Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge
Abstract
:1. Introduction
Methodological Consideration
2. ICI Induced Cardiac Adverse Events
3. ICI-Associated Myocarditis
3.1. Clinical Presentation of ICI-Associated Myocarditis
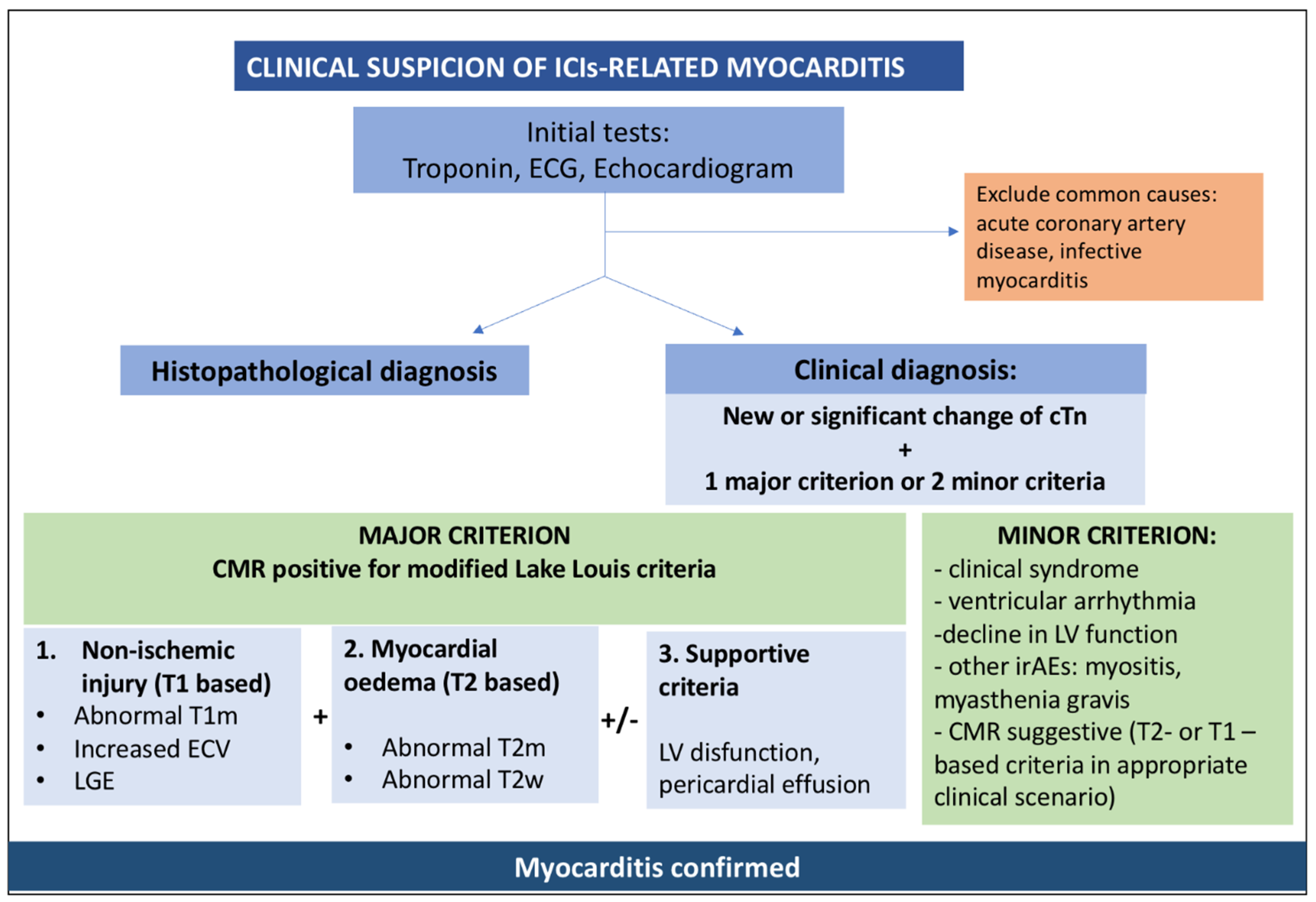
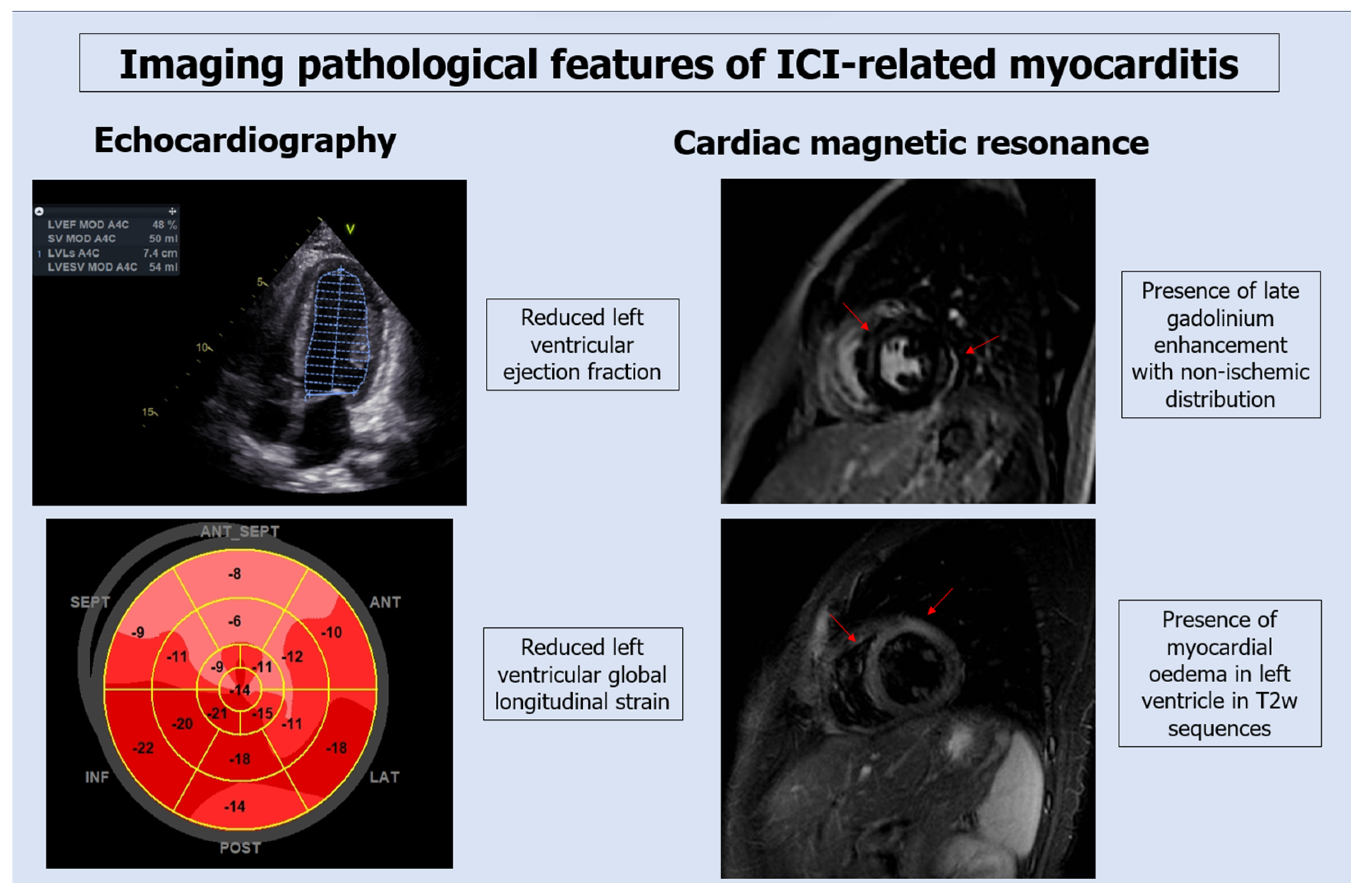
3.2. Diagnostic Testing for ICI-Associated Myocarditis
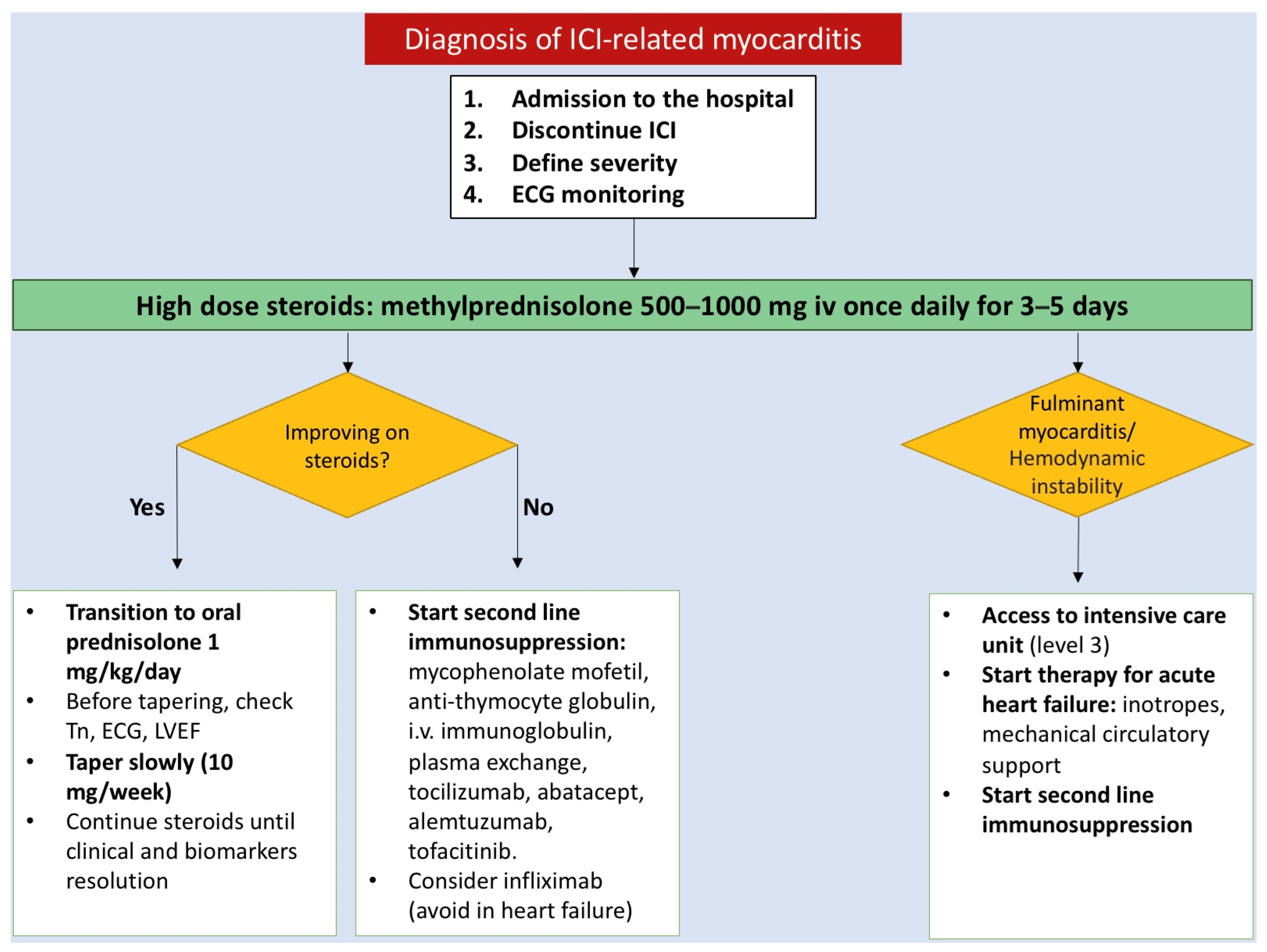
3.3. Treatment
3.4. Outcomes
3.5. Relapse and Therapy Rechallenge
3.6. Review of Case Reports on Rechallenge after ICI Myocarditis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zlotoff, D.A.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zubiri, L.; Chen, C.L.; Sullivan, R.J.; et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor–Associated Myocarditis. Circulation 2020, 141, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Liblik, K.; Gevaert, S. Immune checkpoint inhibitors—The revolutionary cancer immunotherapy comes with a cardiotoxic price. Trends Cardiovasc. Med. 2022, S1050173822001219. [Google Scholar] [CrossRef]
- Gan, L.; Liu, D.; Ma, Y.; Chen, X.; Dai, A.; Zhao, S.; Jin, X.; Gu, G. Cardiotoxicity associated with immune checkpoint inhibitors: Current status and future challenges. Front. Pharmacol. 2022, 13, 962596. [Google Scholar] [CrossRef] [PubMed]
- Suero-Abreu, G.A.; Zanni, M.V.; Neilan, T.G. Atherosclerosis with Immune Checkpoint Inhibitor Therapy. JACC CardioOncol. 2022, 4, 598–615. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef]
- Ball, S.; Ghosh, R.K.; Wongsaengsak, S.; Bandyopadhyay, D.; Ghosh, G.C.; Aronow, W.S.; Fonarow, G.C.; Lenihan, D.J.; Bhatt, D.L. Cardiovascular Toxicities of Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2019, 74, 1714–1727. [Google Scholar] [CrossRef]
- Nardi Agmon, I.; Itzhaki Ben Zadok, O.; Kornowski, R. The Potential Cardiotoxicity of Immune Checkpoint Inhibitors. JCM 2022, 11, 865. [Google Scholar] [CrossRef]
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune checkpoint inhibitor–associated myocarditis: Manifestations and mechanisms. J. Clin. Investig. 2021, 131, e145186. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Gao, J.; Zhao, S.; Li, Q.; Cui, Y.H.; Liu, Q.; Wu, D.; Wang, Y.; Jin, X.; Ji, Y.; et al. Nivolumab-associated DRESS in a genetic susceptible individual. J. Immunother. Cancer 2021, 9, e002879. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.H.; Toh, M.M.X.; Thian, Y.L.; Vellayappan, B.A.; Fairhurst, A.M.; Chan, Y.H.; Aminkeng, F.; Bharwani, L.D.; Huang, Y.; Mak, A.; et al. Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature. Front. Immunol. 2022, 13, 807050. [Google Scholar] [CrossRef] [PubMed]
- Radovaic, M.; Jevtic, D.; Calvin, A.D.; Petrovic, M.; Paulson, M.; Rueda Prada, L.; Sprecher, L.; Savic, I.; Dumic, I. “Heart in DRESS”: Cardiac Manifestations, Treatment and Outcome of Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Abitbol, J.; Vallet, A.; Routier, E.; Smaali, S.; Robert, C. Immune checkpoint inhibitors-associated myocarditis without cardiovascular symptoms. Eur. J. Cancer 2023, 194, 113319. [Google Scholar] [CrossRef]
- Xu, A.; Yuan, M.; Zhan, X.; Zhao, G.; Mu, G.; Wang, T.; Hu, H.; Fu, H. Early detection of immune checkpoint inhibitor-related subclinical cardiotoxicity: A pilot study by using speckle tracking imaging and three-dimensional echocardiography. Front. Cardiovasc. Med. 2022, 9, 1087287. [Google Scholar] [CrossRef]
- Tamura, Y.; Tamura, Y. Usefulness of Longitudinal Strain to Assess Cancer Therapy-Related Cardiac Dysfunction and Immune Checkpoint Inhibitor-Induced Myocarditis. Pharmaceuticals 2023, 16, 1297. [Google Scholar] [CrossRef]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.O.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Bonaca, M.P.; Cautela, J. What Is the Evidence of the Diagnostic Criteria and Screening of Immune Checkpoint Inhibitor–Induced Myocarditis? JACC CardioOncol. 2022, 4, 624–628. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. JAHA 2020, 9, e013757. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Cadour, F.; Cautela, J.; Rapacchi, S.; Varoquaux, A.; Habert, P.; Arnaud, F.; Jacquier, A.; Meilhac, A.; Paganelli, F.; Lalevée, N.; et al. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor–induced Myocarditis. Radiology 2022, 303, 512–521. [Google Scholar] [CrossRef]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-ín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Moslehi, J.; Salem, J.E. Immune Checkpoint Inhibitor Myocarditis Treatment Strategies and Future Directions. JACC CardioOncol. 2022, 4, 704–707. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Y.; Xu, L. Drug therapy for myocarditis induced by immune checkpoint inhibitors. Front. Pharmacol. 2023, 14, 1161243. [Google Scholar] [CrossRef]
- Cautela, J.; Zeriouh, S.; Gaubert, M.; Bonello, L.; Laine, M.; Peyrol, M.; Paganelli, F.; Lalevee, N.; Barlesi, F.; Thuny, F. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J. Immunother. Cancer 2020, 8, e001887. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Parikh, R.; Gunturu, K.S.; Tariq, R.Z.; Dani, S.S.; Ganatra, S.; Nohria, A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2021, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- de Filippi, C.; Barac, A. Cardiac Troponins for Diagnosis and Prognostic Assessment of Immune Checkpoint Inhibitor Myocarditis and Myositis: The Emerging Importance of Peripheral Vision. Circulation 2023, 148, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors after Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, G.; Zhang, Z.; Yang, L.; Liu, S.; Li, G.; Wang, H.; Huang, J.; Wang, S.; Li, N. Immune checkpoint inhibitor–associated myocarditis: A systematic analysis of case reports. Front. Immunol. 2023, 14, 1275254. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of Immune Therapy. Cardiol. Clin. 2019, 37, 385–397. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, A.; Zhang, R.; Fu, X.; Liu, N.; Shi, C.; Wang, J.; Zheng, X.; Tian, T.; Liang, X.; et al. Immune Checkpoint Inhibitor-Associated Cardiotoxicity in Solid Tumors: Real-World Incidence, Risk Factors, and Prognostic Analysis. Front. Cardiovasc. Med. 2022, 9, 882167. [Google Scholar] [CrossRef]
- Peleg Hasson, S.; Salwen, B.; Sivan, A.; Shamai, S.; Geva, R.; Merimsky, O.; Raphael, A.; Shmilovich, H.; Moshkovits, Y.; Kapusta, L.; et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin. Res. Cardiol. 2021, 110, 50–60. [Google Scholar] [CrossRef]
- Lee, D.H.; Armanious, M.; Huang, J.; Jeong, D.; Druta, M.; Fradley, M.G. Case of pembrolizumab-induced myocarditis presenting as torsades de pointes with safe re-challenge. J. Oncol. Pharm. Pract. 2020, 26, 1544–1548. [Google Scholar] [CrossRef]
- Menachery, S.M.; Hang, Y.; Pritchard, L.; Poklepovic, A.; Bottinor, W. Immune Checkpoint Inhibitor Rechallenge in a Patient with Previous Fulminant Myocarditis. Am. J. Cardiol. 2023, 199, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Eslinger, C.; Walden, D.; Barry, T.; Shah, S.; Samadder, N.J. Rechallenge with Switching Immune Checkpoint Inhibitors Following Autoimmune Myocarditis in a Patient with Lynch Syndrome. J. Natl. Compr. Cancer Netw. 2023, 21, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.A.; Gawinecka, J.; Dimitriou, F.; von Eckardstein, A.; Dummer, R.; Ruschitzka, F.; Matter, C.M. Value of troponin T versus I in the diagnosis of immune checkpoint inhibitor-related myocarditis and myositis: Rechallenge? ESC Heart Fail. 2023, 10, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, A.; Yang, Q.; Yu, J.; Li, G. Case report: A successful re-challenge report of GLS-010 (Zimberelimab), a novel fully humanized mAb to PD-1, in a case of recurrent endometrial cancer. Front. Immunol. 2022, 13, 987345. [Google Scholar] [CrossRef]
- Balanescu, D.V.; Donisan, T.; Palaskas, N.; Lopez-Mattei, J.; Kim, P.Y.; Buja, L.M.; McNamara, D.M.; Kobashigawa, J.A.; Durand, J.-B.; Iliescu, C.A. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: Pathway toward precision-based therapy. Cardiovasc. Pathol. 2020, 47, 107211. [Google Scholar] [CrossRef]
- Shen, L.; Chen, H.; Wei, Q. Immune-therapy-related toxicity events and dramatic remission after a single dose of pembrolizumab treatment in metastatic thymoma: A case report. Front. Immunol. 2021, 12, 621858. [Google Scholar] [CrossRef]
| Reference | Number of Patients Rechallenged | Grade of Myocarditis | Adverse Event after Rechallenging | ||
|---|---|---|---|---|---|
| Grade 1 | Grade 2–3 | Grade 4 | |||
| Xue Chen et al., 2022 [38] | 5 | 0 | 5 | 0 | 1 patient: grade 2 myocarditis 1 patient: cancer death after 1 year |
| Peleg Hasson S. et al., 2021 [39] | 3 | 1 | 2 | 0 | 1 patient: worsening cardiac symptoms |
| Dae Hyun Lee et al., 2020 [40] | 1 | 0 | 0 | 1 | None |
| Menachery Sherin M. et al., 2023 [41] | 1 | 0 | 0 | 1 | Cancer death after <1 year |
| Eslinger Cody et al., 2023 [42] | 1 | 0 | 0 | 1 | None |
| Rossi A. Valentina et al., 2023 [43] | 1 | 0 | 1 | 0 | Grade 1 myocarditis |
| Yeshan Chen et al., 2022 [44] | 1 | 0 | 1 | 0 | None |
| Dinu Valentin Balanescu et al., 2020 [45] | 2 | 0 | 2 | 0 | None |
| Shen et. al., 2021 [46] | 1 | 0 | 1 | 0 | Grade 2 myocarditis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frascaro, F.; Bianchi, N.; Sanguettoli, F.; Marchini, F.; Meossi, S.; Zanarelli, L.; Tonet, E.; Serenelli, M.; Guardigli, G.; Campo, G.; et al. Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. J. Clin. Med. 2023, 12, 7737. https://doi.org/10.3390/jcm12247737
Frascaro F, Bianchi N, Sanguettoli F, Marchini F, Meossi S, Zanarelli L, Tonet E, Serenelli M, Guardigli G, Campo G, et al. Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. Journal of Clinical Medicine. 2023; 12(24):7737. https://doi.org/10.3390/jcm12247737
Chicago/Turabian StyleFrascaro, Federica, Nicola Bianchi, Federico Sanguettoli, Federico Marchini, Sofia Meossi, Luca Zanarelli, Elisabetta Tonet, Matteo Serenelli, Gabriele Guardigli, Gianluca Campo, and et al. 2023. "Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge" Journal of Clinical Medicine 12, no. 24: 7737. https://doi.org/10.3390/jcm12247737
APA StyleFrascaro, F., Bianchi, N., Sanguettoli, F., Marchini, F., Meossi, S., Zanarelli, L., Tonet, E., Serenelli, M., Guardigli, G., Campo, G., Calabrò, L., & Pavasini, R. (2023). Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. Journal of Clinical Medicine, 12(24), 7737. https://doi.org/10.3390/jcm12247737








