Extrapulmonary Neuroendocrine Carcinomas: Current Management and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. GEP-NECs
3.1. Epidemiology and Staging
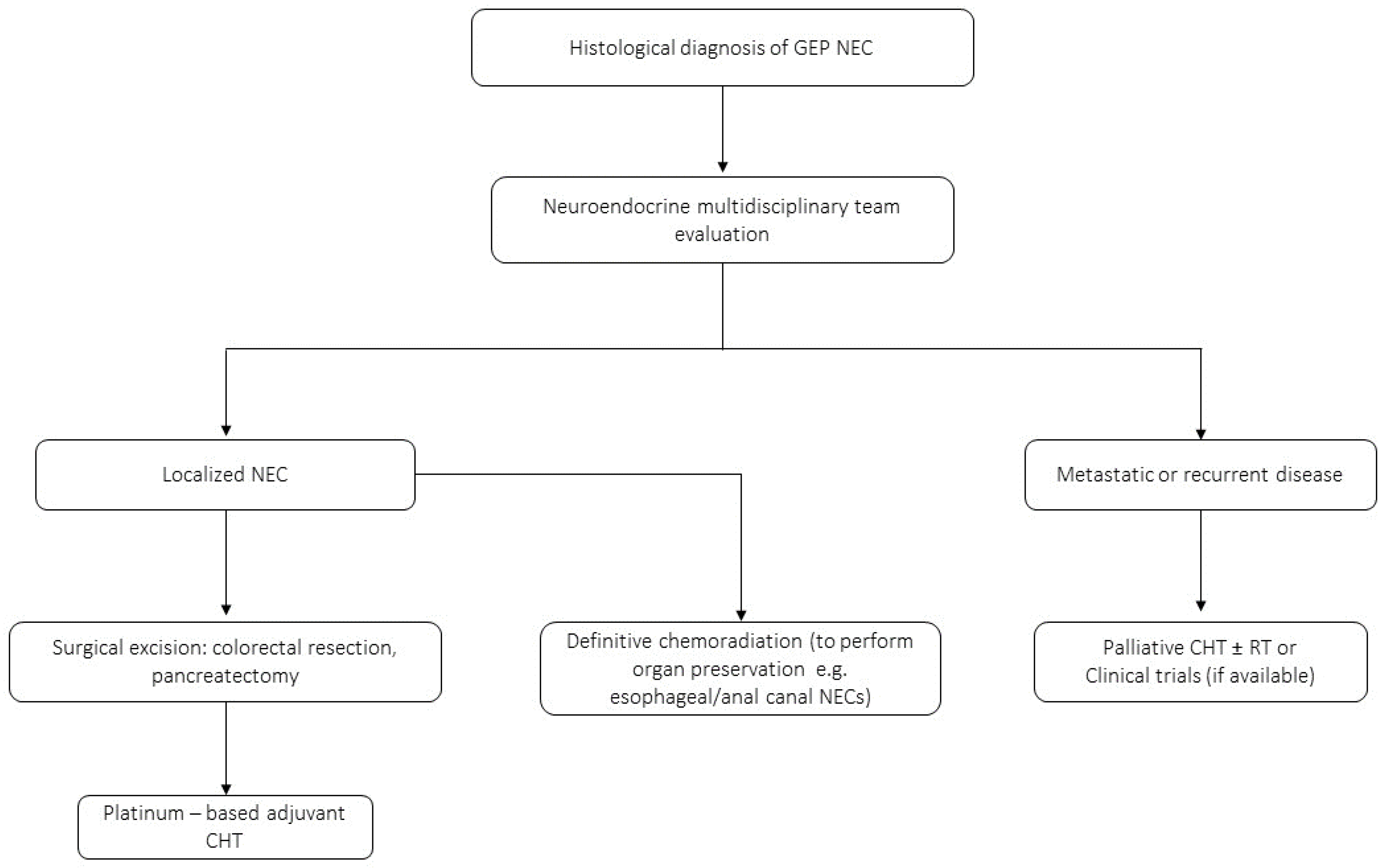
3.2. Management of GEP-NECs
4. GY-NECs
4.1. Epidemiology and Staging
4.2. NECs of the Cervix
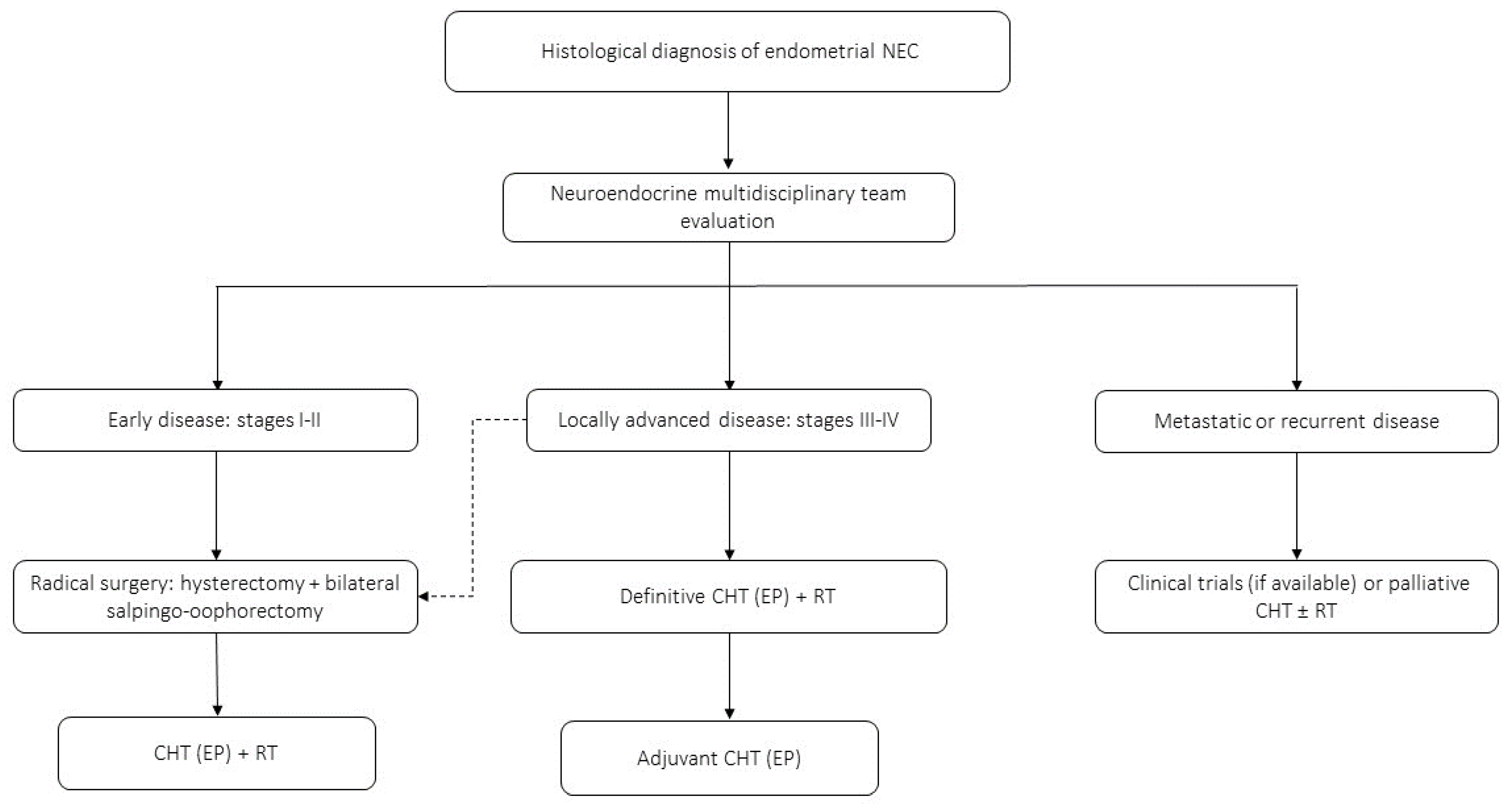
4.3. NECs of the Endometrium
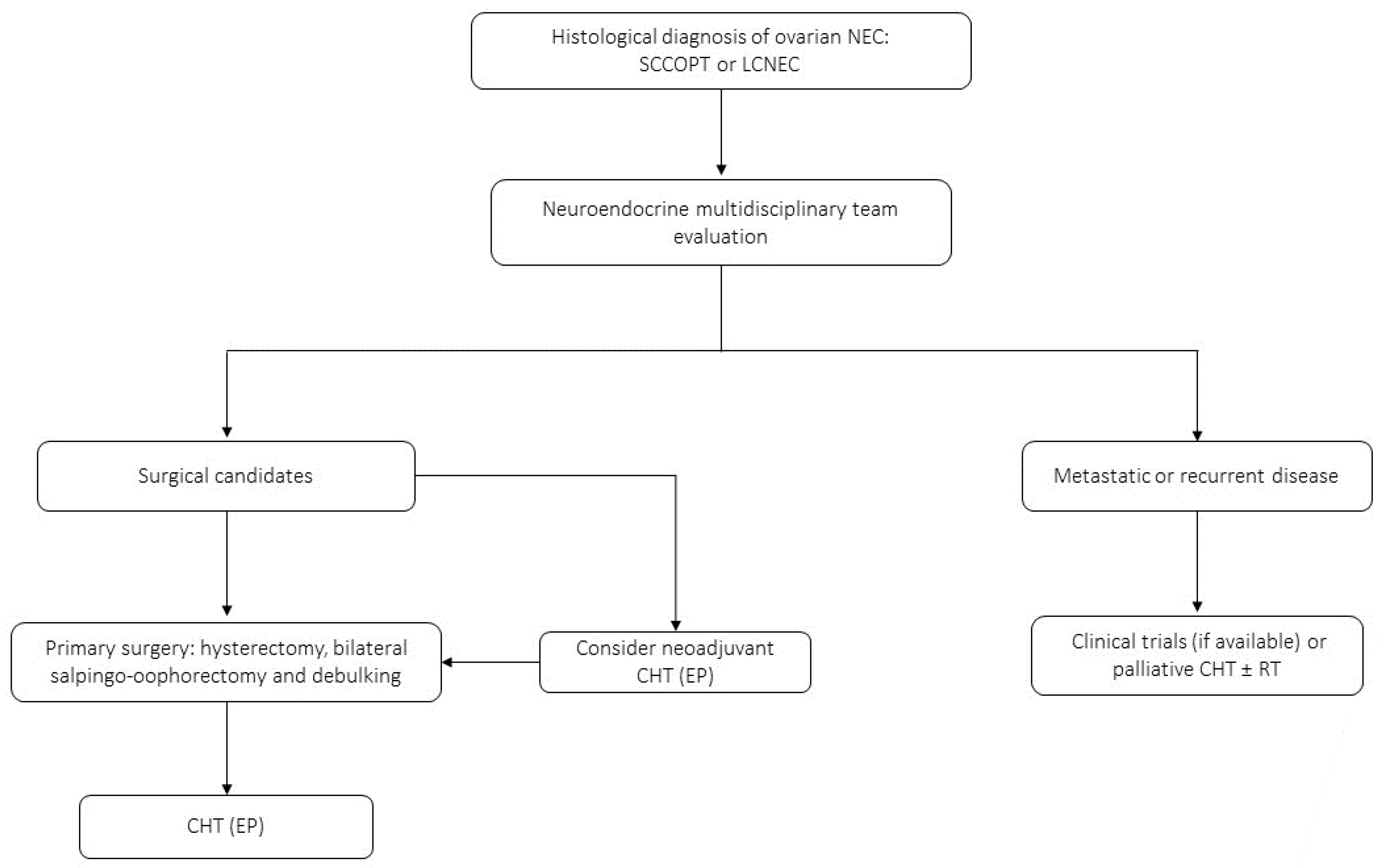
4.4. NECs of the Ovary
4.5. Other GY-NECs
5. NECs of the Genitourinary Tract
5.1. NECs of the Urinary Bladder
5.2. NECs of the Prostate
5.3. NECs of the Kidney
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms; Springer: New York, NY, USA, 2022; Volume 33, ISBN 1202202209. [Google Scholar]
- Classification of Tumours Editorial Board. WHO Classification of Endocrine and Neuroendocrine Tumours; WHO: Lyone, France, 2022. [Google Scholar]
- Rindi, G.; Wiedenmann, B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: Towards precision medicine. Nat. Rev. Endocrinol. 2020, 16, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldin, A.N.; Ro, J.Y. Neuroendocrine tumors of genitourinary tract: Recent advances. Ann. Diagn. Pathol. 2019, 42, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Livesey, D.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. Future therapeutic strategies in the treatment of extrapulmonary neuroendocrine carcinoma: A review. Ther. Adv. Med. Oncol. 2023, 15, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef]
- Uccella, S.; La Rosa, S.; Metovic, J.; Marchiori, D.; Scoazec, J.Y.; Volante, M.; Mete, O.; Papotti, M. Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr. Pathol. 2021, 32, 192–210. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Sigal, D.S.; Bhangoo, M.S.; Hermel, J.A.; Pavlick, D.C.; Frampton, G.; Miller, V.A.; Ross, J.S.; Ali, S.M. Comprehensive genomic profiling identifies novel NTRK fusions in neuroendocrine tumors. Oncotarget 2018, 9, 35809–35812. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, P271–P282. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: Results of the NCI-MATCH trial subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Mortel, C.; Kvols, L.; O’Connell, M. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Cancer 1991, 68, 227–232. [Google Scholar] [CrossRef]
- Ferro, L.B.; Wolf, I.; Peleg Hasson, S.; Golomb, I.; Osher, E.; Berlin, A.; Gutfeld, O.; Ospovat, I.; Soyfer, V. Extrapulmonary Small Cell Cancer: A New Insight into a Rare Disease. Oncology 2021, 99, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.; Teupe, F.; Michl, P.; Gress, T.M.; Rinke, A. Brain metastases in patients with neuroendocrine neoplasms: Risk factors and outcome. BMC Cancer 2019, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Devabhaktuni, A.; Nighot, R.; Sorbye, H. Survival According to Primary Tumor Location, Stage, and Treatment Patterns in Locoregional Gastroenteropancreatic High-grade Neuroendocrine Carcinomas. Oncologist 2022, 27, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Alese, O.B.; Jiang, R.; Shaib, W.; Wu, C.; Akce, M.; Behera, M.; El-Rayes, B.F. High-Grade Gastrointestinal Neuroendocrine Carcinoma Management and Outcomes: A National Cancer Database Study. Oncologist 2019, 24, 911–920. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Anton-Pascual, B.; Modrego, A.; Del Carmen Riesco-Martinez, M.; Lens-Pardo, A.; Carretero-Puche, C.; Rubio-Cuesta, B.; Soldevilla, B. Advances in the Treatment of Gastroenteropancreatic Neuroendocrine Carcinomas: Are we Moving Forward? Endocr. Rev. 2023, 44, 724–736. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Deng, H.Y.; Ni, P.Z.; Wang, Y.C.; Wang, W.P.; Chen, L.Q. Neuroendocrine carcinoma of the esophagus: Clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J. Thorac. Dis. 2016, 8, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Eads, J.R.; Halfdanarson, T.R.; Asmis, T.; Belizzi, A.M.; Bergsland, E.K.; Dasari, A.; El-Haddad, G.; Frumovitz, M.; Meyer, J.; Mittra, E.; et al. Expert Consensus Practice Recommendations of the North American Neuroendocrine Tumor Society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. Endocr. Relat. Cancer 2023, 30, e220206. [Google Scholar] [CrossRef] [PubMed]
- Borbon, L.C.; Tran, C.G.; Sherman, S.K.; Ear, P.H.; Chandrasekharan, C.; Bellizzi, A.M.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. ASO Visual Abstract: Is There a Role for Surgical Resection of Grade 3 Neuroendocrine Neoplasms? Ann. Surg. Oncol. 2022, 29, 6947–6948. [Google Scholar] [CrossRef] [PubMed]
- Fields, A.C.; Lu, P.; Vierra, B.M.; Hu, F.; Irani, J.; Bleday, R.; Goldberg, J.E.; Nash, G.M. Carcinomas: The Role of Surgery and Chemotherapy. Ann. Surg. Oncol. 2019, 26, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Reidy, D.L.; Goodman, K.A.; Shia, J.; Nash, G.M. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann. Surg. Oncol. 2014, 21, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Holmager, P.; Langer, S.W.; Kjaer, A.; Ringholm, L.; Garbyal, R.S.; Pommergaard, H.C.; Hansen, C.P.; Federspiel, B.; Andreassen, M.; Knigge, U. Surgery in Patients with Gastro-Entero-Pancreatic Neuroendocrine Carcinomas, Neuroendocrine Tumors G3 and High Grade Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Curr. Treat. Options Oncol. 2022, 23, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Ravizza, D.; Milione, M.; Massironi, S.; Grana, C.M.; Zerini, D.; Piccioli, A.N.; Spinoglio, G.; Fazio, N. Neuroendocrine neoplasms of rectum: A management update. Cancer Treat. Rev. 2018, 66, 45–55. [Google Scholar] [CrossRef]
- Katada, C.; Komori, S.; Yoshida, T.; Kawakami, S.; Watanabe, A.; Ishido, K.; Azuma, M.; Wada, T.; Hosoda, K.; Yamashita, K.; et al. A retrospective study of definitive chemoradiotherapy in patients with resectable small cell neuroendocrine carcinoma of the esophagus. Esophagus 2020, 17, 135–140. [Google Scholar] [CrossRef]
- Modrek, A.S.; Hsu, H.C.; Leichman, C.G.; Du, K.L. Radiation therapy improves survival in rectal small cell cancer—Analysis of Surveillance Epidemiology and End Results (SEER) data. Radiat. Oncol. 2015, 10, 101. [Google Scholar] [CrossRef][Green Version]
- Brieau, B.; Lepere, C.; Walter, T.; Lecomte, T.; Guimbaud, R.; Manfredi, S.; Tougeron, D.; Desseigne, F.; Lourenco, N.; Afchain, P.; et al. Radiochemotherapy versus surgery in nonmetastatic anorectal neuroendocrine carcinoma A multicenter study by the association des gastro-entérologues oncologues. Medicine 2015, 94, e1864. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Konukiewitz, B.; Schlitter, A.M.; Jesinghaus, M.; Pfister, D.; Steiger, K.; Segler, A.; Agaimy, A.; Sipos, B.; Zamboni, G.; Weichert, W.; et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. Mod. Pathol. 2017, 30, 587–598. [Google Scholar] [CrossRef]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef]
- Nicolini, S.; Severi, S.; Ianniello, A.; Sansovini, M.; Ambrosetti, A.; Bongiovanni, A.; Scarpi, E.; Di Mauro, F.; Rossi, A.; Matteucci, F.; et al. Investigation of receptor radionuclide therapy with 177Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 923–930. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat. Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Guerrera, F.; Evangelista, A.; Galassi, C.; Welter, S.; Rendina, E.A.; Travis, W.; Lim, E.; Sarkaria, I.; Thomas, P.A.; et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: Results from the European Society for Thoracic Surgeons Lung Neuroendocrine Tumours Retrospective Database. Eur. J. Cardio-Thorac. Surg. 2017, 52, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Rinke, A.; Partelli, S.; Gress, T.M.; Andreasi, V.; Kollár, A.; Perren, A.; Christ, E.; Panzuto, F.; Pascher, A.; et al. Surgery with Radical Intent: Is There an Indication for G3 Neuroendocrine Neoplasms? Ann. Surg. Oncol. 2020, 27, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Mitry, E.; Rougier, P. The treatment of undifferentiated neuroendocrine tumors. Crit. Rev. Oncol. Hematol. 2001, 37, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Li, J.; Zhang, X.; Zhou, J.; Wang, X.; Peng, Z.; Shen, L.; Lu, M. Etoposide and cisplatin versus irinotecan and cisplatin as the first-line therapy for patients with advanced, poorly differentiated gastroenteropancreatic neuroendocrine carcinoma: A randomized phase 2 study. Cancer 2020, 126, 2086–2092. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Tiseo, M.; Boni, L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: A pooled analysis of topotecan second-line trials. Eur. J. Cancer 2014, 50, 2211–2218. [Google Scholar] [CrossRef]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Lévy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef]
- Hadoux, J.; Malka, D.; Planchard, D.; Scoazec, J.Y.; Caramella, C.; Guigay, J.; Boige, V.; Leboulleux, S.; Burtin, P.; Berdelou, A.; et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr. Relat. Cancer 2015, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Öberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef]
- Couronne, T.; Girot, P.; Hadoux, J.; Lecomte, T.; Durand, A.; Fine, C.; Vandevoorde, K.; Lombard-Bohas, C.; Walter, T. Post first-line dacarbazine or temozolomide ineuroendocrine carcinoma. Endocr. Connect. 2020, 9, 498–505. [Google Scholar] [CrossRef]
- Apostolidis, L.; Bergmann, F.; Jäger, D.; Winkler, E.C. Efficacy of topotecan in pretreated metastatic poorly differentiated extrapulmonary neuroendocrine carcinoma. Cancer Med. 2016, 5, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; Swain, J.; Craig, Z.; Sharma, R.; Faluyi, O.; Wadsley, J.; Morgan, C.; Wall, L.R.; Chau, I.; Reed, N.; et al. NET-02: A randomised, non-comparative, phase II trial of nal-IRI/5-FU or docetaxel as second-line therapy in patients with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma. eClinicalMedicine 2023, 60, 102015. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Lievre, A.; Coriat, R.; Malka, D.; Elhajbi, F.; Di Fiore, F.; Hentic, O.; Smith, D.; Hautefeuille, V.; Roquin, G.; et al. Bevacizumab plus FOLFIRI after failure of platinum–etoposide first-line chemotherapy in patients with advanced neuroendocrine carcinoma (PRODIGE 41-BEVANEC): A randomised, multicentre, non-comparative, open-label, phase 2 trial. Lancet Oncol. 2023, 24, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Liverani, C.; Pusceddu, S.; Leo, S.; Di Meglio, G.; Tamberi, S.; Santini, D.; Gelsomino, F.; Pucci, F.; Berardi, R.; et al. Randomised phase II trial of CAPTEM or FOLFIRI as SEcond-line therapy in NEuroendocrine CArcinomas and exploratory analysis of predictive role of PET/CT imaging and biological markers (SENECA trial): A study protocol. BMJ Open 2020, 10, e034393. [Google Scholar] [CrossRef]
- Xing, J.; Ying, H.; Li, J.; Gao, Y.; Sun, Z.; Li, J.; Bai, C.; Cheng, Y.; Wu, H. Immune Checkpoint Markers in Neuroendocrine Carcinoma of the Digestive System. Front. Oncol. 2020, 10, 132. [Google Scholar] [CrossRef]
- Venizelos, A.; Elvebakken, H.; Perren, A.; Nikolaienko, O.; Deng, W.; Lothe, I.M.B.; Couvelard, A.; Hjortland, G.O.; Sundlöv, A.; Svensson, J.; et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Capdevila, J.; Hernando, J.; Teule, A.; Lopez, C.; Garcia-Carbonero, R.; Benavent, M.; Custodio, A.; Garcia-Alvarez, A.; Cubillo, A.; Alonso, V.; et al. Durvalumab plus tremelimumab for the treatment of advanced neuroendocrine neoplasms of gastroenteropancreatic and lung origin. Nat. Commun. 2023, 14, 2973. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Mazieres, J.; Otto, J.; Lena, H.; Lepage, C.; Egenod, T.; Smith, D.; Madelaine, J.; Gérinière, L.; El Hajbi, F.; et al. LBA41 Nivolumab (nivo) ± ipilimumab (ipi) in pre-treated patients with advanced, refractory pulmonary or gastroenteropancreatic poorly differentiated neuroendocrine tumors (NECs) (GCO-001 NIPINEC). Ann. Oncol. 2021, 32, S1318. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Riesco Martinez, M.C.; Capdevila Castillon, J.; Alonso, V.; Jimenez-Fonseca, P.; Teule, A.; Grande, E.; Sevilla, I.; Benavent, M.; Alonso-Gordoa, T.; Custodio, A.; et al. 496MO Final overall survival results from the NICE-NEC trial (GETNE-T1913): A phase II study of nivolumab and platinum-doublet chemotherapy (CT) in untreated advanced G3 neuroendocrine neoplasms (NENs) of gastroenteropancreatic (GEP) or unknown (UK) origi. Ann. Oncol. 2022, 33, S769. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS G12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Yaeger, R.; Spira, A.I.; Pelster, M.S.; Sabari, J.K.; Hafez, N.; Barve, M.; Velastegui, K.; Yan, X.; Shetty, A.; et al. Adagrasib in Advanced Solid Tumors Harboring a KRAS G12C Mutation. J. Clin. Oncol. 2023, 41, 4097–4106. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Puzanov, I.; Blay, J.Y.; Chau, I.; Lockhart, A.C.; Raje, N.S.; Wolf, J.; Baselga, J.; Meric-Bernstam, F.; Roszik, J.; et al. Pan-cancer efficacy of vemurafenib in brafv600-mutant non-melanoma cancers. Cancer Discov. 2020, 10, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Sennino, B.; Ishiguro-oonuma, T.; Wei, Y.; Naylor, R.M.; Williamson, C.W. Suppression of Tumor Invasion and Metastasis by Concurrent Inhibition of c-Met and VEGF Signaling in Pancreatic Neuroendocrine Tumors. Cancer Discov. 2012, 2, 270–287. [Google Scholar] [CrossRef]
- Pellat, A.; Dreyer, C.; Couffignal, C.; Walter, T.; Lombard-Bohas, C.; Niccoli, P.; Seitz, J.F.; Hentic, O.; André, T.; Coriat, R.; et al. Clinical and Biomarker Evaluations of Sunitinib in Patients with Grade 3 Digestive Neuroendocrine Neoplasms. Neuroendocrinology 2018, 107, 24–31. [Google Scholar] [CrossRef]
- Alifieris, C.E.; Griniatsos, J.; Delis, S.G.; Nikolaou, M.; Avgoustou, C.; Panagiotidis, M.I.; Souferi-Chronopoulou, E.; Trafalis, D.T. Capecitabine, Oxaliplatin, Irinotecan, and Bevacizumab Combination Followed by Pazopanib plus Capecitabine Maintenance for High-Grade Gastrointestinal Neuroendocrine Carcinomas. Am. J. Clin. Oncol. Cancer Clin. Trials 2020, 43, 305–310. [Google Scholar] [CrossRef]
- Patibandla, J.R.; Fehniger, J.E.; Levine, D.A.; Jelinic, P. Small cell cancers of the female genital tract: Molecular and clinical aspects. Gynecol. Oncol. 2018, 149, 420–427. [Google Scholar] [CrossRef]
- Georgescu, T.A.; Bohiltea, R.E.; Munteanu, O.; Furtunescu, F.; Lisievici, A.C.; Grigoriu, C.; Gherghiceanu, F.; Vlădăreanu, E.M.; Berceanu, C.; Ducu, I.; et al. Emerging therapeutic concepts and latest diagnostic advancements regarding neuroendocrine tumors of the gynecologic tract. Medicina 2021, 57, 1338. [Google Scholar] [CrossRef]
- Bermúdez, A.; Vighi, S.; García, A.; Sardi, J. Neuroendocrine cervical carcinoma: A diagnostic and therapeutic challenge. Gynecol. Oncol. 2001, 82, 32–39. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, L.; Cheng, Z.; Guo, X.; Cao, D. INSM1 Is Less Sensitive but More Specific Than Synaptophysin in Gynecologic High-grade Neuroendocrine Carcinomas. Am. J. Surg. Pathol. 2021, 45, 147–159. [Google Scholar] [CrossRef]
- Crane, E.K.; Ramos, P.; Farley, J.H.; Naumann, R.W.; Tait, D.L.; Higgins, R.V.; Brown, J. Molecular profiling in a large cohort of gynecologic neuroendocrine tumors. Gynecol. Oncol. 2020, 159, 262. [Google Scholar] [CrossRef]
- Caruso, G.; Sassu, C.M.; Tomao, F.; Di Donato, V.; Perniola, G.; Fischetti, M.; Benedetti Panici, P.; Palaia, I. The puzzle of gynecologic neuroendocrine carcinomas: State of the art and future directions. Crit. Rev. Oncol. Hematol. 2021, 162, 103344. [Google Scholar] [CrossRef]
- Gadducci, A.; Carinelli, S.; Aletti, G. Neuroendrocrine tumors of the uterine cervix: A therapeutic challenge for gynecologic oncologists. Gynecol. Oncol. 2017, 144, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Pierz, A.; Stoler, M.H. A systematic review and meta-analysis on the attribution of human papillomavirus (HPV) in neuroendocrine cancers of the cervix. Gynecol. Oncol. 2018, 148, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Alejo, M.; Alemany, L.; Clavero, O.; Quiros, B.; Vighi, S.; Seoud, M.; Cheng-Yang, C.; Garland, S.M.; Juanpere, N.; Lloreta, J.; et al. Contribution of Human papillomavirus in neuroendocrine tumors from a series of 10,575 invasive cervical cancer cases. Papillomavirus Res. 2018, 5, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Burzawa, J.K.; Byers, L.A.; Lyons, Y.A.; Ramalingam, P.; Coleman, R.L.; Brown, J. Sequencing of mutational hotspots in cancer-related genes in small cell neuroendocrine cervical cancer. Gynecol. Oncol. 2016, 141, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Zheng, G.; Schoolmeester, J.K.; Li, Z.; Pallavajjala, A.; Haley, L.; Conner, M.G.; Vang, R.; Hung, C.F.; Wu, T.C.; et al. Next-generation Sequencing Reveals Recurrent Somatic Mutations in Small Cell Neuroendocrine Carcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2018, 42, 750–760. [Google Scholar] [CrossRef]
- Winer, I.; Kim, C.; Gehrig, P. Neuroendocrine tumors of the gynecologic tract update. Gynecol. Oncol. 2021, 162, 210–219. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.E.; Coté, T.R.; Clegg, L.X.; Tavassoli, F.J. Endocrine tumors of the uterine cervix: Incidence, demographics, and survival with comparison to squamous cell carcinoma. Gynecol. Oncol. 2003, 88, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Vulasala, S.S.; Morani, A.C.; Waters, R.; Gopireddy, D.R.; Kumar, S.; Bhosale, P.; Lall, C. Neuroendocrine Neoplasms of the Gynecologic Tract. Cancers 2022, 14, 1835. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine carcinoma of the cervix: A systematic review of the literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.J.; Reidy-Lagunes, D.; Gehrig, P.A. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011, 122, 190–198. [Google Scholar] [CrossRef]
- Chu, T.; Meng, Y.; Wu, P.; Li, Z.; Wen, H.; Ren, F.; Zou, D.; Lu, H.; Wu, L.; Zhou, S.; et al. The prognosis of patients with small cell carcinoma of the cervix: A retrospective study of the SEER database and a Chinese multicentre registry. Lancet Oncol. 2023, 24, 701–708. [Google Scholar] [CrossRef]
- Kim, C.; Salvo, G.; Ishikawa, M.; Chen, T.C.; Jhingran, A.; Bhosale, P.; Ramalingam, P.; Frumovitz, M. The role of postoperative radiation after radical hysterectomy for women with early-stage neuroendocrine carcinoma of the cervix: A meta-analysis. Gynecol. Oncol. 2023, 170, 328–332. [Google Scholar] [CrossRef]
- Boruta, D.M.; Schorge, J.O.; Duska, L.A.; Crum, C.P.; Castrillon, D.H.; Sheets, E.E. Multimodality therapy in early-stage neuroendocrine carcinoma of the uterine cervix. Gynecol. Oncol. 2001, 81, 82–87. [Google Scholar] [CrossRef]
- Hoskins, P.J.; Swenerton, K.D.; Pike, J.A.; Lim, P.; Aquino-Parsons, C.; Wong, F.; Lee, N. Small-cell carcinoma of the cervix: Fourteen years of experience at a single institution using a combined-modality regimen of involved-field irradiation and platinum-based combination chemotherapy. J. Clin. Oncol. 2003, 21, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Martin, A.G.; Gonzales, N.R.; Frumovitz, M. Updates and management algorithm for neuroendocrine tumors of the uterine cervix. Int. J. Gynecol. Cancer 2019, 29, 986–995. [Google Scholar] [CrossRef]
- Frumovitz, M.; Munsell, M.F.; Burzawa, J.K.; Byers, L.A.; Ramalingam, P.; Brown, J.; Coleman, R.L. Combination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in recurrent small cell neuroendocrine carcinoma of the cervix. Gynecol. Oncol. 2017, 144, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. 1Final overall survival of the phase III randomised trial of chemotherapy with and without bevacizumab for advanced cervical cancer: An NRG oncology/gynecologic oncology group study. Obstet. Gynecol. Surv. 2017, 69, 331–332. [Google Scholar] [CrossRef][Green Version]
- Tangjitgamol, S.; Ramirez, P.T.; Sun, C.C.; See, H.T.; Jhingran, A.; Kavanagh, J.J.; Deavers, M.T. Expression of HER-2/neu, epidermal growth factor receptor, vascular endothelial growth factor, cyclooxygenase-2, estrogen receptor, and progesterone receptor in small cell and large cell neuroendocrine carcinoma of the uterine cervix: A clinicopathologic. Int. J. Gynecol. Cancer 2005, 15, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Aguilar, E.J.; Ricciuti, B.; Gainor, J.F.; Kehl, K.L.; Kravets, S.; Dahlberg, S.; Nishino, M.; Sholl, L.M.; Adeni, A.; Subegdjo, S.; et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann. Oncol. 2019, 30, 1653–1659. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bjarne, R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Brandon, S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Carroll, M.R.; Ramalingam, P.; Salvo, G.; Fujimoto, J.; Solis Soto, L.M.; Phoolcharoen, N.; Hillman, R.T.; Cardnell, R.; Byers, L.; Frumovitz, M. Evaluation of PARP and PDL-1 as potential therapeutic targets for women with high-grade neuroendocrine carcinomas of the cervix. Int. J. Gynecol. Cancer 2020, 30, 1303–1307. [Google Scholar] [CrossRef]
- Mahdi, H.; Joehlin-Price, A.; Elishaev, E.; Dowlati, A.; Abbas, A. Genomic analyses of high-grade neuroendocrine gynecological malignancies reveal a unique mutational landscape and therapeutic vulnerabilities. Mol. Oncol. 2021, 15, 3545–3558. [Google Scholar] [CrossRef]
- Wu, H.-X.; Wang, Z.-X.; Zhao, Q.; Chen, D.-L.; He, M.-M.; Yang, L.-P.; Wang, Y.-N.; Jin, Y.; Ren, C.; Luo, H.-Y.; et al. Tumor mutational and indel burden: A systematic pan-cancer evaluation as prognostic biomarkers. Ann. Transl. Med. 2019, 7, 640. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non-Small Cell Lung Cancers with Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.; Kim, S.S.; Kato, S.; Sanders, P.D.; Patel, S.P.; Sanghvi, P.; Weihe, E.; Kurzrock, R. Exceptional Response to Nivolumab and Stereotactic Body Radiation Therapy (SBRT) in Neuroendocrine Cervical Carcinoma with High Tumor Mutational Burden: Management Considerations from the Center for Personalized Cancer Therapy at UC San Diego Moores Cance. Oncologist 2017, 22, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Paraghamian, S.E.; Longoria, T.C.; Eskander, R.N. Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: A case report. Gynecol. Oncol. Res. Pract. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, T.A.; Dorr, K.; Ullah, A.; White, J.; Williams, H.; Ghamande, S. Complete Response to Combination Nivolumab and Ipilimumab in Recurrent Neuroendocrine Carcinoma of the Cervix. Obstet. Gynecol. 2021, 138, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Sui, L.; Song, K.; Lv, T.; Zhao, H.; Yao, Q. PD-L1, PARP1, and MMRs as potential therapeutic biomarkers for neuroendocrine cervical cancer. Cancer Med. 2021, 10, 4743–4751. [Google Scholar] [CrossRef]
- Frumovitz, M.; Westin, S.N.; Salvo, G.; Zarifa, A.; Xu, M.; Yap, T.A.; Rodon, A.J.; Karp, D.D.; Abonofal, A.; Jazaeri, A.A.; et al. Phase II study of pembrolizumab efficacy and safety in women with recurrent small cell neuroendocrine carcinoma of the lower genital tract. Gynecol. Oncol. 2020, 158, 570–575. [Google Scholar] [CrossRef]
- Frumovitz, M.; Chisholm, G.B.; Jhingran, A.; Ramalingam, P.; Flores-Legarreta, A.; Bhosale, P.; Gonzales, N.R.; Hillman, R.T.; Salvo, G. Combination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in patients with recurrent high-grade neuroendocrine cervical cancer: A Neuroendocrine Cervical Tumor Registry (NeCTuR) study. Am. J. Obstet. Gynecol. 2023, 228, 445.e1–445.e8. [Google Scholar] [CrossRef]
- Lyons, Y.A.; Frumovitz, M.; Soliman, P.T. Response to MEK inhibitor in small cell neuroendocrine carcinoma of the cervix with a KRAS mutation. Gynecol. Oncol. Rep. 2014, 10, 28–29. [Google Scholar] [CrossRef]
- Schlechtweg, K.; Chen, L.; St. Clair, C.M.; Tergas, A.I.; Khoury-Collado, F.; Hou, J.Y.; Melamed, A.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Neuroendocrine carcinoma of the endometrium: Disease course, treatment, and outcomes. Gynecol. Oncol. 2019, 155, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Akgor, U.; Kuru, O.; Sakinci, M.; Boyraz, G.; Sen, S.; Cakır, I.; Turan, T.; Gokcu, M.; Gultekin, M.; Sayhan, S.; et al. Neuroendocrine carcinoma of the endometrium: A very rare gynecologic malignancy. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Wu, X.; Liu, Y.; Xi, X. Clinical characteristic and prognostic factors in high-grade endometrial neuroendocrine carcinoma. J. Obstet. Gynaecol. Res. 2022, 48, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Lopes Dias, J.; Cunha, T.M.; Gomes, F.V.; Callé, C.; Félix, A. Neuroendocrine tumours of the female genital tract: A case-based imaging review with pathological correlation. Insights Imaging 2015, 6, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Pocrnich, C.E.; Ramalingam, P.; Euscher, E.D.; Malpica, A. Neuroendocrine carcinoma of the endometrium: A clinicopathologic study of 25 cases. Am. J. Surg. Pathol. 2016, 40, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pang, L. Primary Neuroendocrine Tumors of the Endometrium: Management and Outcomes. Front. Oncol. 2022, 12, 921615. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, L.; Goudie, C.; Ramos, P.; Boshari, T.; Brunet, J.S.; Karnezis, A.N.; Longy, M.; Knost, J.A.; Saloustros, E.; McCluggage, W.G.; et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol. Oncol. 2016, 141, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Bagga, R.; Rai, B.; Srinivasan, R. Primary pure large cell neuroendocrine carcinoma of the ovary: Histopathologic and immunohistochemical analysis with review of the literature. Int. J. Clin. Exp. Pathol. 2021, 14, 1000–1009. [Google Scholar]
- Pang, L.; Guo, Z. Primary neuroendocrine tumors of the ovary: Management and outcomes. Cancer Med. 2021, 10, 8558–8569. [Google Scholar] [CrossRef]
- Jang, A.; Newell, M.J.; Phaeton, R.; Kesterson, P.J. Large cell neuroendocrine carcinoma (LCNEC) of the ovary: A case report and review of the literature. Integr. Cancer Sci. Ther. 2016, 3, 1–3. [Google Scholar] [CrossRef]
- Bhalodia, J.N.; Kapapura, D.V.; Parekh, M.N. Primary Small Cell Neuroendocrine Carcinoma of Vagina: A Rare Case Report. Patholog. Res. Int. 2011, 2011, 306921. [Google Scholar] [CrossRef] [PubMed]
- Bing, Z.; Levine, L.; Lucci, J.A.; Hatch, S.S.; Eltorky, M.A. Primary small cell neuroendocrine carcinoma of the vagina: A clinicopathologic study. Arch. Pathol. Lab. Med. 2004, 128, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Bócoli, M.C.; Saldanha, J.C.; Murta, E.F.C.; Nomelini, R.S. Primary Small Cell Carcinoma of the Vagina. Case Rep. Obstet. Gynecol. 2013, 2013, 827037. [Google Scholar] [CrossRef]
- Chen, P.P.; Ramalingam, P.; Alvarado-Cabrero, I.; Euscher, E.D.; Nagarajan, P.; Lawson, B.C.; Malpica, A. High-grade Neuroendocrine Carcinomas of the Vulva: A Clinicopathologic Study of 16 Cases. Am. J. Surg. Pathol. 2021, 45, 304–316. [Google Scholar] [CrossRef]
- Bobos, M.; Hytiroglou, P.; Kostopoulos, I.; Karkavelas, G.; Papadimitriou, C.S. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am. J. Dermatopathol. 2006, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Maggio, I.; Manuzzi, L.; Lamberti, G.; Ricci, A.D.; Tober, N.; Campana, D. Landscape and future perspectives of immunotherapy in neuroendocrine neoplasia. Cancers 2020, 12, 832. [Google Scholar] [CrossRef]
- Eckert, F.; Fehm, T.; Bamberg, M.; Müller, A.C. Small cell carcinoma of vulva: Curative multimodal treatment in face of resistance to initial standard chemotherapy. Strahlenther. Onkol. 2010, 186, 521–524. [Google Scholar] [CrossRef]
- Virarkar, M.; Vulasala, S.S.; Gopireddy, D.; Morani, A.C.; Daoud, T.; Waters, R.; Bhosale, P. Neuroendocrine Neoplasms of the Female Genitourinary Tract: A Comprehensive Overview. Cancers 2022, 14, 3218. [Google Scholar] [CrossRef]
- Le, B.K.; McGarrah, P.; Paciorek, A.; Mohamed, A.; Apolo, A.B.; Chan, D.L.; Reidy-Lagunes, D.; Hauser, H.; Rivero, J.D.; Whitman, J.; et al. Urinary Neuroendocrine Neoplasms Treated in the “Modern Era”: A Multicenter Retrospective Review. Clin. Genitourin. Cancer 2023, 21, 403–414.e5. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Blomjous, C.E.; Vos, W.; De Voogt, H.J.; Van der Valk, P.; Meijer, C.J. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer 1989, 64, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Erdem, G.U.; Özdemir, N.Y.; Demirci, N.S.; Şahin, S.; Bozkaya, Y.; Zengin, N. Small cell carcinoma of the urinary bladder: Changing trends in the current literature. Curr. Med. Res. Opin. 2016, 32, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, M.; Aben, K.K.; Hulsbergen-van de Kaa, C.A.; Schoenberg, M.P.; Witjes, J.A.; Kiemeney, L.A. Clinical Epidemiology of Nonurothelial Bladder Cancer: Analysis of The Netherlands Cancer Registry. J. Urol. 2010, 183, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Pompas-Veganzones, N.; Gonzalez-Peramato, P.; Sanchez-Carbayo, M. The neuroendocrine component in bladder tumors. Curr. Med. Chem. 2014, 21, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Grignon, D.J.; Ro, J.Y.; Ayala, A.G.; Shum, D.T.; Ordóñez, N.G.; Logothetis, C.J.; Johnson, D.E.; Mackay, B. Small cell carcinoma of the urinary bladder. A clinicopathologic analysis of 22 cases. Cancer 1992, 69, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Marchiori, D.; Cricca, A.; Garofalo, M.; Giovannini, C.; Manferrari, F.; Gerace, T.G.; Pernetti, R.; Martorana, G. Neuroendocrine carcinoma of the urinary bladder: Case report and review of the literature. Anticancer Res. 2008, 28, 1369–1372. [Google Scholar] [CrossRef]
- Cheng, L.; Pan, C.X.; Yang, X.J.; Lopez-Beltran, A.; MacLennan, G.T.; Lin, H.; Kuzel, T.M.; Papavero, V.; Tretiakova, M.; Nigro, K.; et al. Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 64 patients. Cancer 2004, 101, 957–962. [Google Scholar] [CrossRef]
- Sjödahl, G. Molecular subtype profiling of urothelial carcinoma using a subtype-specific immunohistochemistry panel. Methods Mol. Biol. 2018, 1655, 53–64. [Google Scholar] [CrossRef]
- Wang, X.; MacLennan, G.T.; Lopez-Beltran, A.; Cheng, L. Small cell carcinoma of the urinary bladder—Histogenesis, genetics, diagnosis, biomarkers, treatment, and prognosis. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 8–18. [Google Scholar] [CrossRef]
- Ghervan, L.; Zaharie, A.; Ene, B.; Elec, F.I. Small-cell carcinoma of the urinary bladder: Where do we stand? Clujul Med. 2017, 90, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Liu, L.Y.; Yu, G.H.; Qu, G.M.; Gong, P.Y.; Yu, X.; Yang, P. Analysis of clinicopathological features and prognostic factors in 39 cases of bladder neuroendocrine carcinoma. Anticancer Res. 2017, 37, 4529–4537. [Google Scholar] [CrossRef] [PubMed]
- Naturale, R.T.; MacLennan, G.T. Small Cell Carcinoma of the Bladder. J. Urol. 2006, 176, 781. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Massari, F.; Andrini, E.; Rosellini, M.; Marchetti, A.; Nuvola, G.; Tassinari, E.; Lamberti, G.; Campana, D. Prognostic Factors of Survival for High-Grade Neuroendocrine Neoplasia of the Bladder: A SEER Database Analysis. Curr. Oncol. 2022, 29, 5846–5854. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Reidy-Lagunes, D. The management of extrapulmonary poorly differentiated (high-grade) neuroendocrine carcinomas. Semin. Oncol. 2013, 40, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Vetterlein, M.W.; Wankowicz, S.A.M.; Seisen, T.; Lander, R.; Löppenberg, B.; Chun, F.K.H.; Menon, M.; Sun, M.; Barletta, J.A.; Choueiri, T.K.; et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 2017, 123, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Brizzi, M.P.; Pusceddu, S.; Gelsomino, F.; Di Meglio, G.; Massari, F.; Badalamenti, G.; Riccardi, F.; Ibrahim, T.; Ciccarese, C.; et al. Perioperative Chemotherapy in Poorly Differentiated Neuroendocrine Neoplasia of the Bladder: A Multicenter Analysis. J. Clin. Med. 2020, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Ghatalia, P.; Litwin, S.; Horwitz, E.M.; Uzzo, R.G.; Greenberg, R.E.; Viterbo, R.; Geynisman, D.M.; Kutikov, A.; Plimack, E.R.; et al. Small-Cell Carcinoma of the Bladder: 20-Year Single-Institution Retrospective Review. Clin. Genitourin. Cancer 2017, 15, e337–e343. [Google Scholar] [CrossRef]
- Mondal, K.; Mandal, R. A carcinoid tumor in the urinary bladder with uncommon clinicopathological presentation. Iran. J. Pathol. 2017, 12, 277–280. [Google Scholar] [CrossRef]
- Coelho, H.M.P.; Pereira, B.A.G.J.; Caetano, P.A.S.T. Large cell neuroendocrine carcinoma of the urinary bladder: Case report and review. Curr. Urol. 2014, 7, 155–159. [Google Scholar] [CrossRef]
- Akdeniz, E.; Bakirtas, M.; Bolat, M.S.; Akdeniz, S.; Özer, I. Pure large cell neuroendocrine carcinoma of the bladder without urological symptoms. Pan Afr. Med. J. 2018, 30, 134. [Google Scholar] [CrossRef]
- Gupta, S.; Thompson, R.H.; Boorjian, S.A.; Thapa, P.; Hernandez, L.P.H.; Jimenez, R.E.; Costello, B.A.; Frank, I.; Cheville, J.C. High grade neuroendocrine carcinoma of the urinary bladder treated by radical cystectomy: A series of small cell, mixed neuroendocrine and large cell neuroendocrine carcinoma. Pathology 2015, 47, 533–542. [Google Scholar] [CrossRef]
- Radović, N.; Turner, R.; Bacalja, J. Primary “Pure” Large Cell Neuroendocrine Carcinoma of the Urinary Bladder: A Case Report and Review of the Literature. Clin. Genitourin. Cancer 2015, 13, e375–e377. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, B.; Kuthi, L.; Varga, L.; Laczo, I.; Révész, J.; Kránicz, R.; Maráz, A. The colorful palette of neuroendocrine neoplasms in the genitourinary tract. Anticancer Res. 2018, 38, 3243–3254. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, R.; Morichetti, D.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Kirkali, Z.; Montironi, R. Neuroendocrine tumours of the urinary system and male genital organs: Clinical significance. BJU Int. 2009, 103, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef]
- Zou, M.; Toivanen, R.; Mitrofanova, A.; Floch, N.; Hayati, S.; Sun, Y.; Le Magnen, C.; Chester, D.; Mostaghel, E.A.; Califano, A.; et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017, 7, 736–749. [Google Scholar] [CrossRef]
- Guo, C.C.; Dancer, J.Y.; Wang, Y.; Aparicio, A.; Navone, N.M.; Troncoso, P.; Czerniak, B.A. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum. Pathol. 2011, 42, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mounir, Z.; Lin, F.; Lin, V.G.; Korn, J.M.; Yu, Y.; Valdez, R.; Aina, O.H.; Buchwalter, G.; Jaffe, A.B.; Korpal, M.; et al. TMPRSS2:ERG blocks neuroendocrine and luminal cell differentiation to maintain prostate cancer proliferation. Oncogene 2015, 34, 3815–3825. [Google Scholar] [CrossRef]
- Tan, H.L.; Sood, A.; Rahimi, H.A.; Wang, W.; Gupta, N.; Hicks, J.; Mosier, S.; Gocke, C.D.; Epstein, J.I.; Netto, G.J.; et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res. 2014, 20, 890–903. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.S.K.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef]
- Lee, J.K.; Phillips, J.W.; Smith, B.A.; Park, J.W.; Stoyanova, T.; McCaffrey, E.F.; Baertsch, R.; Sokolov, A.; Meyerowitz, J.G.; Mathis, C.; et al. N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells. Cancer Cell 2016, 29, 536–547. [Google Scholar] [CrossRef]
- Lotan, T.L.; Gupta, N.S.; Wang, W.; Toubaji, A.; Haffner, M.C.; Chaux, A.; Hicks, J.L.; Meeker, A.K.; Bieberich, C.J.; De Marzo, A.M.; et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod. Pathol. 2011, 24, 820–828. [Google Scholar] [CrossRef]
- Rodrigues, L.U.; Rider, L.; Nieto, C.; Romero, L.; Karimpour-Fard, A.; Loda, M.; Lucia, M.S.; Wu, M.; Shi, L.; Cimic, A.; et al. Coordinate loss of MAP3K7 and CHD1 promotes aggressive prostate cancer. Cancer Res. 2015, 75, 1021–1034. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53-and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, S.; Wyatt, A.W.; Lin, D.; Lysakowski, S.; Zhang, F.; Kim, S.; Tse, C.; Wang, K.; Mo, F.; Haegert, A.; et al. The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep. 2015, 12, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.; Bratu, O.; Spînu, D.; Marcu, D.; Farcaș, C.; Dinu, M.; Mischianu, D. Neuroendocrine differentiation in prostate cancer—A review. Rom. J. Mil. Med. 2015, 118, 16–19. [Google Scholar] [CrossRef]
- Aparicio, A.M.; Harzstark, A.L.; Corn, P.G.; Wen, S.; Araujo, J.C.; Tu, S.M.; Pagliaro, L.C.; Kim, J.; Millikan, R.E.; Ryan, C.; et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 3621–3630. [Google Scholar] [CrossRef] [PubMed]
- Fléchon, A.; Pouessel, D.; Ferlay, C.; Perol, D.; Beuzeboc, P.; Gravis, G.; Joly, F.; Oudard, S.; Deplanque, G.; Zanetta, S.; et al. Phase ii study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: Results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann. Oncol. 2011, 22, 2476–2481. [Google Scholar] [CrossRef]
- Beltran, H.; Oromendia, C.; Danila, D.C.; Montgomery, B.; Hoimes, C.; Szmulewitz, R.Z.; Vaishampayan, U.; Armstrong, A.J.; Stein, M.; Pinski, J.; et al. A phase II trial of the aurora kinase a inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: Efficacy and biomarkers. Clin. Cancer Res. 2019, 25, 43–51. [Google Scholar] [CrossRef]
- Brown, L.C.; Halabi, S.; Somarelli, J.A.; Humeniuk, M.; Wu, Y.; Oyekunle, T.; Howard, L.; Huang, J.; Anand, M.; Davies, C.; et al. A phase 2 trial of avelumab in men with aggressive-variant or neuroendocrine prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 762–769. [Google Scholar] [CrossRef]
- Chin, A.I.; Ly, A.; Rodriguez, S.; Sachdeva, A.; Zomorodian, N.; Zhang, H.; Kim, J.; Li, G.; Rettig, M.; Liu, S.; et al. Updated results of a phase Ib single-center study of pembrolizumab in combination with chemotherapy in patients with locally advanced or metastatic small cell/neuroendocrine cancers of the prostate and urothelium. J. Clin. Oncol. 2023, 41, 165. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Zhang, J.; Zhu, X.; Monk, P.; Jones, R.J.; Linch, M.D.; Costin, D.; De Bono, J.S.; Karsh, L.I.; Petrylak, D.P.; et al. First-in-class oral innate immune activator BXCL701 combined with pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC) of small cell neuroendocrine (SCNC) phenotype: Phase 2a final results. J. Clin. Oncol. 2023, 41, 176. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Hong, D.S.; Hann, C.L.; Farago, A.F.; Beltran, H.; Waqar, S.N.; Hendifar, A.E.; Anthony, L.B.; Taylor, M.H.; Bryce, A.H.; et al. A phase I/II study of rovalpituzumab tesirine in delta-like 3—Expressing advanced solid tumors. npj Precis. Oncol. 2021, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Choi, J.E.; Tien, J.C.; Simko, S.A.; Rajendiran, T.; Vo, J.N.; Delekta, A.D.; Wang, L.; Xiao, L.; Hodge, N.B.; et al. Autophagy inhibition by targeting PIKfyve potentiates response to immune checkpoint blockade in prostate cancer. Nat. Cancer 2022, 2, 978–993. [Google Scholar] [CrossRef]
- Sultana, Q.; Kar, J.; Verma, A.; Sanghvi, S.; Kaka, N.; Patel, N.; Sethi, Y.; Chopra, H.; Kamal, M.A.; Greig, N.H. A Comprehensive Review on Neuroendocrine Neoplasms: Presentation, Pathophysiology and Management. J. Clin. Med. 2023, 12, 5138. [Google Scholar] [CrossRef]
- Paisey, S.A.; Weerasuriya, S.; Palmer, K.; White, B.E.; Srirajaskanthan, R.; Chandrakumaran, K.; Ramage, J.K. Primary renal neuroendocrine neoplasms: A systematic literature review, report of four local cases, and original survival analysis of 63 patients from a national registry 2012–2018. J. Neuroendocrinol. 2022, 34, e13215. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Hu, C.Y.; Batra, R.; Lin, A.Y. Small cell carcinoma of the kidney: A case report and analysis of data from the Surveillance, Epidemiology, and End Results registry. J. Med. Case Rep. 2019, 13, 71. [Google Scholar] [CrossRef]
- Shimbori, M.; Osaka, K.; Kawahara, T.; Kasahara, R.; Kawabata, S.; Makiyama, K.; Kondo, K.; Nakaigawa, N.; Yamanaka, S.; Yao, M. Large cell neuroendocrine carcinoma of the kidney with cardiac metastasis: A case report. J. Med. Case Rep. 2017, 11, 7–11. [Google Scholar] [CrossRef][Green Version]


| Study Design | NCT | Drug | Number of Patients | Primary Endpoint | Results | Study Completion | |
|---|---|---|---|---|---|---|---|
| Avelumab [164] | Phase II single-arm | NCT03179410 | Avelumab | 15 | ORR | ORR: 6.7% median rPFS 1.8 months (95% CI 1.6–3.6 months) Median OS: 7.4 months (85% CI 2.8–12.6 months) | 2020 |
| Pembrolizumab + Platin-based chemotherapy [165] | Phase I single-arm | NCT03582475 | Pembrolizumab + platinum-based chemotherapy | 14 (7 in cohort NEPC) | ORR, PFS, and OS | ORR: 43% PFS rate at 12 months: 43% OS rate at 12 months: 71% | 2022 |
| BXCL701 (Talabostat) + Pembrolizumab [166] | Phase IIa single-arm | NCT03910660 | Talabostat + Pembrolizumab | 34 (25 evaluable for response) | Composite response (either OR by RECIST 1.1 or PSA50 or CTC count conversion from ≥5/7.5 mL to <5/7.5 mL from baseline) | ORR 20% DCR 48% | Primary completion 2023 Study completion estimated 2025 |
| Alisertib [163] | phase II single-arm | NCT01799278 | Alisertib | 60 | 6-month rPFS | mPFS:2.2 months (95% CI 2.0–2.6) mOS 9.5 months (95% CI 7.4–13.0) | 2017 |
| Rovalpituzumab tesirine (Rova-t) | Phase I/II | NCT02709889 | Rovalpituzumab tesirine (Rova-t) | 101 (NEC/NET cohort from multiple primary sites, 21 patiens with NEPC) | TEAEs | Grade 3/4 AEs: 54% [pleural effusion (5%), pericardial effusion (4%), and dyspnea (3%)] ORR overall population: 10% ORR NEC/NET: 13% | 2019 |
| Name of Study and Population | Phase | Study Interventions | NCT Identifiers | Estimated Study Completion |
|---|---|---|---|---|
| Lu177-Dotatate in metastatic Prostate Cancer with Neuroendocrine Differentiation | II | Lutetium Lu 177-dotate (4 cycles) | NCT05691465 | 2024 |
| BXCL701 and Pembrolizumab in Patients with mCRPC Small Cell Neuroendocrine Prostate Cancer Phenotype | IIb | BXCL701 +/− Pembrolizumab | NCT03910660 | 2025 |
| PLANE-PC: Pembrolizumab and Lenvatinib in Advanced/Metastatic Neuroendocrine Prostate Cancer | II | Lenvatinib + Pembrolizumab | NCT04848337 | 2023 |
| A Phase 1 Study of PT217 in Patients with Advanced Refractory Cancers Expressing DLL3 (NEPC, GEP-NET, SCLC, LCNEC) | I | PT217 (bispecific antibody against DDL3 and CD47) | NCT05652686 | 2025 |
| Apalutamide Plus Cetrelimab in Patients with Treatment-Emergent Small Cell Neuroendocrine Prostate Cancer | II | Apalutamide + Cetrelimab (anti-PD1 antibody) | NCT04926181 | 2026 |
| DeLLpro-300: A Study of Tarlatamab in Participants with Neuroendocrine Prostate Cancer | I | Tarlatamab (DLL3-rargeting BITE) | NCT04702737 | 2025 |
| CHAMP: A Study of Chemoimmunotherapy for the Treatment of Men with Neuroendocrine or Aggressive Variant Metastatic Prostate Cancer | II | Nivolumab (w3), ipilimumab (w6), carboplatin (w3) and cabazitaxel (w3) × 10 cycles followed by maintenance nivolumab and ipilimumab (max 3 years) | NCT04709276 | 2027 |
| Multicenter Trial of ESK981 in Patients with Select Solid Tumors (included cohort of NEPC) | II | ESK981 | NCT05988918 | 2029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stumpo, S.; Formelli, M.G.; Persano, I.; Parlagreco, E.; Lauricella, E.; Rodriquenz, M.G.; Guerrera, L.P.; Zurlo, I.V.; Campana, D.; Brizzi, M.P.; et al. Extrapulmonary Neuroendocrine Carcinomas: Current Management and Future Perspectives. J. Clin. Med. 2023, 12, 7715. https://doi.org/10.3390/jcm12247715
Stumpo S, Formelli MG, Persano I, Parlagreco E, Lauricella E, Rodriquenz MG, Guerrera LP, Zurlo IV, Campana D, Brizzi MP, et al. Extrapulmonary Neuroendocrine Carcinomas: Current Management and Future Perspectives. Journal of Clinical Medicine. 2023; 12(24):7715. https://doi.org/10.3390/jcm12247715
Chicago/Turabian StyleStumpo, Sara, Maria Giovanna Formelli, Irene Persano, Elena Parlagreco, Eleonora Lauricella, Maria Grazia Rodriquenz, Luigi Pio Guerrera, Ina Valeria Zurlo, Davide Campana, Maria Pia Brizzi, and et al. 2023. "Extrapulmonary Neuroendocrine Carcinomas: Current Management and Future Perspectives" Journal of Clinical Medicine 12, no. 24: 7715. https://doi.org/10.3390/jcm12247715
APA StyleStumpo, S., Formelli, M. G., Persano, I., Parlagreco, E., Lauricella, E., Rodriquenz, M. G., Guerrera, L. P., Zurlo, I. V., Campana, D., Brizzi, M. P., Cives, M., La Salvia, A., & Lamberti, G. (2023). Extrapulmonary Neuroendocrine Carcinomas: Current Management and Future Perspectives. Journal of Clinical Medicine, 12(24), 7715. https://doi.org/10.3390/jcm12247715







