet 2010, 375, 1634–1639. [Google Scholar] [CrossRef]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1353. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef]
- Klempfner, R.; Erez, A.; Sagit, B.Z.; Goldenberg, I.; Fisman, E.; Kopel, E.; Shlomo, N.; Israel, A.; Tenenbaum, A. Elevated Triglyceride Level Is Independently Associated with Increased All-Cause Mortality in Patients with Established Coronary Heart Disease: Twenty-Two-Year Follow-Up of the Bezafibrate Infarction Prevention Study and Registry. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 100–108. [Google Scholar] [CrossRef]
- Carrasquilla, G.D.; Christiansen, M.R.; Kilpeläinen, T.O. The Genetic Basis of Hypertriglyceridemia. Curr. Atheroscler. Rep. 2021, 23, 39. [Google Scholar] [CrossRef]
- Bissonnette, S.; Salem, H.; Wassef, H.; Saint-Pierre, N.; Tardif, A.; Baass, A.; Dufour, R.; Faraj, M. Low density lipoprotein delays clearance of triglyceride-rich lipoprotein by human subcutaneous adipose tissue. J. Lipid Res. 2013, 54, 1466. [Google Scholar] [CrossRef]
- Bard, J.M.; Charles, M.A.; Juhan-Vague, I.; Vague, P.; André, P.; Safar, M.; Fruchart, J.C.; Eschwege, E. Accumulation of Triglyceride-Rich Lipoprotein in Subjects with Abdominal Obesity. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 407–414. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Patsch, J.R.; Miesenböck, G.; Hopferwieser, T.; Mühlberger, V.; Knapp, E.; Kay Dunn, J.; Gotto, A.M.; Patsch, W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb. A J. Vasc. Biol. 1992, 12, 1336–1345. [Google Scholar] [CrossRef]
- Simons, L.A.; Dwyer, T.; Simons, J.; Bernstein, L.; Mock, P.; Pooma, N.S.; Balasubramaniam, S.; Baron, D.; Branson, J.; Morgan, J.; et al. Chylomicrons and chylomicron remnants in coronary artery disease: A case-control study. Atherosclerosis 1987, 65, 181–189. [Google Scholar] [CrossRef]
- Ryu, J.E.; Howard, G.; Craven, T.E.; Bond, M.G.; Hagaman, A.P.; Crouse, J.R. Postprandial triglyceridemia and carotid atherosclerosis in middle-aged subjects. Stroke 1992, 23, 823–828. [Google Scholar] [CrossRef]
- Karpe, F.; Steiner, G.; Uffelman, K.; Olivecrona, T.; Hamsten, A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis 1994, 106, 83–97. [Google Scholar] [CrossRef]
- Groot, P.H.E.; Van Stiphout, W.A.H.J.; Krauss, X.H.; Jansen, H.; Van Tol, A.; Van Ramshorst, E.; Chin-On, S.; Hofman, A.; Cresswell, S.R.; Havekes, L. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler. Thromb. A. J. Vasc. Biol. 1991, 11, 653–662. [Google Scholar] [CrossRef]
- Ellsworth, J.L.; Fong, L.G.; Kraemer, F.B.; Cooper, A.D. Differences in the processing of chylomicron remnants and beta-VLDL by macrophages. J. Lipid Res. 1990, 31, 1399–1411. [Google Scholar] [CrossRef]
- Cabezas, M.C.; De Bruin, T.W.A.; De Valk, H.W.; Shoulders, C.C.; Jansen, H.; Erkelens, D.W. Impaired fatty acid metabolism in familial combined hyperlipidemia. A mechanism associating hepatic apolipoprotein B overproduction and insulin resistance. J. Clin. Investig. 1993, 92, 160–168. [Google Scholar] [CrossRef]
- Castro Cabezas, M.; de Bruin, T.W.A.; Kock, L.A.W.; Kortlandt, W.; Van Linde-Sibenius Trip, M.; Jansen, H.; Willem Erkelens, D. Simvastatin improves chylomicron remnant removal in familial combined hyperlipidemia without changing chylomicron conversion. Metabolism 1993, 42, 497–503. [Google Scholar] [CrossRef]
- Capell, W.H.; Zambon, A.; Austin, M.A.; Brunzell, J.D.; Hokanson, J.E. Compositional differences of LDL particles in normal subjects with LDL subclass phenotype A and LDL subclass phenotype B. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1040–1046. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Krauss, R.M.; Albers, J.J.; Austin, M.A.; Brunzell, J.D. LDL physical and chemical properties in familial combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 452–459. [Google Scholar] [CrossRef]
- Arner, P.; Rydén, M. Human white adipose tissue: A highly dynamic metabolic organ. J. Intern. Med. 2022, 291, 611–621. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Rydén, M.; Arner, P. Subcutaneous Adipocyte Lipolysis Contributes to Circulating Lipid Levels. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1782–1787. [Google Scholar] [CrossRef]
- Sajuthi, S.P.; Sharma, N.K.; Comeau, M.E.; Chou, J.W.; Bowden, D.W.; Freedman, B.I.; Langefeld, C.D.; Parks, J.S.; Das, S.K. Genetic regulation of adipose tissue transcript expression is involved in modulating serum triglyceride and HDL-cholesterol. Gene 2017, 632, 50–58. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, E1082–E1143. [Google Scholar] [CrossRef]
- Andreu, A.; Moizé, V.; Rodríguez, L.; Flores, L.; Vidal, J. Protein intake, body composition, and protein status following bariatric surgery. Obes. Surg. 2010, 20, 1509–1515. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Bermúdez, V.; Calvo, M.; Olivar, L.C.; Luzardo, E.; Navarro, C.; Mencia, H.; Martínez, M.; Rivas-Ríos, J.; Wilches-Durán, S.; et al. Optimal cutoff for the evaluation of insulin resistance through triglyceride-glucose index: A cross-sectional study in a Venezuelan population. F1000Research 2018, 6, 1337. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Shrestha, Y.B.; Vaughan, C.H.; Schwartz, G.J.; Song, C.K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 2010, 318, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ramseyer, V.D.; Granneman, J.G. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 2016, 5, 119–129. [Google Scholar] [CrossRef]
- Warfel, J.D.; Vandanmagsar, B.; Dubuisson, O.S.; Hodgeson, S.M.; Elks, C.M.; Ravussin, E.; Mynatt, R.L. Examination of carnitine palmitoyltransferase 1 abundance in white adipose tissue: Implications in obesity research. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R816–R820. [Google Scholar] [CrossRef]
- Mayas, M.D.; Ortega, F.J.; MacÍas-Gonzlez, M.; Bernal, R.; Gámez-Huelgas, R.; Fernndez-Real, J.M.; Tinahones, F.J. Inverse relation between FASN expression in human adipose tissue and the insulin resistance level. Nutr. Metab. 2010, 7, 3. [Google Scholar] [CrossRef]
- Sievert, H.; Krause, C.; Geiβler, C.; Grohs, M.; El-Gammal, A.T.; Wolter, S.; Mann, O.; Lehnert, H.; Kirchner, H. Epigenetic Downregulation of FASN in Visceral Adipose Tissue of Insulin Resistant Subjects. Exp. Clin. Endocrinol. Diabetes 2021, 129, 674–682. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Jessen, N.; Jørgensen, J.O.L.; Møller, N.; Lund, S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014, 52, R199–R222. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E.; Watt, M.J. Adipocyte triglyceride lipase expression in human obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E958–E964. [Google Scholar] [CrossRef] [PubMed]
- Yao-Borengasser, A.; Varma, V.; Coker, R.H.; Ranganathan, G.; Phanavanh, B.; Rasouli, N.; Kern, P.A. Adipose triglyceride lipase expression in human adipose tissue and muscle. Role in insulin resistance and response to training and pioglitazone. Metabolism. 2011, 60, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- De Naeyer, H.; Ouwens, D.M.; Van Nieuwenhove, Y.; Pattyn, P.; ’T Hart, L.M.; Kaufman, J.M.; Sell, H.; Eckel, J.; Cuvelier, C.; Taes, Y.E.; et al. Combined gene and protein expression of hormone-sensitive lipase and adipose triglyceride lipase, mitochondrial content, and adipocyte size in subcutaneous and visceral adipose tissue of morbidly obese men. Obes. Facts 2011, 4, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.E.; Langin, D.; Smit, E.; Saris, W.H.M.; Valle, C.; Hul, G.B.; Holm, C.; Arner, P.; Blaak, E.E. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J. Clin. Endocrinol. Metab. 2007, 92, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Liss, K.H.H.; Lutkewitte, A.J.; Pietka, T.; Finck, B.N.; Franczyk, M.; Yoshino, J.; Klein, S.; Hall, A.M. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J. Lipid Res. 2018, 59, 1630–1639. [Google Scholar] [CrossRef]
- Terra, X.; Quintero, Y.; Auguet, T.; Porras, J.A.; Hernández, M.; Sabench, F.; Aguilar, C.; Luna, A.M.; Del Castillo, D.; Richart, C. FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. Eur. J. Endocrinol. 2011, 164, 539–547. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Pérusse, J.R.; Lavoie, M.È.; Sladek, R.; Madiraju, S.R.M.; Ruderman, N.B.; Coulombe, B.; Prentki, M.; Rabasa-Lhoret, R. Increased subcutaneous adipose tissue expression of genes involved in glycerolipid-fatty acid cycling in obese insulin-resistant versus -sensitive individuals. J. Clin. Endocrinol. Metab. 2014, 99, E2518–E2528. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M. Adipocyte fatty acid binding protein in a Caucasian population: A new marker of metabolic syndrome? Eur. J. Clin. Investig. 2006, 36, 621–625. [Google Scholar] [CrossRef]
- Xu, A.; Tso, A.W.K.; Cheung, B.M.Y.; Wang, Y.; Wat, N.M.S.; Fong, C.H.Y.; Yeung, D.C.Y.; Janus, E.D.; Sham, P.C.; Lam, K.S.L. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: A 5-year prospective study. Circulation 2007, 115, 1537–1543. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.S.; Wong, W.K.; Lam, K.S.L. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Kralisch, S.; Fasshauer, M. Adipocyte fatty acid binding protein: A novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013, 56, 10–21. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Ek, B.A.; Cistola, D.P.; Hamilton, J.A.; Kaduce, T.L.; Spector, A.A. Fatty acid binding proteins reduce 15-lipoxygenase-induced oxygenation of linoleic acid and arachidonic acid. Biochim. Biophys. Acta 1997, 1346, 75–85. [Google Scholar] [CrossRef]
- Dickinson Zimmer, J.S.; Dyckes, D.F.; Bernlohr, D.A.; Murphy, R.C. Fatty acid binding proteins stabilize leukotriene A4: Competition with arachidonic acid but not other lipoxygenase products. J. Lipid Res. 2004, 45, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Furuhashi, M.; Hiramitsu, S.; Ishii, J.; Hoshina, K.; Ishimura, S.; Fuseya, T.; Watanabe, Y.; Tanaka, M.; Ohno, K.; et al. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity 2015, 23, 359–367. [Google Scholar] [CrossRef]
- Kim, Y.C.; Cho, Y.K.; Lee, W.Y.; Kim, H.J.; Park, J.H.; Park, D.I.; Sohn, C., II; Jeon, W.K.; Kim, B.I.; Park, S.E.; et al. Serum adipocyte-specific fatty acid-binding protein is associated with nonalcoholic fatty liver disease in apparently healthy subjects. J. Nutr. Biochem. 2011, 22, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.B.; Kim, S.M.; Cho, G.J.; Choi, K.M. Serum AFBP levels are elevated in patients with nonalcoholic fatty liver disease. Scand. J. Gastroenterol. 2014, 49, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chan, H.L.Y.; Wong, G.L.H.; Choi, P.C.L.; Chan, A.W.H.; Chan, H.Y.; Chim, A.M.L.; Yeung, D.K.W.; Chan, F.K.L.; Woo, J.; et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J. Hepatol. 2012, 56, 1363–1370. [Google Scholar] [CrossRef]
- Koh, J.H.; Shin, Y.G.; Nam, S.M.; Lee, M.Y.; Chung, C.H.; Shin, J.Y. Serum adipocyte fatty acid-binding protein levels are associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Diabetes Care 2009, 32, 147–152. [Google Scholar] [CrossRef]
- Jeon, W.S.; Park, S.E.; Rhee, E.-J.; Park, C.-Y.; Oh, K.-W.; Park, S.-W.; Lee, W.-Y. Association of serum adipocyte-specific Fatty Acid binding protein with Fatty liver index as a predictive indicator of nonalcoholic Fatty liver disease. Endocrinol. Metab. 2013, 28, 283. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, X.; Pan, X.; He, X.; Wang, Y.; Bao, Y. Serum adipocyte fatty acid-binding protein levels: An indicator of non-alcoholic fatty liver disease in Chinese individuals. Liver Int. 2019, 39, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calvo, R.; Moreno-Vedia, J.; Girona, J.; Ibarretxe, D.; Martínez-Micaelo, N.; Merino, J.; Plana, N.; Masana, L. Relationship Between Fatty Acid Binding Protein 4 and Liver Fat in Individuals at Increased Cardiometabolic Risk. Front. Physiol. 2021, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Milner, K.L.; van der Poorten, D.; Xu, A.; Bugianesi, E.; Kench, J.G.; Lam, K.S.L.; Chisholm, D.J.; George, J. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vedia, J.; Girona, J.; Ibarretxe, D.; Masana, L.; Rodríguez-Calvo, R. Unveiling the Role of the Fatty Acid Binding Protein 4 in the Metabolic-Associated Fatty Liver Disease. Biomedicines 2022, 10, 197. [Google Scholar] [CrossRef]
- Baar, R.A.; Dingfelder, C.S.; Smith, L.A.; Bernlohr, D.A.; Wu, C.; Lange, A.J.; Parks, E.J. Investigation of in vivo fatty acid metabolism in AFABP/aP2(-/-) mice. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E187–E193. [Google Scholar] [CrossRef]
- Coe, N.R.; Simpson, M.A.; Bernlohr, D.A. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J. Lipid Res. 1999, 40, 967–972. [Google Scholar] [CrossRef]
- Scheja, L.; Makowski, L.; Uysal, K.T.; Wiesbrock, S.M.; Shimshek, D.R.; Meyers, D.S.; Morgan, M.; Parker, R.A.; Hotamisligil, G.S. Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/- mice. Diabetes 1999, 48, 1987–1994. [Google Scholar] [CrossRef]
- Lan, H.; Cheng, C.C.; Kowalski, T.J.; Pang, L.; Shan, L.; Chuang, C.C.; Jackson, J.; Rojas-Triana, A.; Bober, L.; Liu, L.; et al. Small-molecule inhibitors of FABP4/5 ameliorate dyslipidemia but not insulin resistance in mice with diet-induced obesity. J. Lipid Res. 2011, 52, 646–656. [Google Scholar] [CrossRef]
- Uysal, K.T.; Scheja, L.; Wiesbrock, S.M.; Bonner-Weir, S.; Hotamisligil, G.S. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology 2000, 141, 3388–3396. [Google Scholar] [CrossRef]
- Yang, R.; Castriota, G.; Chen, Y.; Cleary, M.A.; Ellsworth, K.; Shin, M.K.; Tran, J.L.; Vogt, T.F.; Wu, M.; Xu, S.; et al. RNAi-mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet-induced obese mice. Int. J. Obes. 2011, 35, 217–225. [Google Scholar] [CrossRef]
- Boord, J.B.; Maeda, K.; Makowski, L.; Babaev, V.R.; Fazio, S.; Linton, M.F.; Hotamisligil, G.S. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Boord, J.B.; Maeda, K.; Makowski, L.; Babaev, V.R.; Fazio, S.; Linton, M.F.; Hotamisligil, G.S. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation 2004, 110, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Furuhashi, M.; Koyama, M.; Higashiura, Y.; Akasaka, H.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; Ura, N.; et al. Elevated circulating FABP4 concentration predicts cardiovascular death in a general population: A 12-year prospective study. Sci. Rep. 2021, 11, 4008. [Google Scholar] [CrossRef] [PubMed]
- Cabré, A.; Lázaro, I.; Cofán, M.; Jarauta, E.; Plana, N.; Garcia-Otín, A.L.; Ascaso, J.F.; Ferré, R.; Civeira, F.; Ros, E.; et al. FABP4 plasma levels are increased in familial combined hyperlipidemia. J. Lipid Res. 2010, 51, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.M.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J. Lipid Res. 2008, 49, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.M.; Eriksson, P.; Hoffstedt, J.; Hotamisligil, G.S.; Thörne, A.; Rydén, M.; Hamsten, A.; Arner, P. Fatty acid binding protein expression in different adipose tissue depots from lean and obese individuals. Diabetologia 2001, 44, 1268–1273. [Google Scholar] [CrossRef]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Sung, J.M.; Song, C.S.; Lee, W.Y.; Rhee, E.J.; Shin, J.H.; Yoo, C.H.; Chae, S.W.; Kim, J.Y.; Jin, W.; et al. Enhanced A-FABP expression in visceral fat: Potential contributor to the progression of NASH. Clin. Mol. Hepatol. 2012, 18, 279–286. [Google Scholar] [CrossRef]
- Coilly, A.; Desterke, C.; Guettier, C.; Samuel, D.; Chiappini, F. FABP4 and MMP9 levels identified as predictive factors for poor prognosis in patients with nonalcoholic fatty liver using data mining approaches and gene expression analysis. Sci. Rep. 2019, 9, 19785. [Google Scholar] [CrossRef]
- Furuhashi, M.; Fuseya, T.; Murata, M.; Hoshina, K.; Ishimura, S.; Mita, T.; Watanabe, Y.; Omori, A.; Matsumoto, M.; Sugaya, T.; et al. Local Production of Fatty Acid-Binding Protein 4 in Epicardial/Perivascular Fat and Macrophages Is Linked to Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 825–834. [Google Scholar] [CrossRef]
- Rolph, M.S.; Young, T.R.; Shum, B.O.V.; Gorgun, C.Z.; Schmitz-Peiffer, C.; Ramshaw, I.A.; Hotamisligil, G.S.; Mackay, C.R. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J. Immunol. 2006, 177, 7794–7801. [Google Scholar] [CrossRef] [PubMed]
| Ob-NTG (n = 67) | Ob-HTG (n = 37) | p | |
|---|---|---|---|
| Women | 49 (73%) | 25 (67%) | 0.618 a |
| Age (years) | 50.03 ± 10.17 | 51.87 ± 11.21 | 0.319 |
| Weight (kg) | 117.68 ± 20.03 | 122.52 ± 25.24 | 0.209 |
| BMI (kg/m2) | 44.04 ± 4.69 | 45.78 ± 6.96 | 0.075 |
| Waist:hip ratio | 0.93 ± 0.08 | 0.96 ± 0.06 | 0.222 |
| CUN-BAE index | 53.01 (51.14–55.41) | 54.16 (48.98–56.15) | 0.398 |
| hs-CRP (mg/dL) | 0.62 (0.34–1.14) | 0.87 (0.58–1.46) | 0.021 |
| FPG (mg/dL) | 99 (90–113) | 104.5 (96–133) | 0.028 |
| HbA1c (%) | 5.6 (5.3–6.2) | 6.1 (5.6–6.8) | 0.002 |
| T2D | 19 (29%) | 17 (46%) | 0.158 a |
| T2D medications | 0 (0–1) | 0 (0–2) | 0.114 |
| Insulin treatment | 8 (12%) | 5 (13%) | 0.999 a |
| HTN | 25 (38%) | 24 (66%) | 0.014 a |
| HTN medications | 0 (0–1.5) | 1 (0–2) | 0.062 |
| AST (IU/L) | 20 (17–25) | 21 (17–26) | 0.653 |
| ALT (IU/L) | 22 (17–32) | 24 (18–34) | 0.394 |
| GGT (IU/L) | 24 (17–36) | 29 (23–54) | 0.001 |
| AST:ALT ratio | 0.88 (0.70–1.12) | 0.80 (0.63–1.13) | 0.313 |
| MAFLD | 48 (72%) | 33 (90%) | 0.060 a |
| FIB4 score | 0.82 (0.61–1.07) | 0.82 (0.58–1.07) | 0.856 |
| APRI score | 0.18 (0.14–0.24) | 0.19 (0.15–0.25) | 0.545 |
| HSI index | 55.25 (52.00–58.93) | 56.66 (53.99–62.40) | 0.055 |
| TyG index | 4.6 ± 0.21 | 4.99 ± 0.14 | <0.0001 |
| FLI index | 97.64 (94.29–99.02) | 98.5 (97.36–99.64) | 0.040 |
| Total cholesterol (mg/dL) | 177.72 ± 31.83 | 195.29 ± 41.68 | 0.005 |
| TG (mg/dL) | 99 (80.25–122.50) | 181 (164.5–202.5) | <0.0001 |
| HDL (mg/dL) | 48.22 ± 10.43 | 45.39 ± 10.27 | 0.123 |
| LDL (mg/dL) | 110.24 ± 27.89 | 115.34 ± 37.8 | 0.347 |
| Hypercolesterolemia | 25 (38%) | 16 (43%) | 0.818 a |
| Statins treatment | 18 (28%) | 15 (40%) | 0.335 a |
| GFR (ml/min/1.73 m2) | 90 (90–90) | 90 (90–90) | 0.270 |
| Creatinine (mg/dL) | 0.69 (0.63–0.75) | 0.67 (0.62–0.80) | 0.934 |
| Ob-NTG | Ob-HTG | p | |
|---|---|---|---|
| SAT | |||
| FABP4 | 3.237 (2.732–4.942) | 4.177 (3.119–6.793) | 0.032 |
| MOGAT1 | 0.002 (0.001–0.003) | 0.001 (0.0008–0.002) | 0.044 |
| ATGL | 4.192 ± 2.111 | 5.456 ± 2.886 | 0.040 |
| DIO2 | 0.006 ± 0.003 | 0.011 ± 0.005 | 0.024 |
| MMP13 | 0.0009 (0.0007–0.002) | 0.0005 (0.00004–0.001) | 0.009 |
| MMP15 | 0.024 (0.015–0.030) | 0.016 (0.013–0.023) | 0.029 |
| VAT | |||
| CD80 | 0.015 (0.012–0.022) | 0.012 (0.011–0.013) | 0.045 |
| F13A1 | 0.335 (0.190–0.416) | 0.187 (0.123–0.220) | 0.048 |
| PTEN | 1.048 ± 0.389 | 0.798 ± 0.196 | 0.001 |
| VEGFR2 | 0.001 ± 0.001 | 0.002 ± 0.0007 | 0.042 |
| ANGPT1 | 0.026 (0.017–0.039) | 0.014 (0.012–0.022) | 0.028 |
| PDGFRB | 0.002 (0.0009–0.006) | 0.0007 (0.0004–0.001) | 0.032 |
| ADIPOR1 | 0.150 (0.100–0.191) | 0.202 (0.140–0.244) | 0.012 |
| FASN | 0.082 (0.076–0.099) | 0.067 (0.063–0.090) | 0.008 |
| IRS1 | 0.109 (0.086–0.140) | 0.131 (0.115–0.157) | 0.017 |
| CPT1A | 0.019 (0.014–0.027) | 0.027 (0.019–0.038) | 0.031 |
| ADRB3 | 0.156 (0.124–0.197) | 0.197 (0.153–0.242) | 0.006 |
| ADRB1 | 0.356 (0.230–0.609) | 0.512 (0.412–0.676) | 0.027 |
| Correlation Coefficient (95%CI) | p | R2 | |
|---|---|---|---|
| SAT | |||
| FABP4 | |||
| Unadjusted | 2.967 (0.773, 5.162) | 0.009 | 0.054 |
| Adjusted 1 a | 2.870 (0.688, 5.053) | 0.010 | 0.084 |
| Adjusted 2 b | 3.058 (0.931, 5.184) | 0.005 | 0.137 |
| Adjusted 3 c | 2.828 (0.74, 4.92) | 0.008 | 0.194 |
| VAT | |||
| F13A1 | |||
| Unadjusted | 72.704 (8.8, 136.609) | 0.027 | 0.114 |
| Adjusted 1 a | 64.55 (1.813, 127.286) | 0.044 | 0.177 |
| Adjusted 2 b | 52.272 (−19.133, 123.676) | 0.181 | 0.238 |
| Adjusted 3 c | 23.816 (−30.041, 77.674) | 0.293 | 0.033 |
| Model | Predictor | B | SE | 95% CI | p | Adjusted R2 | p |
|---|---|---|---|---|---|---|---|
| 1 | Constant | 128.903 | 4.979 | (119.03, 138.77) | <0.0001 | 0.054 | 0.009 |
| SAT-FABP4 | 2.967 | 1.107 | (0.77, 5.16) | 0.009 | |||
| 2 | Constant | −1.561 | 40.706 | (−82.14, 79.02) | 0.969 | 0.143 | <0.001 |
| BMI | 2.276 | 0.694 | (0.9, 3.65) | 0.001 | |||
| HbA1c | 0.836 | 4.636 | (−8.34, 10.01) | 0.857 | |||
| MAFLD | 21.204 | 10.492 | (0.43, 41.97) | 0.045 | |||
| Creatinine | −45.560 | 35.564 | (−115.96, 24.84) | 0.203 | |||
| GGT | 22.030 | 10.562 | (1.12, 42.94) | 0.039 | |||
| Statins | 14.440 | 10.280 | (−5.91, 34.79) | 0.163 | |||
| 3 | Constant | −4.613 | 41.243 | (−86.4, 77.17) | 0.911 | 0.194 | <0.001 |
| SAT-FABP4 | 2.828 | 1.054 | (0.74, 4.92) | 0.008 | |||
| BMI | 2.108 | 0.72 | (0.68, 3.54) | 0.004 | |||
| HbA1c | 0.345 | 4.747 | (−9.07, 9.76) | 0.942 | |||
| MAFLD | 21.925 | 11.403 | (−0.69, 44.54) | 0.057 | |||
| Creatinine | −42.67 | 34.886 | (−111.85, 26.51) | 0.224 | |||
| GGT | 15.188 | 10.997 | (−6.62, 37) | 0.17 | |||
| Statins | 20.566 | 10.735 | (−0.72, 41.85) | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Conles, Ó.; Ibarzabal, A.; Balibrea, J.M.; Vidal, J.; Ortega, E.; de Hollanda, A. FABP4 Expression in Subcutaneous Adipose Tissue Is Independently Associated with Circulating Triglycerides in Obesity. J. Clin. Med. 2023, 12, 1013. https://doi.org/10.3390/jcm12031013
Osorio-Conles Ó, Ibarzabal A, Balibrea JM, Vidal J, Ortega E, de Hollanda A. FABP4 Expression in Subcutaneous Adipose Tissue Is Independently Associated with Circulating Triglycerides in Obesity. Journal of Clinical Medicine. 2023; 12(3):1013. https://doi.org/10.3390/jcm12031013
Chicago/Turabian StyleOsorio-Conles, Óscar, Ainitze Ibarzabal, José María Balibrea, Josep Vidal, Emilio Ortega, and Ana de Hollanda. 2023. "FABP4 Expression in Subcutaneous Adipose Tissue Is Independently Associated with Circulating Triglycerides in Obesity" Journal of Clinical Medicine 12, no. 3: 1013. https://doi.org/10.3390/jcm12031013
APA StyleOsorio-Conles, Ó., Ibarzabal, A., Balibrea, J. M., Vidal, J., Ortega, E., & de Hollanda, A. (2023). FABP4 Expression in Subcutaneous Adipose Tissue Is Independently Associated with Circulating Triglycerides in Obesity. Journal of Clinical Medicine, 12(3), 1013. https://doi.org/10.3390/jcm12031013







