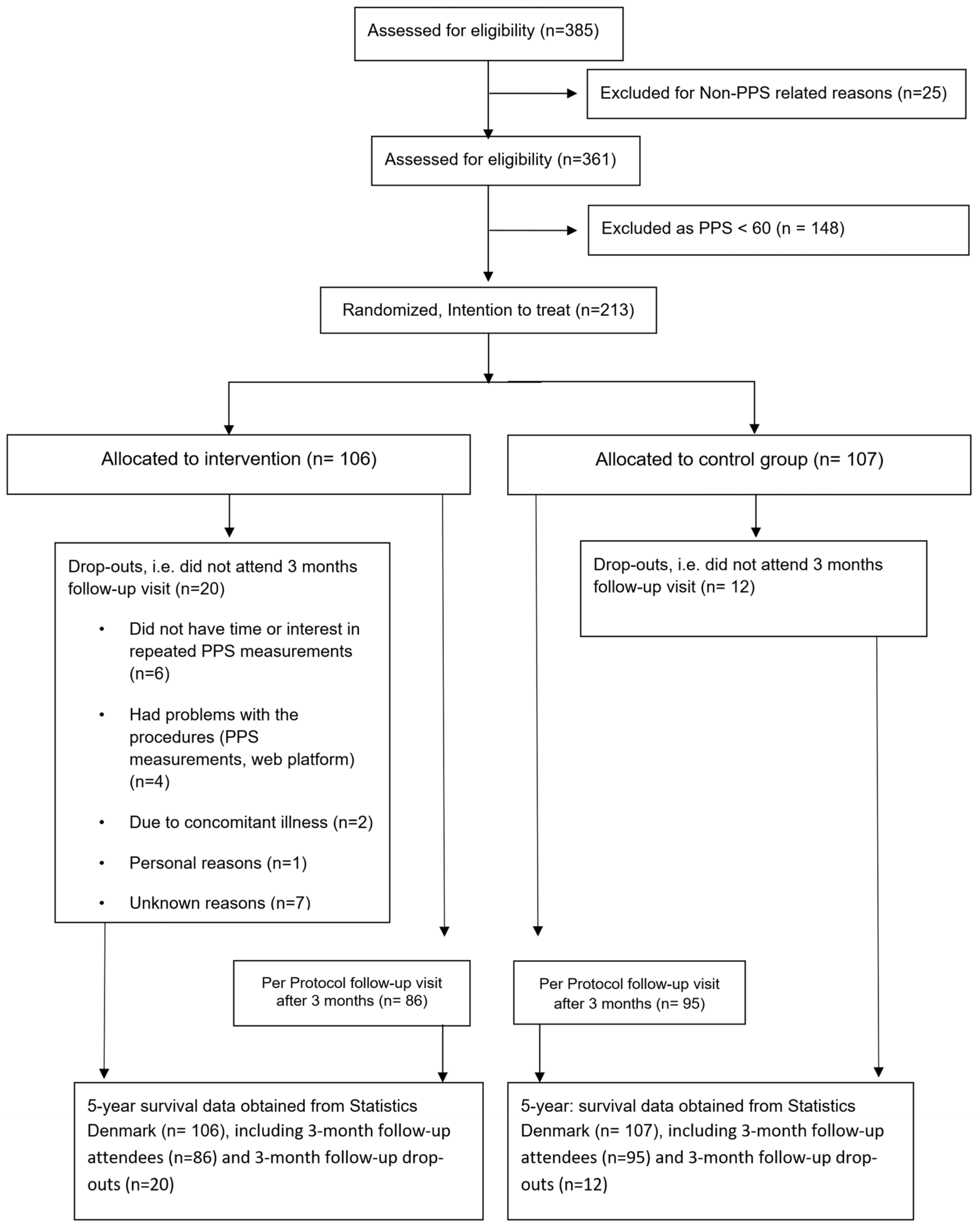
In Ischemic Heart Disease, Reduced Sensitivity to Pressure at the Sternum Accompanies Lower Mortality after Five Years: Evidence from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Design and Participants
2.3. Outcome Measures
2.4. PPS Measurement
2.5. Interventions
2.6. Active Intervention
- (1)
- Mandatory daily PPS measurements at home with instruction of how to perform PPS measurements, including a guideline for interpretation of the PPS measure, how to reflect on the measure, and a guide to clinical signs of alarm that require immediate attention.
- (2)
- Mandatory daily cutaneous sensory nerve stimulation at specific sites on the body surface aimed at a reduction in elevated baseline PPS values and subsequent maintenance of low resting PPS.
- (3)
- Daily recording of PPS measures in a web journal as a personal guide to the effect of the intervention, with ad hoc cognitive reflection in cases of sudden elevations of the PPS measure.
- (4)
- Ongoing professional surveillance based on a personal web journal allowing pro-active professional intervention in cases of missing or deviating PPS measurements.
- (5)
- A range of free-choice mental and physical exercises presented in the book of general stress management aimed at reducing stress in support of persistent lowering of resting PPS.
2.7. Passive Intervention
2.8. Statistics
2.9. Meta-Analysis
3. Results
3.1. Active and Passive Intervention Groups versus General Population
3.2. Active versus Passive Intervention Groups
4. Discussion
4.1. PPS Measure, ANSD, and Mortality of Patients with Ischemic Heart Disease
4.2. Modulation of Sensitivity to Periosteal Pressure
4.3. Meta-Analysis of Pooled Data
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Autonomic nervous system |
| ANSD | Autonomic nervous system dysfunction |
| CCS | Canadian Cardiovascular Society grading of angina pectoris |
| CONSORT | Consolidated Standards of Reporting Trials |
| IHD | Ischemic heart disease |
| ITT | Intention-to-treat |
| PP | Per protocol |
| PPS | Periosteal pressure sensitivity |
| T2D | Type 2 diabetes mellitus |
References
- Moran, A.E.; Forouzanfar, M.H.; Roth, G.A.; Mensah, G.A.; Ezzati, M.; Flaxman, A.; Murray, C.J.; Naghavi, M. The global burden of ischemic heart disease in 1990 and 2010: The Global Burden of Disease 2010 study. Circulation 2014, 129, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.D. Neural control of the circulation. Adv. Physiol. Educ. 2011, 35, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Fox, K.; Ford, I.; Steg, P.G.; Tendera, M.; Robertson, M.; Ferrari, R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet 2008, 372, 817–821. [Google Scholar] [CrossRef] [PubMed]
- De Backer, G.; Ambrosioni, E.; Borch-Johnsen, K.; Brotons, C.; Cifkova, R.; Dallongeville, J.; Ebrahim, S.; Faergeman, O.; Graham, I.; Mancia, G.; et al. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis 2003, 171, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L.; Morris, R.W. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch. Intern. Med. 2005, 165, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Song, C.L.; Bin, L.; Diao, H.Y.; Wang, J.H.; Shi, Y.F.; Lu, Y.; Wang, G.; Guo, Z.Y.; Li, Y.X.; Liu, J.G.; et al. Diagnostic Value of Serum YKL-40 Level for Coronary Artery Disease: A Meta-Analysis. J. Clin. Lab. Anal. 2016, 30, 23–31. [Google Scholar] [CrossRef]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef]
- Grippo, A.J.; Johnson, A.K. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009, 12, 1–21. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef]
- Goldstein, D.S. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R301–R317. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. In pursuit of resilience: Stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci. 2016, 1373, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Koenig, J.; Sgoifo, A.; Ottaviani, C. Autonomic and Brain Morphological Predictors of Stress Resilience. Front. Neurosci. 2018, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab. J. 2019, 43, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Novak, P. Quantitative autonomic testing. J. Vis. Exp. 2011, 53, e2502. [Google Scholar] [CrossRef]
- Graham, L.N.; Smith, P.A.; Stoker, J.B.; Mackintosh, A.F.; Mary, D.A. Time course of sympathetic neural hyperactivity after uncomplicated acute myocardial infarction. Circulation 2002, 106, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Boundy, E.O.; Dastjerdi, R.; Spiegelman, D.; Fawzi, W.W.; Missmer, S.A.; Lieberman, E.; Kajeepeta, S.; Wall, S.; Chan, G.J. Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis. Pediatrics 2016, 137, e20152238. [Google Scholar] [CrossRef] [PubMed]
- Moberg, K.U.; Handlin, L.; Petersson, M. Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact—With a particular focus on the oxytocinergic system. Infant. Behav. Dev. 2020, 61, 101482. [Google Scholar] [CrossRef]
- Salvini, V.; Accioli, R.; Lazzerini, P.E.; Acampa, M. New challenges and future perspectives in autonomic neuroscience. Front. Neurosci. 2023, 17, 1271499. [Google Scholar] [CrossRef]
- Rawstorn, J.C.; Gant, N.; Direito, A.; Beckmann, C.; Maddison, R. Telehealth exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Heart 2016, 102, 1183–1192. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Pena-Gil, C.; Abu-Assi, E.; Raposeiras, S.; van’t Hof, A.; Meindersma, E.; Prescott, E.I.B.; González-Juanatey, J.R. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016, 223, 436–443. [Google Scholar] [CrossRef]
- Ballegaard, S.; Borg, E.; Karpatschof, B.; Nyboe, J.; Johannessen, A. Long-term effects of integrated rehabilitation in patients with advanced angina pectoris: A nonrandomized comparative study. J. Altern. Complement. Med. 2004, 10, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, G.; Ballegaard, S.; Karpatschof, B.; Nyboe, J. Long-term effects of integrated rehabilitation in patients with stroke: A nonrandomized comparative feasibility study. J. Altern. Complement. Med. 2010, 16, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, N.; Ballegaard, S.; Holmager, P.; Kristiansen, J.; Gyntelberg, F.; Andersen, L.J.; Hjalmarson, A.; Bech, P.; Arendt-Nielsen, L.; Faber, J. Pressure pain sensitivity: A new method of stress measurement in patients with ischemic heart disease. Scand. J. Clin. Lab. Investig. 2013, 73, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, N.; Ballegaard, S.; Bech, P.; Hjalmarson, A.; Krogh, J.; Gyntelberg, F.; Faber, J. The effect of daily self-measurement of pressure pain sensitivity followed by acupressure on depression and quality of life versus treatment as usual in ischemic heart disease: A randomized clinical trial. PLoS ONE 2014, 9, e97553. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, S.; Bergmann, N.; Karpatschof, B.; Kristiansen, J.; Gyntelberg, F.; Arendt-Nielsen, L.; Bech, P.; Hjalmarson, Å.; Faber, J. Association between pressure pain sensitivity and autonomic function as assessed by a tilt table test. Scand. J. Clin. Lab. Investig. 2015, 75, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Bannister, L.; Berry, M.; Collins, P.; Dyson, M.; Dussek, J.; Ferguson, M. The anatomical basis of medicine and surgery. Gray’s Anat. 1995, 38111, 512–514. [Google Scholar]
- Lewis, J.A.; Machin, D. Intention to treat--who should use ITT? Br. J. Cancer 1993, 68, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R.; Spjøtvoll, E.; Johansen, S.; van Zwet, W.R.; Bithell, J.F.; Barndorff-Nielsen, O.; Keuls, M. The Role of Significance Tests. Scand. J. Stat. 1977, 4, 49–70. [Google Scholar]
- Hochman, J.S.; Anthopolos, R.; Reynolds, H.R.; Bangalore, S.; Xu, Y.; O’Brien, S.M.; Mavromichalis, S.; Chang, M.; Contreras, A.; Rosenberg, Y. Survival after invasive or conservative management of stable coronary disease. Circulation 2023, 147, 8–19. [Google Scholar] [CrossRef]
- Sidney, S.; Sorel, M.E.; Quesenberry, C.P.; Jaffe, M.G.; Solomon, M.D.; Nguyen-Huynh, M.N.; Go, A.S.; Rana, J.S. Comparative Trends in Heart Disease, Stroke, and All-Cause Mortality in the United States and a Large Integrated Healthcare Delivery System. Am. J. Med. 2018, 131, 829–836.e1. [Google Scholar] [CrossRef] [PubMed]
- Quinones, A.; Lobach, I.; Maduro, G.A., Jr.; Smilowitz, N.R.; Reynolds, H.R. Diabetes and ischemic heart disease death in people age 25–54: A multiple-cause-of-death analysis based on over 400,000 deaths from 1990 to 2008 in New York City. Clin. Cardiol. 2015, 38, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, S.; Petersen, P.B.; Harboe, G.S.; Karpatschof, B.; Gyntelberg, F.; Faber, J. The association between changes in pressure pain sensitivity and changes in cardiovascular physiological factors associated with persistent stress. Scand. J. Clin. Lab. Investig. 2014, 74, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Eldrup, E.; Selmer, C.; Pichat, C.; Hecquet, S.K.; Watt, T.; Kreiner, S.; Karpatschof, B.; Gyntelberg, F.; Ballegaard, S. Reduction of pressure pain sensitivity as novel non-pharmacological therapeutic Approach to type 2 diabetes: A randomized trial. Front. Neurosci. 2021, 15, 613858. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Ballegaard, S.; Ørsted, N.; Eldrup, E.; Karpatschof, B.; Gyntelberg, F.; Hecquet, S.K.; Gjedde, A. In Type 2 Diabetes Mellitus, normalization of hemoglobin A1c accompanies reduced sensitivity to pressure at the sternum. Front. Neurosci. 2023, 17, 1067098. [Google Scholar] [CrossRef] [PubMed]
- Winchester, D.E.; Pepine, C.J. Usefulness of Beta blockade in contemporary management of patients with stable coronary heart disease. Am. J. Cardiol. 2014, 114, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, Cd003177. [Google Scholar] [CrossRef]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Dong, S.Y.; Yan, S.T.; Wang, M.L.; Li, Z.B.; Fang, L.Q.; Zeng, Q. Associations of body weight and weight change with cardiovascular events and mortality in patients with coronary heart disease. Atherosclerosis 2018, 274, 104–111. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, Cd011737. [Google Scholar] [CrossRef]
- Koenig, H.G. Religion, spirituality, and health: The research and clinical implications. ISRN Psychiatry 2012, 2012, 278730. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Fry, J.S.; Thornton, A.J. Updating the evidence relating smoking bans to incidence of heart disease. Regul. Toxicol. Pharmacol. 2019, 101, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Chandrasekaran, A.M.; Singh, K.; Mohan, B.; Chattopadhyay, K.; Chadha, D.S.; Negi, P.C.; Bhat, P.; Sadananda, K.S.; Ajay, V.S.; et al. Yoga-Based Cardiac Rehabilitation After Acute Myocardial Infarction: A Randomized Trial. J. Am. Coll. Cardiol. 2020, 75, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, Cd009825. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.H.; Anderson, L.; Jenkinson, C.E.; Whalley, B.; Rees, K.; Davies, P.; Bennett, P.; Liu, Z.; West, R.; Thompson, D.R.; et al. Psychological interventions for coronary heart disease: Cochrane systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2018, 25, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, N.; Saab, P.G.; Catellier, D.J.; Powell, L.H.; DeBusk, R.F.; Williams, R.B.; Carney, R.M.; Raczynski, J.M.; Cowan, M.J.; Berkman, L.F.; et al. Psychosocial treatment within sex by ethnicity subgroups in the Enhancing Recovery in Coronary Heart Disease clinical trial. Psychosom. Med. 2004, 66, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina: From Pathophysiology to New Therapeutic Nonpharmacological Technologies. JACC Cardiovasc. Interv. 2020, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kupers, R.; Fumal, A.; de Noordhout, A.M.; Gjedde, A.; Schoenen, J.; Ptito, M. Transcranial magnetic stimulation of the visual cortex induces somatotopically organized qualia in blind subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13256–13260. [Google Scholar] [CrossRef]
- Lundby, L.; Møller, A.; Buntzen, S.; Krogh, K.; Vang, K.; Gjedde, A.; Laurberg, S. Relief of fecal incontinence by sacral nerve stimulation linked to focal brain activation. Dis. Colon Rectum 2011, 54, 318–323. [Google Scholar] [CrossRef]
- Landau, A.M.; Dyve, S.; Jakobsen, S.; Alstrup, A.K.; Gjedde, A.; Doudet, D.J. Acute Vagal Nerve Stimulation Lowers α2 Adrenoceptor Availability: Possible Mechanism of Therapeutic Action. Brain Stimul. 2015, 8, 702–707. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Richner, M.; Pallesen, L.T.; Ulrichsen, M.; Poulsen, E.T.; Holm, T.H.; Login, H.; Castonguay, A.; Lorenzo, L.E.; Goncalves, N.P.; Andersen, O.M.; et al. Sortilin gates neurotensin and BDNF signaling to control peripheral neuropathic pain. Sci. Adv. 2019, 5, eaav9946. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K.; Handlin, L.; Petersson, M. Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 2014, 5, 1529. [Google Scholar] [CrossRef]
- Walker, S.C.; Trotter, P.D.; Swaney, W.T.; Marshall, A.; McGlone, F.P. C-tactile afferents: Cutaneous mediators of oxytocin release during affiliative tactile interactions? Neuropeptides 2017, 64, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Yarnitsky, D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J. Pain 2009, 10, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, C.K.; Ballegaard, S.; Karpatschof, B.; Schousen, P. Pressure pain sensitivity as a marker for stress and pressure pain sensitivity-guided stress management in women with primary breast cancer. Scand. J. Clin. Lab. Investig. 2014, 74, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Blais, M.; Baer, L. Understanding rating scales and assessment instruments. In Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health; Humana Press: New York, NY, USA, 2010; pp. 1–6. [Google Scholar]
- Murray, E.J.; Caniglia, E.C.; Petito, L.C. Causal survival analysis: A guide to estimating intention-to-treat and per-protocol effects from randomized clinical trials with non-adherence. Res. Methods Med. Health Sci. 2021, 2, 39–49. [Google Scholar] [CrossRef]
- Califf, R.M.; DeMets, D.L. Principles from clinical trials relevant to clinical practice: Part I. Circulation 2002, 106, 1015–1021. [Google Scholar] [CrossRef]
- Sawilowsky, S.S.; Fahoome, G. Statistics through Monte Carlo Simulation with FORTRAN. Journal of Modern Applied Statistical Methods Inc, Michigan. 2003. Available online: https://digitalcommons.wayne.edu/jmasm/ (accessed on 3 December 2023).
- Mwangi, M. A Comparison of Cox and Poisson Regression in the Analysis of Survival Data. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2010. [Google Scholar]
- Crowther, M.J.; Riley, R.D.; Staessen, J.A.; Wang, J.; Gueyffier, F.; Lambert, P.C. Individual patient data meta-analysis of survival data using Poisson regression models. BMC Med Res Methodol. 2012, 12, 34. [Google Scholar] [CrossRef]

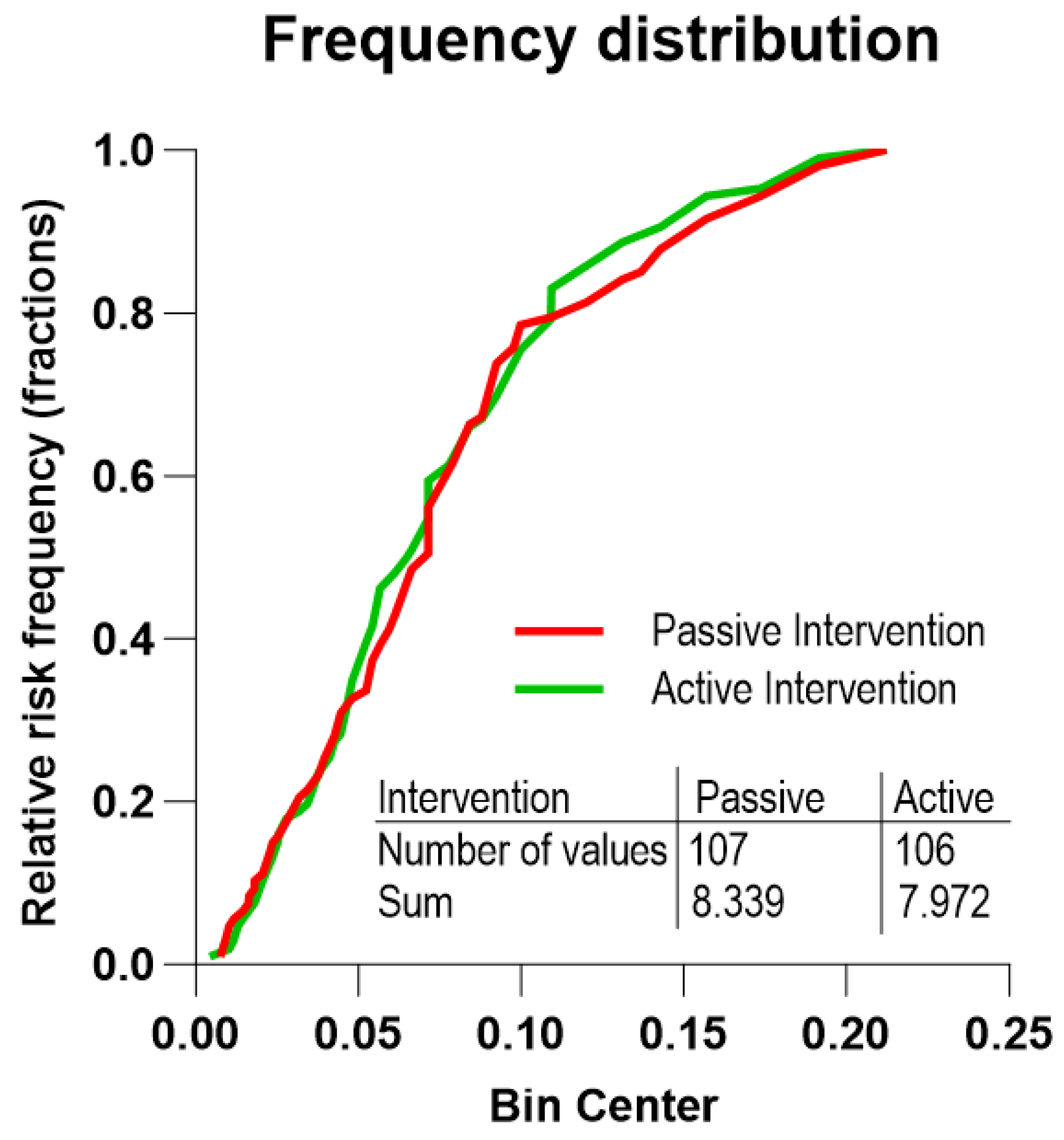
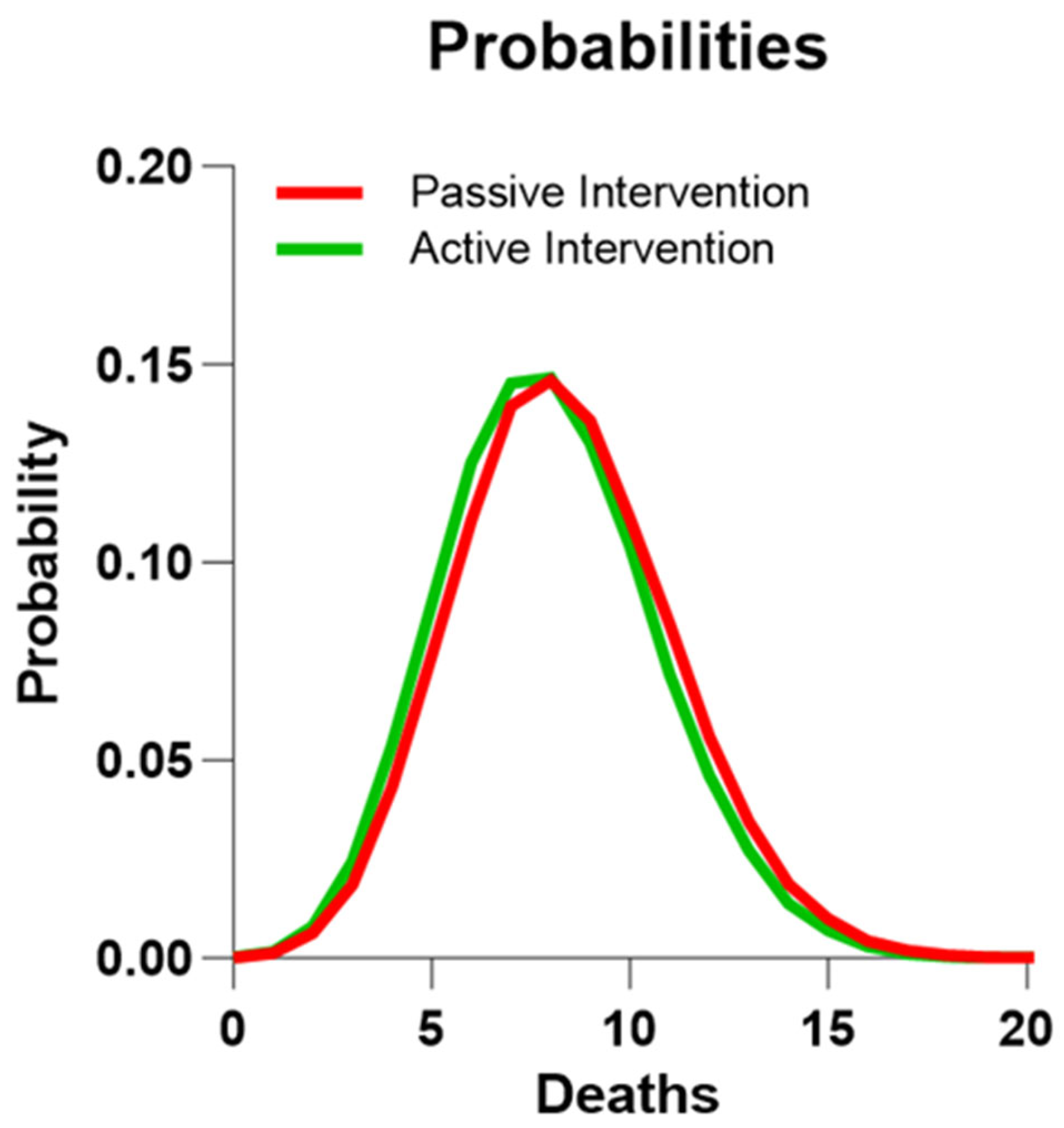
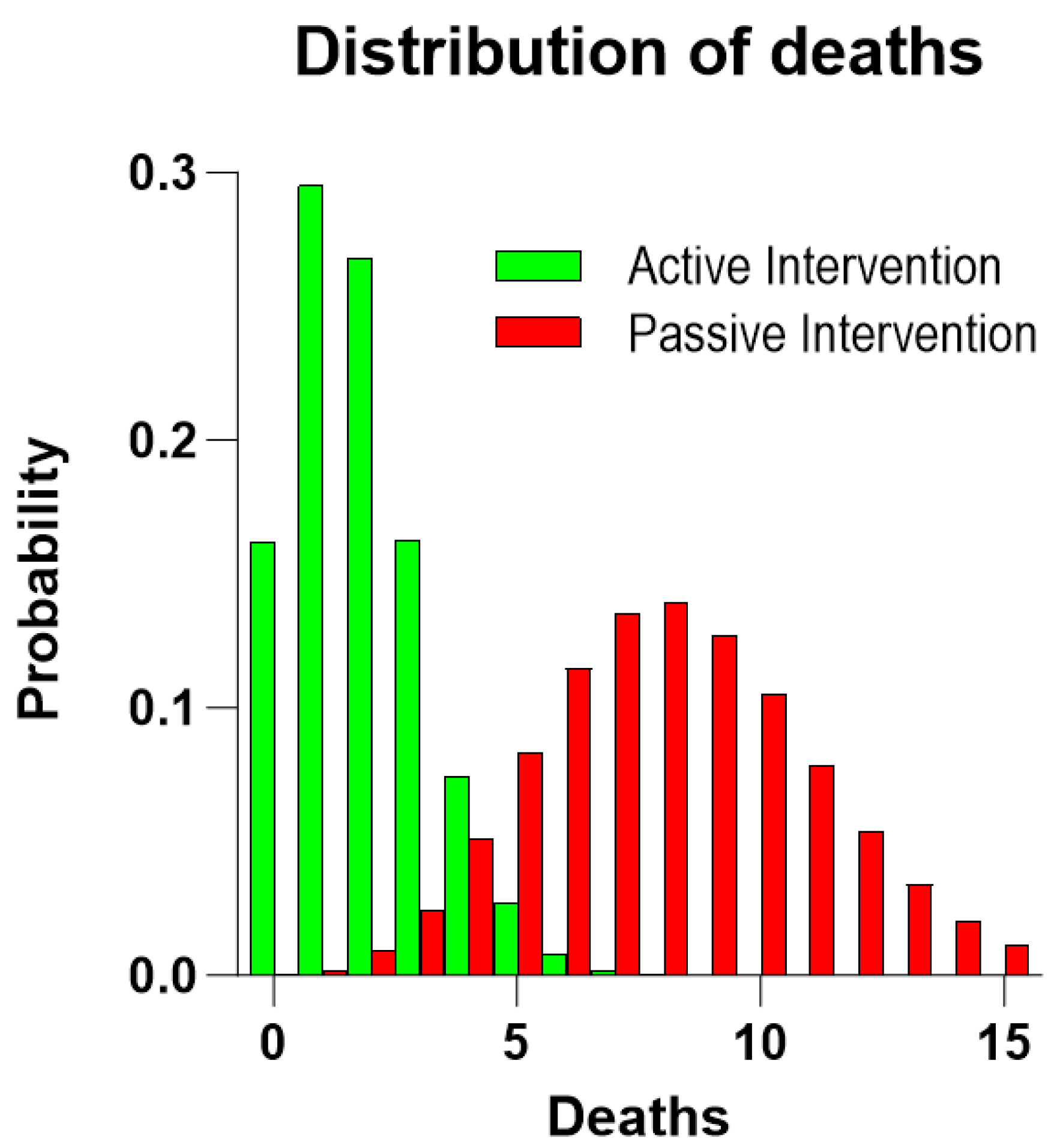

| Full Sample | Active Group | Passive Group | p-Value | |
|---|---|---|---|---|
| Number of participants (n) | 213 | 106 | 107 | |
| Male (n, %) | 156 (73%) | 78 (74%) | 78 (73%) | NS * |
| Age in years (mean, SD) | 62 (8.1) | 62 (8.1) | 62 (8.2) | NS |
| Psychometrics | ||||
| MDI (mean, SD) | 8.9 (7.4) | 8.4 (7.7) | 9.4 (7.0) | NS |
| WHO-5 (mean, SD) | 65 (19) | 67 (19) | 63 (19) | NS |
| PPS (mean, SD) | 81 (13) | 81 (13) | 81 (13) | NS |
| SF-36 PCS (mean, SD) | 48 (8.4) | 48 (9.1) | 48 (7.6) | NS |
| SF-36 MCS (mean, SD) | 52 (9.3) | 53 (9.3) | 52 (9.3) | NS |
| CSS (mean, SD) | 9.7 (7.1) | 9.2 (6.5) | 10 (7.6) | NS |
| Social status | ||||
| Married or cohabitating (n, %) | 175 (82%) | 83 (78%) | 92 (86%) | NS |
| Have children (n, %) | 190 (92%) | 97 (91%) | 96 (90%) | NS |
| Employment status | ||||
| Employed (n, %) | 106 (50%) | 54 (51%) | 52 (49%) | NS |
| Unemployed (n, %) | 4 (2%) | 3 (3%) | 1 (1%) | NS |
| Retired (n, %) | 92 (47%) | 46 (44%) | 52 (48%) | NS |
| Cardiac variables | ||||
| Self-reported time (years) since first AMI (mean, SD) | 7.5 (5.8) | 8.2 (6.5) | 6.8 (5.0) | NS |
| Previous AMI (n, %) | 136 (64%) | 69 (65%) | 67 (63%) | NS |
| Treated with PCI (n, %) | 147 (69%) | 73 (69%) | 74 (69%) | NS |
| Treated with CABG (n, %) | 52 (24%) | 27 (25%) | 25 (23%) | NS |
| In-hospital days during last 12 months before inclusion due to cardiac disease (n, (%), mean number of days) | 46 (22%) 6 days | 18 (17%) 6 days | 28 (26%) 7 days | NS |
| Visits to cardiac outpatient clinic during last 12 months before inclusion (n (%), mean number of visits | 68 (32%), 3 visits | 27 (25%) 4 visits | 41 (39%) 3 visits | NS |
| Visits to cardiologist during last 12 months before inclusion (n (%), mean number of visits | 29 (16%) 2 visits | 13 (12%) 2 visits | 16 (15%) 2 visits | NS |
| Visits to general practitioner during last 12 months before inclusion for cardiac disease (n (%), mean number of visits | 90 (47%) 3 visits | 46 (43%) 3 visits | 44 (42%) 2 visits | NS |
| CCS Angina Pectoris Class I | 55 (26%) | 26 (25%) | 29 (27%) | NS |
| CCS Angina Pectoris Class II | 25 (12%) | 14 (14%) | 11 (10%) | NS |
| CCS Angina Pectoris Class III | 5 (2%) | 1 (1%) | 4 (4%) | NS |
| CCS Angina Pectoris Class IV | 2 (1%) | 1 (1%) | 1 (1%) | NS |
| Chest pain at rest | 71 (34%) | 39 (38%) | 32 (30%) | NS |
| Resting pulse (mean, SD) | 61 (11) | 61 (11) | 60 (11) | NS |
| MAP (mean, SD) | 98 (10) | 98 (9.7) | 97 (11) | NS |
| Cardiac risk factors | ||||
| BMI (mean, SD) | 27.6 (4.3) | 27.8 (4.3) | 27.4 (4.4) | NS |
| Triglyceride (mean, SD) | 1.5 (0.9) | 1.4 (0.7) | 1.5 (1.0) | NS |
| Current smoker (n, %) | 22 (10%) | 9 (9%) | 13 (12%) | NS |
| Heart rate ≥ 70 beats/minute (n, %) | 35 (16%) | 20 (19%) | 15 (14%) | NS |
| Self-reported co-morbidity | ||||
| Heart failure (n, %) | 72 (34%) | 29 (27%) | 43 (40%) | NS |
| Chronic obstructive lung disease (n, %) | 13 (6%) | 5 (5%) | 8 (8%) | NS |
| Diabetes (n, %) | 28 (13%) | 20 (19%) | 8 (8%) | p = 0.015 |
| Previous cerebral insults (n, %) | 15 (7%) | 7 (7%) | 8 (8%) | NS |
| Have been treated for depression (n, %) | 32 (15%) | 12 (11%) | 20 (19%) | NS |
| Elevated depression score (i.e., MDI score ≥ 15, indication incipient depression) | 41 (19%) | 21 (20%) | 20 (19%) | NS |
| Medication | ||||
| Beta-blockers (n, %) | 125 (60%) | 65 (61%) | 60 (57%) | NS |
| Cholesterol-lowering medication (n, %) | 188 (90%) | 94 (89%) | 94 (88%) | NS |
| Calcium antagonists (n, %) | 47 (23%) | 26 (25%) | 21 (20%) | NS |
| Angiotensin-II antagonist and/or ACE inhibitors (n, %) | 115 (55%) | 56 (53%) | 59 (55%) | NS |
| Diuretics (thiazide or furosemide) (n, %) | 74 (36%) | 40 (39%) | 34 (33%) | NS |
| Anti-depressive medication (n, %) | 12 (6%) | 4 (4%) | 8 (8%) | NS |
| RCT Group | n | Five-Year Risks of Death Mean (Range) | Predicted Number of Deaths | Number of Deaths (95% CI) | p-Value |
|---|---|---|---|---|---|
| Active group | 106 | 0.075 (0.0043–0.2123) | 7.97 | ≤3 (0.5–6.6) | 0.01 |
| Passive group | 107 | 0.078 (0.0076–0.2123) | 8.33 | 8 (3.8–14.1) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballegaard, S.; Faber, J.; Selmer, C.; Gyntelberg, F.; Kreiner, S.; Karpatschof, B.; Klausen, T.W.; Hjalmarson, Å.; Gjedde, A. In Ischemic Heart Disease, Reduced Sensitivity to Pressure at the Sternum Accompanies Lower Mortality after Five Years: Evidence from a Randomized Controlled Trial. J. Clin. Med. 2023, 12, 7585. https://doi.org/10.3390/jcm12247585
Ballegaard S, Faber J, Selmer C, Gyntelberg F, Kreiner S, Karpatschof B, Klausen TW, Hjalmarson Å, Gjedde A. In Ischemic Heart Disease, Reduced Sensitivity to Pressure at the Sternum Accompanies Lower Mortality after Five Years: Evidence from a Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(24):7585. https://doi.org/10.3390/jcm12247585
Chicago/Turabian StyleBallegaard, Søren, Jens Faber, Christian Selmer, Finn Gyntelberg, Svend Kreiner, Benny Karpatschof, Tobias Wirenfeldt Klausen, Åke Hjalmarson, and Albert Gjedde. 2023. "In Ischemic Heart Disease, Reduced Sensitivity to Pressure at the Sternum Accompanies Lower Mortality after Five Years: Evidence from a Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 24: 7585. https://doi.org/10.3390/jcm12247585
APA StyleBallegaard, S., Faber, J., Selmer, C., Gyntelberg, F., Kreiner, S., Karpatschof, B., Klausen, T. W., Hjalmarson, Å., & Gjedde, A. (2023). In Ischemic Heart Disease, Reduced Sensitivity to Pressure at the Sternum Accompanies Lower Mortality after Five Years: Evidence from a Randomized Controlled Trial. Journal of Clinical Medicine, 12(24), 7585. https://doi.org/10.3390/jcm12247585