Dolutegravir/Lamivudine versus Tenofovir Alafenamide/Emtricitabine/Bictegravir as a Switch Strategy in a Real-Life Cohort of Virogically Suppressed People Living with HIV
Abstract
:1. Introduction
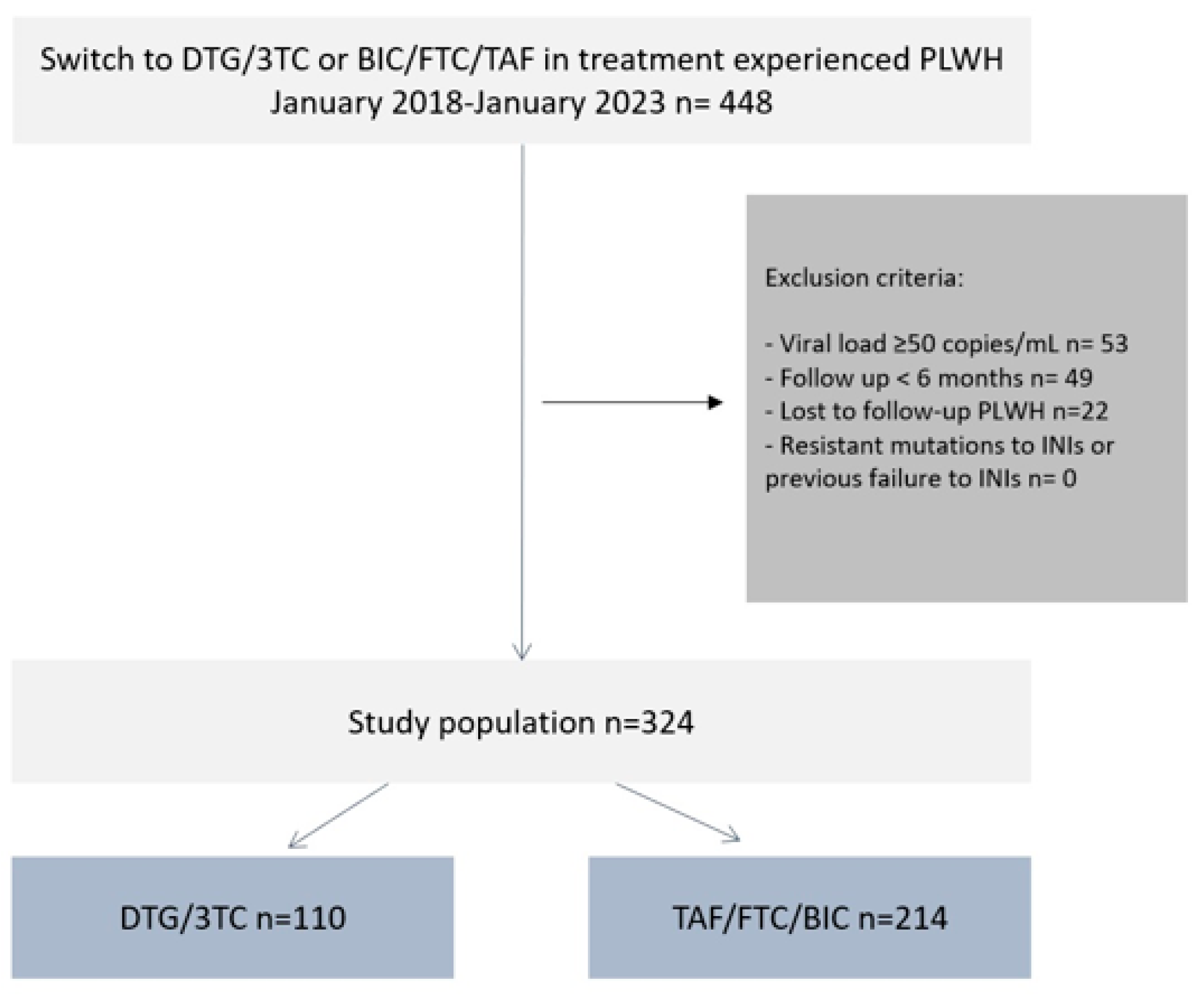
2. Materials and Methods
3. Results
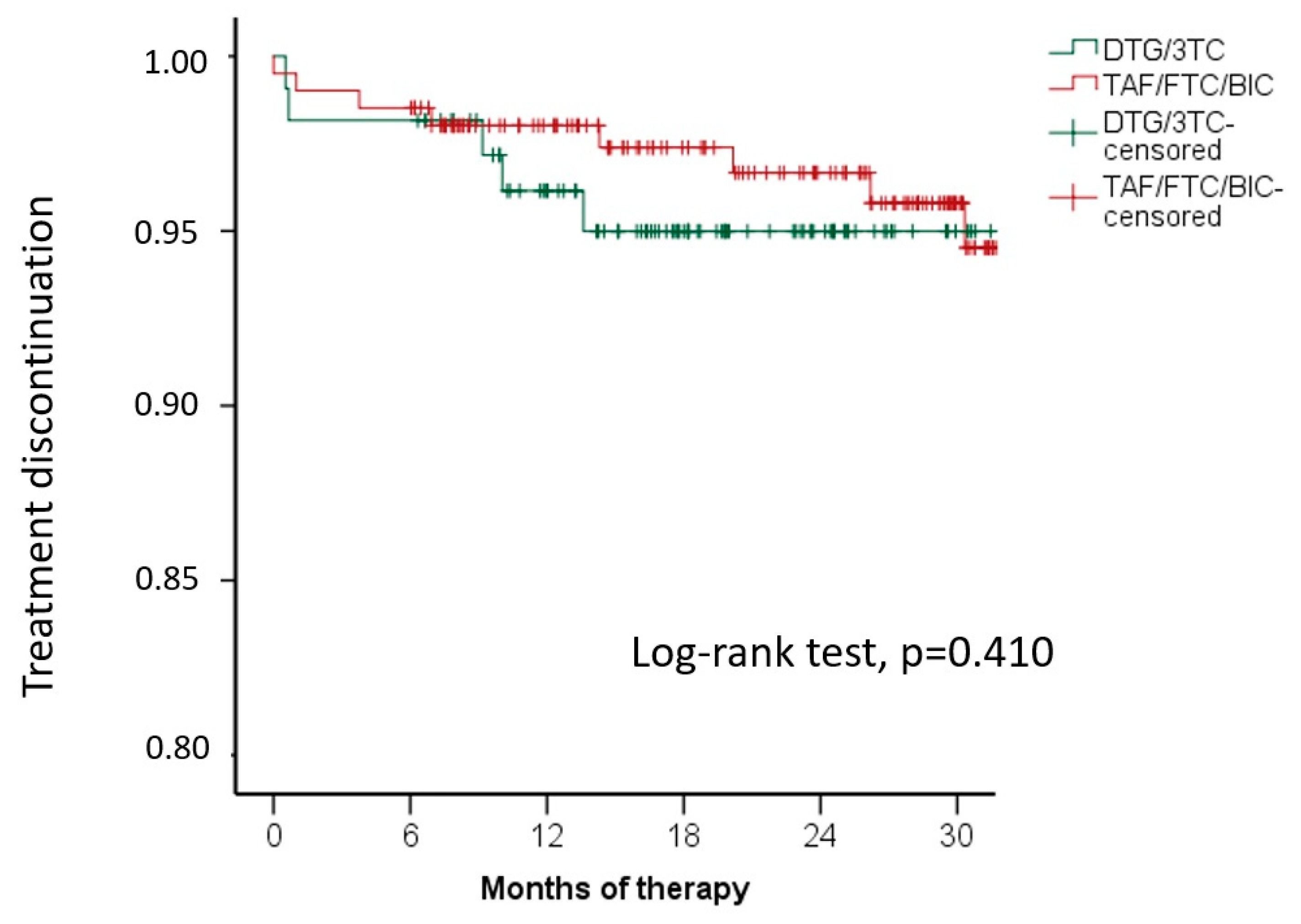
3.1. Treatment Discontinuation and Adverse Events
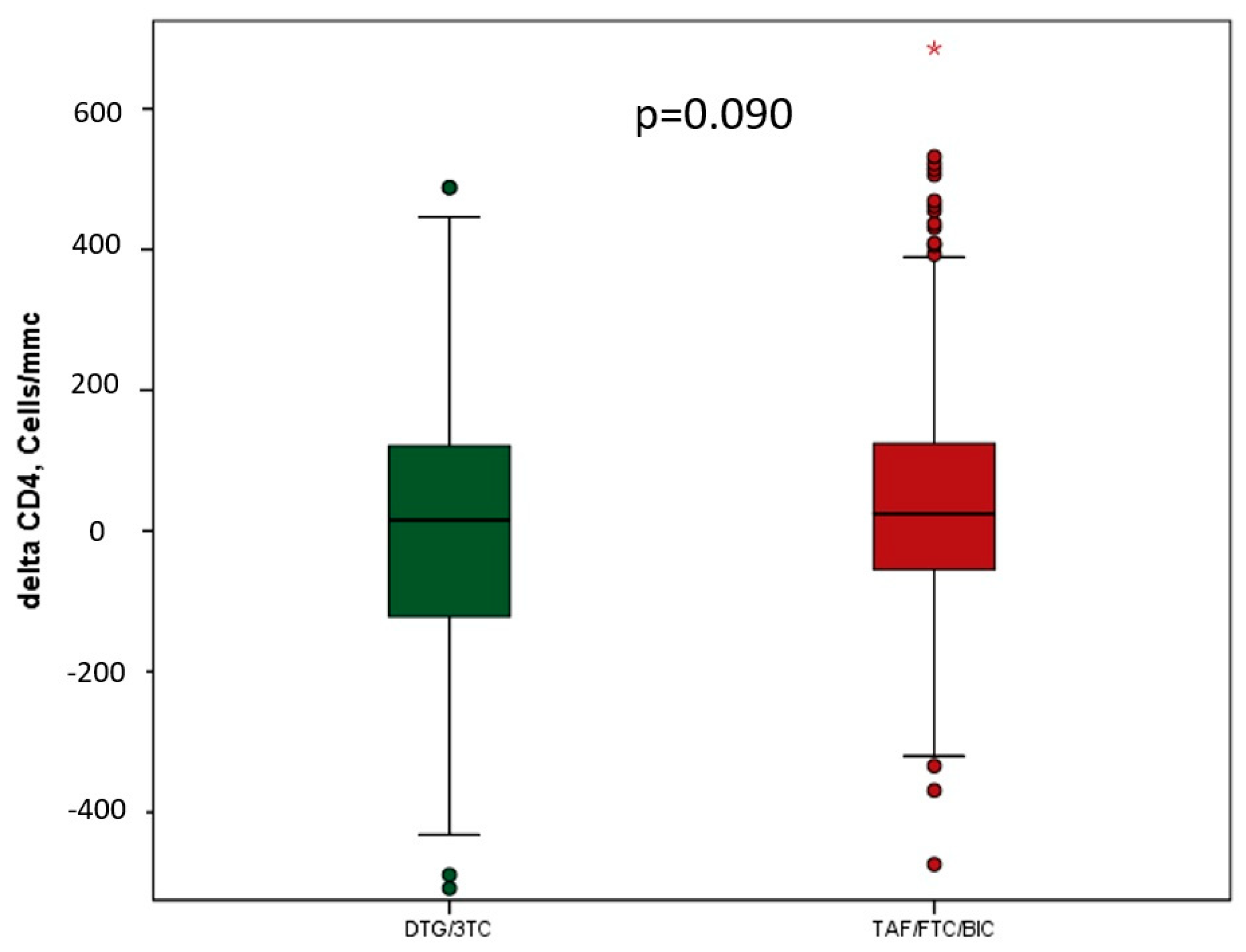
3.2. Effectiveness
3.3. Metabolic Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryom, L.; De Miguel, R.; Cotter, A.G.; Podlekareva, D.; Beguelin, C.; Waalewijn, H.; Arribas, J.R.; Mallon, P.W.G.; Marzolini, C.; Kirk, O.; et al. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022, 23, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Panel on Antiretroviral Guidelines for Adults Adolescents Guidelines for the Use of Antiretroviral Agents in Adults Adolescents with HIV. Department of Health and Human Services. 2023. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv (accessed on 14 December 2023).
- WHO Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 15 June 2023).
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA 2022, 329, 63–84. [Google Scholar] [CrossRef]
- Documento de Consenso de GeSIDA/División de Control de VIH, ITS, Hepatitis Virales y Tuberculosis del Ministerio de Sanidad Respecto al Tratamiento Antirretroviral en Adultos Infectados por el Virus de la Inmunodeficiencia Humana (Actualización enero 2023), GeSIDA 2023. Available online: https://gesida-seimc.org/wp-content/uploads/2023/06/Guia_TAR_V12.pdf (accessed on 15 June 2023).
- Wohl, D.A.; Yazdanpanah, Y.; Baumgarten, A.; Clarke, A.; Thompson, M.A.; Brinson, C.; Hagins, D.; Ramgopal, M.N.; Antinori, A.; Wei, X.; et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: Week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019, 6, e355–e363. [Google Scholar] [CrossRef] [PubMed]
- Orkin, C.; DeJesus, E.; Sax, P.E.; Arribas, J.R.; Gupta, S.K.; Martorell, C.; Stephens, J.L.; Stellbrink, H.J.; Wohl, D.; Maggiolo, F.; et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: Week 144 results from two randomised, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV 2020, 7, e389–e400. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs 2018, 78, 1817–1828, Erratum in Drugs 2019, 79, 687. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, B.; Fabbiani, M.; Di Carlo, D.; Incardona, F.; Abecasis, A.; Gomes, P.; Geretti, A.M.; Seguin-Devaux, C.; Garcia, F.; Kaiser, R.; et al. EuResist Network, INTEGRATE study group. Effectiveness of integrase strand transfer inhibitors in HIV-infected treatment-experienced individuals across Europe. HIV Med. 2022, 23, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649–1676. [Google Scholar] [CrossRef] [PubMed]
- Cahn, P.; Sierra Madero, J.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy–naive adults with HIV-1 infection. AIDS 2022, 36, 39–48. [Google Scholar] [CrossRef]
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment-Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials. J. Acquir. Immune Defic. Syndr. 2020, 83, 310–318, Erratum in J. Acquir. Immune Defic. Syndr. 2020, 84, e21. [Google Scholar] [CrossRef]
- Llibre, J.M.; Pulido, F.; García, F.; García Deltoro, M.; Blanco, J.L.; Delgado, R. Genetic barrier to resistance for dolutegravir. AIDS Rev. 2015, 17, 56–64. [Google Scholar]
- Gibas, K.M.; Kelly, S.G.; Arribas, J.R.; Cahn, P.; Orkin, C.; Daar, E.S.; Sax, P.E.; Taiwo, B.O. Two-drug regimens for HIV treatment. Lancet HIV 2022, 9, e868–e883. [Google Scholar] [CrossRef] [PubMed]
- Osiyemi, O.; De Wit, S.; Ajana, F.; Bisshop, F.; Portilla, J.; Routy, J.P.; Wyen, C.; Ait-Khaled, M.; Leone, P.; Pappa, K.A.; et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Versus Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Results Through Week 144 From the Phase 3, Noninferiority TANGO Randomized Trial. Clin. Infect. Dis. 2022, 75, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Llibre, J.M.; Brites, C.; Cheng, C.Y.; Osiyemi, O.; Galera, C.; Hocqueloux, L.; Maggiolo, F.; Degen, O.; Taylor, S.; Blair, E.; et al. Efficacy and Safety of Switching to the 2-Drug Regimen Dolutegravir/Lamivudine versus Continuing a 3- or 4-Drug Regimen for Maintaining Virologic Suppression in Adults Living With Human Immunodeficiency Virus 1 (HIV-1): Week 48 Results From the Phase 3, Noninferiority SALSA Randomized Trial. Clin. Infect. Dis. 2023, 76, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Serra, A.; De Lazzari, E.; Berrocal, L.; Foncillas, A.; De La Mora, L.; Inciarte, A.; Chivite, I.; González-Cordón, A.; Martínez-Rebollar, M.; Torres, B.; et al. Clinical use and effectiveness of dolutegravir and lamivudine: A long-term, real-world, retrospective study. J. Antimicrob. Chemother. 2023, 78, 1955–1962. [Google Scholar] [CrossRef]
- Patel, R.; Evitt, L.; Mariolis, I.; Di Giambenedetto, S.; d’Arminio Monforte, A.; Casado, J.; Cabello Úbeda, A.; Hocqueloux, L.; Allavena, C.; Barber, T.; et al. HIV Treatment with the Two-Drug Regimen Dolutegravir Plus Lamivudine in Real-world Clinical Practice: A Systematic Literature Review. Infect. Dis. Ther. 2021, 10, 2051–2070. [Google Scholar] [CrossRef]
- Bowman, C.; Ambrose, A.; Kanitkar, T.; Flores, K.; Simoes, P.; Hart, J.; Hunter, A.; Akodu, J.; Barber, T.J. Real world use of dolutegravir two drug regimens. AIDS 2023, 37, 785–788, Erratum in AIDS 2023, 37, 1019. [Google Scholar] [CrossRef]
- Suárez-García, I.; Alejos, B.; Hernando, V.; Viñuela, L.; Vera García, M.; Rial-Crestelo, D.; Pérez Elías, M.J.; Albendín Iglesias, H.; Peraire, J.; Tiraboschi, J.; et al. Effectiveness and tolerability of dolutegravir/lamivudine for the treatment of HIV-1 infection in clinical practice. J. Antimicrob. Chemother. 2023, 78, 1423–1432. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Y.; Xie, X.; Gan, L.; Song, C.; Song, Y.; Li, J.; Long, H. Real-world implementation of dolutegravir plus lamivudine in people living with HIV in Southwest China. Expert Rev. Anti-Infect. Ther. 2022, 20, 1501–1508. [Google Scholar] [CrossRef]
- Cento, V.; Perno, C.F. Dolutegravir Plus Lamivudine Two-Drug Regimen: Safety, Efficacy and Diagnostic Considerations for Its Use in Real-Life Clinical Practice-A Refined Approach in the COVID-19 Era. Diagnostics 2021, 11, 809. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.; Lee, J.A.; Kim, C.H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.S.; Song, Y.G.; et al. Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea. Viruses 2022, 14, 2558. [Google Scholar] [CrossRef]
- Buzón, L.; Dueñas, C.; Pedrero, R.; Iribarren, J.A.; de Los Santos, I.; Díaz de Santiago, A.; Morán, M.Á.; Pousada, G.; Moreno, E.; Ferreira, E.; et al. Dolutegravir Plus 3TC in Virologically Suppressed PLWHIV: Immunological Outcomes in a Multicenter Retrospective Cohort in Spain during the COVID-19 Pandemic. Viruses 2023, 15, 322. [Google Scholar] [CrossRef]
- Borghetti, A.; Baldin, G.; Lombardi, F.; Ciccullo, A.; Capetti, A.; Rusconi, S.; Sterrantino, G.; Latini, A.; Cossu, M.V.; Gagliardini, R.; et al. Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med. 2018, 19, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Punekar, Y.S.; Parks, D.; Joshi, M.; Kaur, S.; Evitt, L.; Chounta, V.; Radford, M.; Jha, D.; Ferrante, S.; Sharma, S.; et al. Effectiveness and safety of dolutegravir two-drug regimens in virologically suppressed people living with HIV: A systematic literature review and meta-analysis of real-world evidence. HIV Med. 2021, 22, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Armenia, D.; Teyssou, E.; Santos, J.R.; Charpentier, C.; Lambert-Niclot, S.; Antinori, A.; Katlama, C.; Descamps, D.; Perno, C.F.; et al. Virological efficacy of switch to DTG plus 3TC in a retrospective observational cohort of suppressed HIV-1 patients with or without past M184V: The LAMRES study. J. Glob. Antimicrob. Resist. 2022, 31, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Gulminetti, R.; Pagnucco, L.; Digaetano, M.; Cervo, A.; Valenti, D.; Callegaro, A.; Mussini, C. Long-term outcome of lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2022, 22, 782. [Google Scholar] [CrossRef] [PubMed]
- Rolle, C.P.; Nguyen, V.; Patel, K.; Cruz, D.; DeJesus, E.; Hinestrosa, F. Real-world efficacy and safety of switching to bictegravir/emtricitabine/tenofovir alafenamide in older people living with HIV. Medicine 2021, 100, e27330. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, R.; Florence, E.; Yombi, J.C.; Henrard, S.; Darcis, G.; Van Praet, J.; Vandekerckhove, L.; Allard, S.D.; Demeester, R.; Messiaen, P.; et al. Efficacy, durability, and tolerability of bictegravir/emtricitabine/tenofovir alafenamide for the treatment of HIV in a real-world setting in Belgium. HIV Med. 2023, 24, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Sun, H.Y.; Chen, C.P.; Lee, C.H.; Lee, C.Y.; Liu, C.E.; Tang, H.J.; Hung, T.C.; Li, C.W.; Lee, Y.T.; et al. Switching to coformulated bictegravir, emtricitabine, and tenofovir alafenamide maintained viral suppression in adults with historical virological failures and K65N/R mutation. Int. J. Infect. Dis. 2023, 126, 39–47. [Google Scholar] [CrossRef]
- Hoffmann, C.; Schewe, K.; Fenske, S.; Buhk, T.; Sabranski, M.; Adam, A.; Hansen, S.; Stellbrink, H.J. Short-term neuropsychiatric tolerability of bictegravir combined with emtricitabine/tenofovir alafenamide in clinical practice. Antivir Ther. 2020, 25, 83–90. [Google Scholar] [CrossRef]
- Chang, H.M.; Chou, P.Y.; Chou, C.H.; Tsai, H.C. Outcomes After Switching to BIC/FTC/TAF in Patients with Virological Failure to Protease Inhibitors or Non-Nucleoside Reverse Transcriptase Inhibitors: A Real-World Cohort Study. Infect Drug Resist. 2021, 14, 4877–4886. [Google Scholar] [CrossRef]
- D’Arminio Monforte, A.; Tavelli, A.; Cingolani, A.; Taramasso, L.; Mussini, C.; Piconi, S.; Calcagno, A.; Orofino, G.; Cicalini, S.; Castagna, A.; et al. Effectiveness of bictregravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) as switch strategy in virologically suppressed: Real-world data from the ICONA cohort. In Proceedings of the HIV Glasgow 2022, Glasgow, UK, 23–26 October 2022. [Google Scholar]
- Baldin, G.; Ciccullo, A.; Lombardi, F.; D’Angelillo, A.; Dusina, A.; Emiliozzi, A.; Farinacci, D.; Moschese, D.; Picarelli, C.; Borghetti, A.; et al. Short Communication: Comparing Lamivudine+Dolutegravir and Bictegravir/Emtricitabine/Tenofovir Alafenamide as Switch Strategies: Preliminary Results from Clinical Practice. AIDS Res. Hum. Retroviruses 2021, 37, 429–432. [Google Scholar] [CrossRef]
- Rocabert, A.; Borjabad, B.; Berrocal, L.; Blanch, J.; Inciarte, A.; Chivite, I.; Gonzalez-Cordon, A.; Torres, B.; Ambrosioni, J.; Martinez-Rebollar, M.; et al. Tolerability of bictegravir/tenofovir alafenamide/emtricitabine versus dolutegravir/lamivudine as maintenance therapy in a real-life setting. J. Antimicrob. Chemother. 2023, 78, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Xie, X.; Fu, Y.; Yang, X.; Ma, S.; Kong, L.; Song, C.; Song, Y.; Ren, T.; Long, H. Bictegravir/Emtricitabine/Tenofovir Alafenamide Versus Dolutegravir Plus Lamivudine for Switch Therapy in Patients with HIV-1 Infection: A Real-World Cohort Study. Infect Dis. Ther. 2023, 12, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Knobel, H.; Cañas-Ruano, E.; Guelar, A.; Knobel, P.; Villar-García, J.; González-Mena, A.; Canepa, C.; Arrieta-Aldea, I.; Marcos, A.; Abalat-Torrres, A.; et al. Switching to Dolutegravir/lamivudine or Bictegravir/Emtricitabine/Tenofovir alafenamide. A comparative real-world study. HIV Res. Clin. Pract. 2023, 24, 2239564. [Google Scholar] [PubMed]
- Molina, J.M.; Ward, D.; Brar, I.; Mills, A.; Stellbrink, H.J.; López-Cortés, L.; Ruane, P.; Podzamczer, D.; Brinson, C.; Custodio, J.; et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e357–e365, Erratum in Lancet HIV 2018, 5, e545. [Google Scholar] [CrossRef] [PubMed]
- Daar, E.S.; DeJesus, E.; Ruane, P.; Crofoot, G.; Oguchi, G.; Creticos, C.; Rockstroh, J.K.; Molina, J.M.; Koenig, E.; Liu, Y.P.; et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e347–e356. [Google Scholar] [CrossRef] [PubMed]
- Kityo, C.; Hagins, D.; Koenig, E.; Avihingsanon, A.; Chetchotisakd, P.; Supparatpinyo, K.; Gankina, N.; Pokrovsky, V.; Voronin, E.; Stephens, J.L.; et al. Switching to Fixed-Dose Bictegravir, Emtricitabine, and Tenofovir Alafenamide (B/F/TAF) in Virologically Suppressed HIV-1 Infected Women: A Randomized, Open-Label, Multicenter, Active-Controlled, Phase 3, Noninferiority Trial. J. Acquir. Immune Defic. Syndr. 2019, 82, 321–328. [Google Scholar] [CrossRef]
- Tang, M.W.; Liu, T.F.; Shafer, R.W. The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology 2012, 55, 98–101. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Cortés, L.L.; Gutiérrez, A.; Santos, J.; Omar, M.; Gálvez, C.; Sequera, S.; Jesús, S.E.; Téllez, F.; Fernández, E.; et al. DOLAMA study: Effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients. Medicine 2019, 98, e16813. [Google Scholar] [CrossRef]
- Joly, V.; Burdet, C.; Landman, R.; Vigan, M.; Charpentier, C.; Katlama, C.; Cabié, A.; Benalycherif, A.; Peytavin, G.; Yeni, P.; et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: Results of the ANRS 167 trial (LAMIDOL). J. Antimicrob. Chemother. 2019, 74, 739–745. [Google Scholar] [CrossRef]
- Wandeler, G.; Buzzi, M.; Anderegg, N.; Sculier, D.; Béguelin, C.; Egger, M.; Calmy, A. Virologic failure and HIV drug resistance on simplified, dolutegravir-based maintenance therapy: Systematic review and meta-analysis. F1000Research 2018, 7, 1359. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valero, I.; Corona, D.; Martínez, N.; López-Cavanillas, M.; Lluis, C.; Luque, I. Real-world discontinuations due to neuropsychiatric symptoms in people living with HIV treated with second-generation integrase inhibitors: A systematic review. Expert Rev. Anti-Infect. Ther. 2023, 21, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Llibre, J.M. Neuropsychiatric adverse events with dolutegravir and other integrase strand transfer inhibitors. AIDS Rev. 2019, 21, 4–10. [Google Scholar] [CrossRef]
- DeGroote, S.; Vanherrewage, S.; Tobback, E.; Caluwé, E.; Vincke, L.; Blomme, E.; Vandekerckhove, L.; De Scheerder, M.A. Understanding changes in metabolic parameters switching to 2DR from 3DR Integrase Strand Inhibitors (InSTIs). In Proceedings of the HIV Glasgow 2022, Glasgow, UK, 23–26 October 2022. [Google Scholar]
- Heseltine, T.; Hughes, E.; Mathew, J.; Murray, S.; Khoo, S. The effect of changing to Bictegravir on lipids using real world data: A brief report. J. Clin. Pharm. Ther. 2022, 47, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; Bonfanti, P.; Ricci, E.; Maggi, P.; Orofino, G.; Squillace, N.; Menzaghi, B.; Madeddu, G.; Molteni, C.; Vichi, F.; et al. Metabolic syndrome and body weight in people living with HIV infection: Analysis of differences observed in three different cohort studies over a decade. HIV Med. 2022, 23, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Lv, S.; Wu, H.; Dai, L. Effects of different integrase strand transfer inhibitors on body weight in patients with HIV/AIDS: A network meta-analysis. BMC Infect Dis. 2022, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Hester, E.K.; Greenlee, S.; Durham, S.H. Weight Changes With Integrase Strand Transfer Inhibitor Therapy in the Management of HIV Infection: A Systematic Review. Ann. Pharmacother. 2022, 56, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Bansi-Matharu, L.; Phillips, A.; Oprea, C.; Grabmeier-Pfistershammer, K.; Günthard, H.F.; De Wit, S.; Guaraldi, G.; Vehreschild, J.J.; Wit, F.; Law, M.; et al. Contemporary antiretrovirals and body-mass index: A prospective study of the RESPOND cohort consortium. Lancet HIV 2021, 8, e711–e722. [Google Scholar] [CrossRef]
- Kileel, E.M.; Malvestutto, C.D.; Lo, J.; Fitch, K.V.; Fichtenbaum, C.J.; Aberg, J.A.; Zanni, M.V.; Martinez, E.; Okeke, N.L.; Kumar, P.; et al. Changes in body mass index with Longer-term Integrase Inhibitor Use: A Longitudinal Analysis of integrase inhibitors: A longitudinal analysis of data from the randomised trial to Prevent Vascular Events in Human Immunodeficiency Virus (REPRIEVE). Clin. Infect. Dis. 2023, 76, 2010–2013. [Google Scholar] [CrossRef]
- Sax, P.E.; JEron, J.; Radtchenko, J.; Dunbar, M.; JGruber, J.; Fridman, M.; Ramgopal, M.; Mounzer, K.; Huhn, G.; Santiago, S.; et al. What influences switching to DTG/3TC vs. B/F/TAF in clinical practice? In Proceedings of the CROI 2023, Seattle, WA, USA, 19–22 February 2023. [Google Scholar]

| Characteristics | 3TC/DTG n = 110 | BIC/FTC/TAF n = 214 | Total n = 324 | p-Value |
|---|---|---|---|---|
| Age, years | 56.0 | 56.5 | 56.3 | 0.653 |
| Median (IQR) | (44.0–63.0) | (48.0–61.5) | (45.9–61.8) | |
| Sex at birth, n (%) | ||||
| Male | 82 (74.5%) | 172 (80.4%) | 254 (78.4%) | 0.227 |
| Female | 28 (25.5%) | 42 (19.6%) | 70 (21.6%) | |
| Nationality, n (%) | ||||
| Italian | 90 (81.8%) | 171 (79.9%) | 261 (80.6%) | 0.681 |
| Non-italian | 20 (18.2%) | 43 (20.1%) | 63 (19.4%) | |
| Sexual orientation, n (%) | ||||
| Heterosexual | 23 (15%) | 57 (37.3%) | 80 (52.3%) | 0.241 |
| MSM | 15 (39.5%) | 58 (50.4%) | 73 (47.7%) | |
| BMI, kg/m2 | 25.6 | 25.7 | 25.6 | 0.770 |
| Median (IQR) | (23.4–28.4) | (23.4–28.6) | (23.4–28.5) | |
| Comorbidities, number Median (IQR) | 2 (1.0–5.0) | 1 (0–2.0) | 1 (0–3.0) | <0.0001 |
| Systemic arterial hypertension | 44 (40%) | 56 (26.2%) | 100 (30.9%) | 0.011 |
| Cardiovascular disease | 26 (23.6%) | 13 (6.1%) | 39 (12%) | <0.0001 |
| Dyslipidaemia | 70 (63.6%) | 65 (30.4%) | 135 (41.7%) | <0.0001 |
| Obesity | 25 (22.7%) | 26 (12.1%) | 51 (15.7%) | 0.013 |
| Chronic kidney disease | 19 (17.3%) | 10 (4.7%) | 29 (9%) | <0.0001 |
| Diabetes mellitus | 15 (13.6%) | 26 (12.1%) | 41 (12.7%) | 0.703 |
| Non-AIDS-defining malignancy | 6 (5.5%) | 13 (6.1%) | 19 (5.9%) | 0.822 |
| HBV co-infection, n (%) | 0 (0%) | 7 (3.3%) | 7 (2.2%) | 0.055 |
| HCV co-infection, n (%) | 12 (10.9%) | 38 (17.8%) | 50 (15.4%) | 0.106 |
| Prior AIDS defining illness, n (%) | 22 (20%) | 60 (28.2%) | 82 (25.4%) | 0.110 |
| Nadir CD4+ T-cell count (cells/μL), | 297.0 | 214.0 | 261.0 | 0.015 |
| Median (IQR) | (172.0–473.0) | (59.0–365.0) | (70.0–406.0) | |
| No drug resistance, n (%) | 95 (94.1%) | 102 (65.8%) | 197 (77%) | |
| Total time on previous cART (months), | 132.0 | 120.0 | 122.0 | 0.108 |
| Median (IQR) | (72.0–228.0) | (72.0–180.0) | (72.0–192.0) | |
| Time of virologic suppression prior to switch (months), | 98.1 | 80.5 | 84.4 | 0.035 |
| Median (IQR) | (57.0–151.0) | (39.5–138.7) | (45.7–142.2) | |
| Previous treatments, n (%) | ||||
| INI | 78 (70.9%) | 172 (80.4%) | 250 (77.2%) | 0.055 |
| PI | 13 (11.8%) | 32 (15.0%) | 45 (13.9%) | 0.440 |
| NNRTI | 26 (23.6%) | 14 (6.5%) | 40 (12.3%) | <0.0001 |
| TAF-based | 45 (40.9%) | 157 (73.4%) | 202 (62.3%) | <0.0001 |
| ABC-based | 50 (45.5%) | 23 (10.7%) | 73 (22.5%) | <0.0001 |
| 3TC/DTG n = 110 | BIC/FTC/TAF n = 214 | Total n = 324 | p-Value | |
|---|---|---|---|---|
| Time of follow-up, months | 19.6 | 27.5 | 24.6 | 0.001 |
| Median (IQR) | (14.2–26.4) | (15.3–32.6) | (14.7–31.4) | |
| Treatment outcomes, n (%) On therapy Discontinuations due to: | 103 (93.6%) | 193 (90.2%) | 296 (91.3%) | 0.295 |
| Failure | 0 (0%) | 0 (0%) | 0 (0%) | |
| Low level viremia | 2 (1.8%) | 2 (0.9%) | 4 (1.2%) | 0.495 |
| Blip | 5 (4.5%) | 25 (11.8%) | 30 (9.3%) | 0.033 |
| Death | 0 (0%) | 3 (1.4%) | 3 (0.9%) | 0.212 |
| Switch | 1 (0.9%) | 11 (5.1%) | 12 (3.7%) | 0.056 |
| Adverse events | 4 (3.6 %) | 5 (2.3%) | 9 (2.8%) | 0.500 |
| Cutaneous | 0 (0%) | 1 (20%) | 1 (11.1%) | |
| Neurological | 1 (25%) | 1 (20%) | 2 (22.2%) | |
| Musculoskeletal | 3 (75%) | 2 (40%) | 4 (44.4%) | |
| Renal | 0 (0%) | 1 (20%) | 2 (22.2%) | |
| CD4+ cell count (cells/μL) baseline, n (%) | 781.5 | 585.0 | 662.0 | <0.0001 |
| Median (IQR) | (598.0–956.0) | (430.0–841.0) | (491.0–892.0) | |
| CD4+ cell count (cells/μL) last control, n (%) | 762 | 651.0 | 693.0 | 0.002 |
| Median (IQR) | (580.0–1014.0) | (473.0–904.0) | (517.0–950.0) | |
| HIV-RNA ˂ 50 cells/μL last control, n (%) | 109 (99.1%) | 205 (97.2%) | 314 (97.8%) | 0.260 |
| Delta Triglycerides last control-baseline, mg/dL Median (IQR) | −11 (−48, 13) | −14 (−52, 12) | −13 (−50, 12) | 0.775 |
| Delta LDL last control-baseline, mg/dL Median (IQR) | −2.3 (−29.1, 16) | −1 (−22, 15.8) | −1.5 (−23.4, 15.8) | 0.682 |
| Delta Cholesterol last control-baseline, mg/dL Median (IQR) | −2 (−36, 20) | −7 (−26, 13) | −6 (−28, 14) | 0.767 |
| Delta BMI last control-baseline, kg/m2 Median (IQR) | 0.0 (0.9, 0.8) | 0.0 (−0.9, 0,7) | 0.0 (−0.9, 0.7) | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Socio, G.V.; Tordi, S.; Altobelli, D.; Gidari, A.; Zoffoli, A.; Francisci, D. Dolutegravir/Lamivudine versus Tenofovir Alafenamide/Emtricitabine/Bictegravir as a Switch Strategy in a Real-Life Cohort of Virogically Suppressed People Living with HIV. J. Clin. Med. 2023, 12, 7759. https://doi.org/10.3390/jcm12247759
De Socio GV, Tordi S, Altobelli D, Gidari A, Zoffoli A, Francisci D. Dolutegravir/Lamivudine versus Tenofovir Alafenamide/Emtricitabine/Bictegravir as a Switch Strategy in a Real-Life Cohort of Virogically Suppressed People Living with HIV. Journal of Clinical Medicine. 2023; 12(24):7759. https://doi.org/10.3390/jcm12247759
Chicago/Turabian StyleDe Socio, Giuseppe Vittorio, Sara Tordi, Debora Altobelli, Anna Gidari, Anastasia Zoffoli, and Daniela Francisci. 2023. "Dolutegravir/Lamivudine versus Tenofovir Alafenamide/Emtricitabine/Bictegravir as a Switch Strategy in a Real-Life Cohort of Virogically Suppressed People Living with HIV" Journal of Clinical Medicine 12, no. 24: 7759. https://doi.org/10.3390/jcm12247759
APA StyleDe Socio, G. V., Tordi, S., Altobelli, D., Gidari, A., Zoffoli, A., & Francisci, D. (2023). Dolutegravir/Lamivudine versus Tenofovir Alafenamide/Emtricitabine/Bictegravir as a Switch Strategy in a Real-Life Cohort of Virogically Suppressed People Living with HIV. Journal of Clinical Medicine, 12(24), 7759. https://doi.org/10.3390/jcm12247759







