Short-Term Heart Rate Variability in Metabolic Syndrome: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Inclusion/Exclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
3. Results
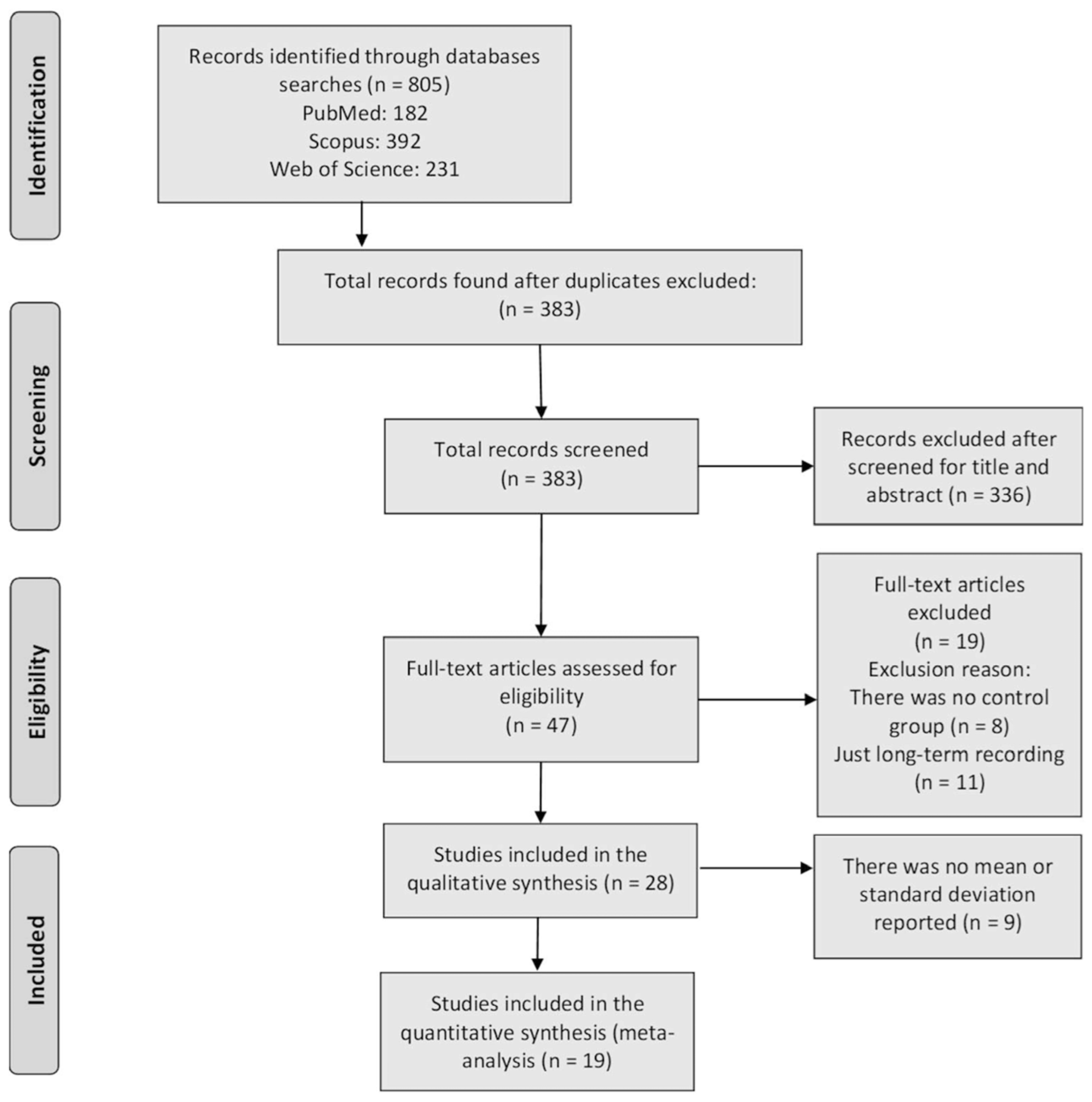
3.1. Identification of Studies
3.2. Quality Assessment
3.3. Study and Patient Characteristics
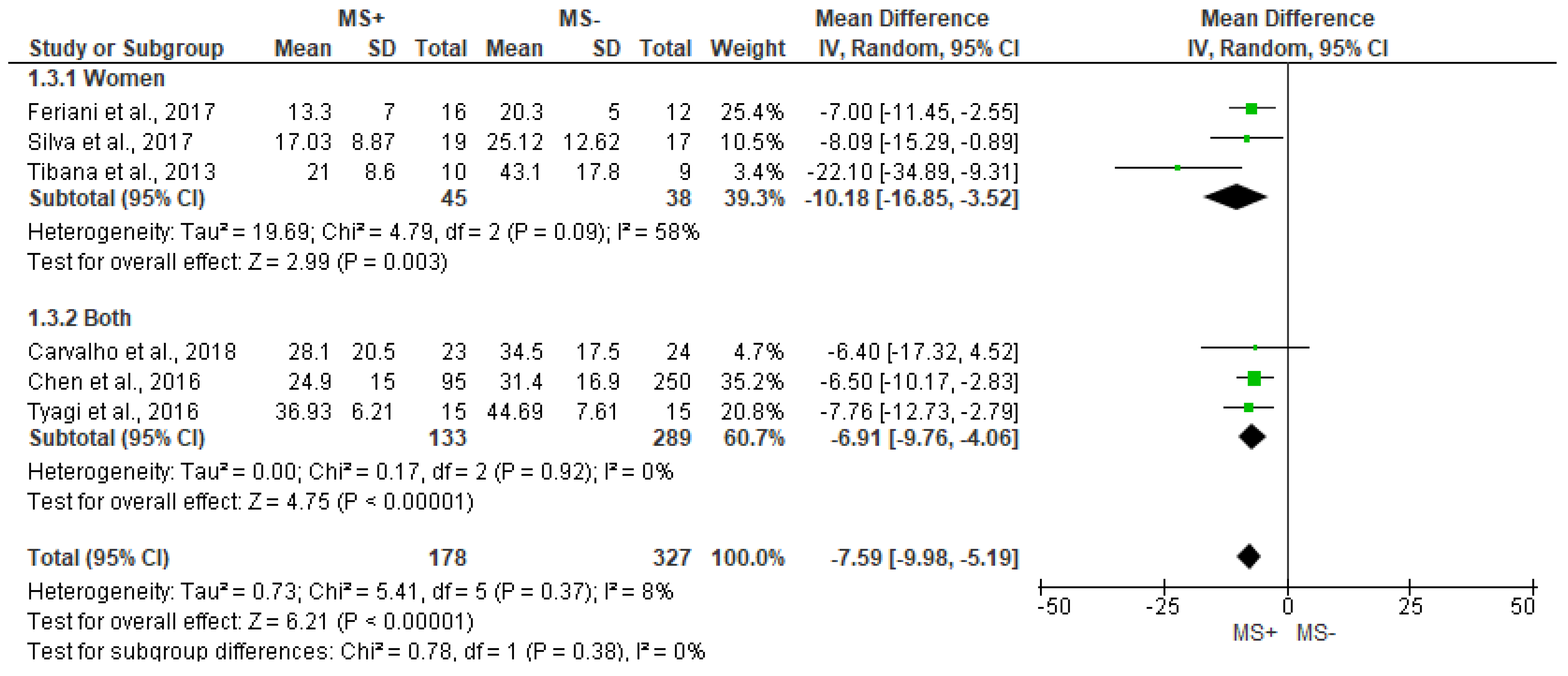
3.4. Time Domain Analysis Outcomes
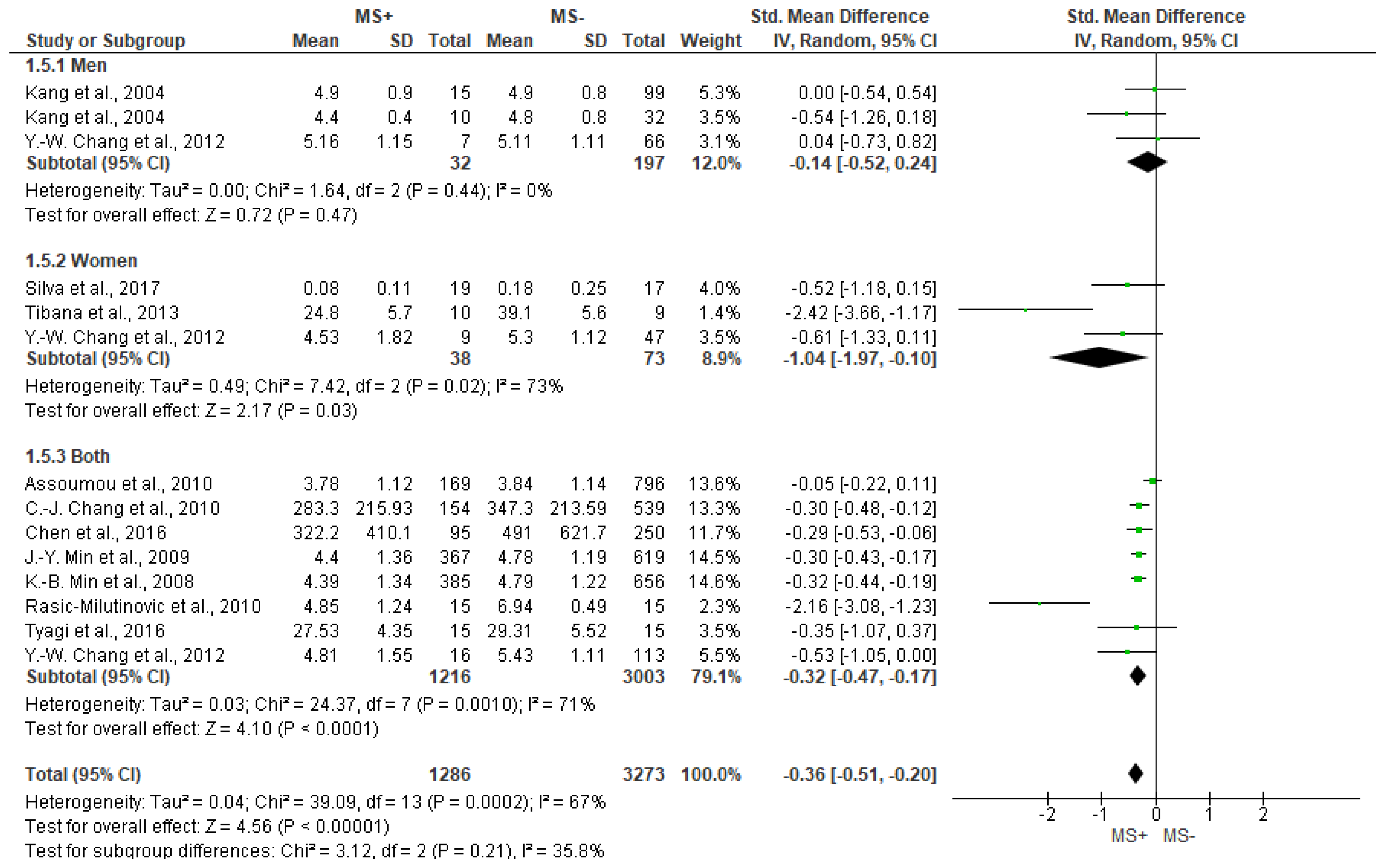
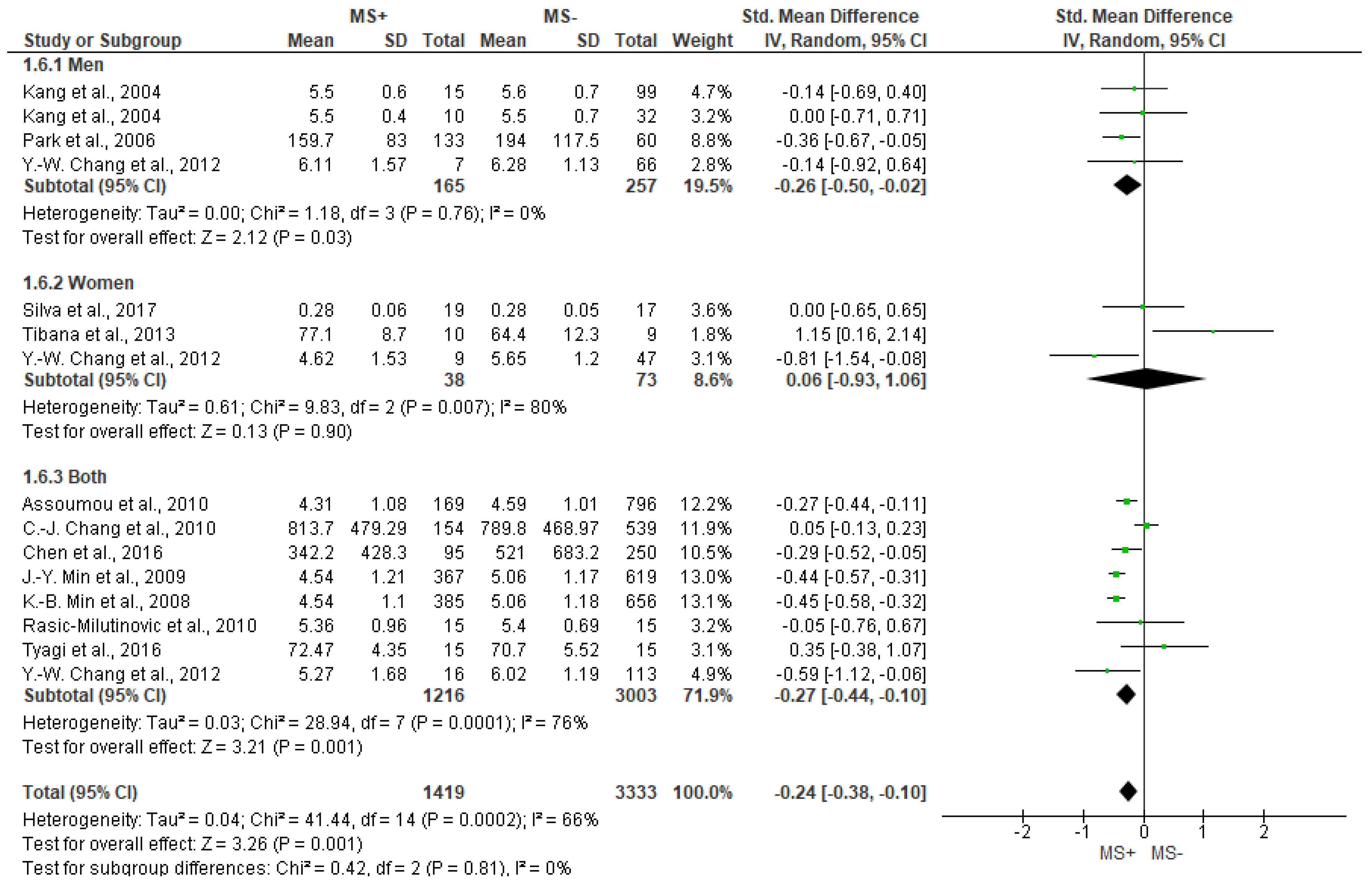
3.5. Frequency Domain Analysis Outcomes
3.6. Non-Linear Analysis Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-Wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Zupkauskiene, J.; Lauceviciene, I.; Navickas, P.; Ryliskyte, L.; Puronaite, R.; Badariene, J.; Laucevicius, A. Changes in Health-Related Quality of Life, Motivation for Physical Activity, and the Levels of Anxiety and Depression after Individualized Aerobic Training in Subjects with Metabolic Syndrome. Hell. J. Cardiol. 2022, 66, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.S.; Mechanick, J.I.; Neeland, I.J.; Herrick, C.J.; Després, J.P.; Ndumele, C.E.; Vijayaraghavan, K.; Handelsman, Y.; Puckrein, G.A.; Araneta, M.R.G.; et al. The CardioMetabolic Health Alliance Working Toward a New Care Model for the Metabolic Syndrome. J. Am. Coll. Cardiol. 2015, 66, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Kumanyika, S.; Afshin, A.; Arimond, M.; Lawrence, M.; McNaughton, S.A.; Nishida, C. Approaches to Defining Healthy Diets: A Background Paper for the International Expert Consultation on Sustainable Healthy Diets. Food Nutr. Bull. 2020, 41, 7S–30S. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Bende, F.; Fofiu, R.; Enache, A.; Pescariu, S.A.; Novacescu, D. The Impact of Metabolic Syndrome and Obesity on the Evolution of Diastolic Dysfunction in Apparently Healthy Patients Suffering from Post-COVID-19 Syndrome. Biomedicines 2022, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Lee, M.K. Autonomic Dysfunction, Diabetes and Metabolic Syndrome. J. Diabetes Investig. 2021, 12, 2108–2111. [Google Scholar] [CrossRef]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular Autonomic Neuropathy in Diabetes: Clinical Impact, Assessment, Diagnosis, and Management. Diabetes. Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef]
- Silva, L.R.B.E.; Zamuner, A.R.; Gentil, P.; Alves, F.M.; Leal, A.G.F.; Soares, V.; Silva, M.S.; Vieira, M.F.; Simoes, K.; Pedrino, G.R.; et al. Cardiac Autonomic Modulation and the Kinetics of Heart Rate Responses in the On- and off-Transient during Exercise in Women with Metabolic Syndrome. Front. Physiol. 2017, 8, 542. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Di Thommazo-Luporini, L.; Mendes, R.G.; Cabiddu, R.; Ricci, P.A.; Basso-Vanelli, R.P.; Oliveira-Junior, M.C.; Vieira, R.P.; Bonjorno-Junior, J.C.; Oliveira, C.R.; et al. Metabolic Syndrome Impact on Cardiac Autonomic Modulation and Exercise Capacity in Obese Adults. Auton. Neurosci. Basic Clin. 2018, 213, 43–50. [Google Scholar] [CrossRef]
- Malik, M.; John Camm, A.; Thomas Bigger, J.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Stuckey, M.I.; Tulppo, M.P.; Kiviniemi, A.M.; Petrella, R.J. Heart Rate Variability and the Metabolic Syndrome: A Systematic Review of the Literature. Diabetes. Metab. Res. Rev. 2014, 30, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in Heart Rate Variability with Exercise Therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2009, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, D.; Letts, L.; Pollock, N.; Bosch, J.; Westmorland, M. Critical Review Form–Quantitative Studies. McMaster Univ. Occup. Ther. Evid.-Based Pract. Res. Group. 1998. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiIodnPhbaBAxXGNt4KHa2QD6wQFnoECBEQAQ&url=https%3A%2F%2Fwww.mcgill.ca%2Fcpengine%2Ffiles%2Fcpengine%2Fquanreview_form1.doc&usg=AOvVaw1XWw38sWQFiLCfZ26bhqWz&opi=89978449 (accessed on 1 February 2023).
- Faber, I.R.; Bustin, P.M.; Oosterveld, F.G.; Elferink-Gemser, M.T.; Nijhuis-Van der Sanden, M.W. Assessing Personal Talent Determinants in Young Racquet Sport Players: A Systematic Review. J Sport. Sci 2016, 34, 395–410. [Google Scholar] [CrossRef]
- Shuster, J.J. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Liao, D.P.; Sloan, R.P.; Cascio, W.E.; Folsom, A.R.; Liese, A.D.; Evans, G.W.; Cai, J.W.; Sharrett, A.R. Multiple Metabolic Syndrome Is Associated with Lower Heart Rate Variability-The Atherosclerosis Risk in Communities Study. Diabetes Care 1998, 21, 2116–2122. [Google Scholar] [CrossRef]
- Park, S.K.; Schwartz, J.; Weisskopf, M.; Sparrow, D.; Vokonas, P.S.; Wright, R.O.; Coull, B.; Nie, H.; Hu, H. Low-Level Lead Exposure, Metabolic Syndrome, and Heart Rate Variability: The VA Normative Aging Study. Environ. Health Perspect. 2006, 114, 1718–1724. [Google Scholar] [CrossRef]
- Pennathur, S.; Jaiswal, M.; Vivekanandan-Giri, A.; White, E.A.; Ang, L.; Raffel, D.M.; Rubenfire, M.; Pop-Busui, R. Structured Lifestyle Intervention in Patients with the Metabolic Syndrome Mitigates Oxidative Stress but Fails to Improve Measures of Cardiovascular Autonomic Neuropathy. J. Diabetes Complicat. 2017, 31, 1437–1443. [Google Scholar] [CrossRef]
- Stuckey, M.I.; Kiviniemi, A.; Gill, D.P.; Shoemaker, J.K.; Petrella, R.J. Associations between Heart Rate Variability, Metabolic Syndrome Risk Factors, and Insulin Resistance. Appl. Physiol. Nutr. Metab. 2015, 40, 734–740. [Google Scholar] [CrossRef]
- Tibana, R.A.; Boullosa, D.A.; Leicht, A.S.; Prestes, J. Women with Metabolic Syndrome Present Different Autonomic Modulation and Blood Pressure Response to an Acute Resistance Exercise Session Compared with Women without Metabolic Syndrome. Clin. Physiol. Funct. Imaging 2013, 33, 364–372. [Google Scholar] [CrossRef]
- Feriani, D.J.; Coelho-Júnior, H.J.; Scapini, K.B.; de Moraes, O.A.; Mostarda, C.; Ruberti, O.M.; Uchida, M.C.; Caperuto, É.C.; Irigoyen, M.C.; Rodrigues, B.; et al. Effects of Inspiratory Muscle Exercise in the Pulmonary Function, Autonomic Modulation, and Hemodynamic Variables in Older Women with Metabolic Syndrome. J. Exerc. Rehabil. 2017, 13, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Maciel, A.W.S.; Pinto, L.M.; Campos, R.C.A.; Ferreira, A.C.; Dias-Filho, C.A.A.; Dias, C.J.M.; Pires, F.d.O.; Urtado, C.B.; Rodrigues, B.; Mostarda, C.T.; et al. Acute Effects of Resistance Exercise With Blood Flow Restriction in Elderly Women: A Pilot Study. J. Aging Phys. Act. 2021, 29, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.J.; Hemingway, H.; Walker, B.R.; Page, M.; Clarke, P.; Juneja, M.; Shipley, M.J.; Kumari, M.; Andrew, R.; Seckl, J.R.; et al. Adrenocortical, Autonomic, and Inflammatory Causes of the Metabolic Syndrome-Nested Case-Control Study. Circulation 2002, 106, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, T.; Kähönen, M.; Jula, A.; Mattsson, N.; Laitinen, T.; Keltikangas-Järvinen, L.; Viikari, J.; Välimäki, I.; Rönnemaa, T.; Raitakari, O.T.; et al. Metabolic Syndrome and Short-Term Heart Rate Variability in Young Adults: The Cardiovascular Risk in Young Finns Study. Diabet. Med. 2009, 26, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, M.H.; Haapala, E.A.; Veijalainen, A.; Seppala, S.; Oliveira, R.S.; Lintu, N.; Laitinen, T.; Tarvainen, M.P.; Lakka, T.A. Associations of Cardiometabolic Risk Factors with Heart Rate Variability in 6- to 8-Year-Old Children: The PANIC Study. Pediatr. Diabetes 2020, 21, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kangas, P.; Tikkakoski, A.; Uitto, M.; Viik, J.; Bouquin, H.; Niemelä, O.; Mustonen, J.; Pörsti, I.; Niemela, O.; Mustonen, J.; et al. Metabolic Syndrome Is Associated with Decreased Heart Rate Variability in a Sex-Dependent Manner: A Comparison between 252 Men and 249 Women. Clin. Physiol. Funct. Imaging 2019, 39, 160–167. [Google Scholar] [CrossRef]
- Assoumou, H.G.N.; Pichot, V.; Barthelemy, J.C.; Dauphinot, V.; Celle, S.; Gosse, P.; Kossovsky, M.; Gaspoz, J.M.; Roche, F. Metabolic Syndrome and Short-Term and Long-Term Heart Rate Variability in Elderly Free of Clinical Cardiovascular Disease: The PROOF Study. Rejuvenation Res. 2010, 13, 653–663. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Sandercock, G.; Vale, S.; Santos, R.; Abreu, S.; Moreira, C.; Mota, J. Metabolic Syndrome, Physical Activity and Cardiac Autonomic Function. Diabetes-Metab. Res. Rev. 2012, 28, 363–369. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.R.; Milicevic, D.R.; Milovanovic, B.D.; Perunicic-Pekovic, G.B.; Pencic, B.D. Do Components of Metabolic Syndrome Contribute to Cardiac Autonomic Neuropathy in Non-Diabetic Patients? SAUDI Med. J. 2010, 31, 650–657. [Google Scholar]
- Chang, C.-J.; Yang, Y.-C.; Lu, F.-H.; Lin, T.-S.; Chen, J.-J.; Yeh, T.-L.; Wu, C.-H.; Wu, J.-S. Altered Cardiac Autonomic Function May Precede Insulin Resistance in Metabolic Syndrome. Am. J. Med. 2010, 123, 432–438. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Lin, J.-D.; Chen, W.-L.; Yen, C.-F.; Loh, C.-H.; Fang, W.-H.; Wu, L.-W. Metabolic Syndrome and Short-Term Heart Rate Variability in Adults with Intellectual Disabilities. Res. Dev. Disabil. 2012, 33, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Lin, C.-L.C.-L.; Li, C.-R.C.-R.; Huang, S.-M.S.-M.; Chan, J.Y.-H.J.Y.-H.; Fang, W.-H.W.-H.; Chen, W.-L.W.-L.W.-L. Associations among Integrated Psychoneuroimmunological Factors and Metabolic Syndrome. Psychoneuroendocrinology 2016, 74, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-M.; Shiao, C.-C.; Huang, Y.-T.; Chen, I.-L.; Yang, C.-L.; Leu, S.-C.; Su, H.-L.; Kao, J.-L.; Tsai, S.-C.; Jhen, R.-N.; et al. Impact of Metabolic Syndrome and Its Components on Heart Rate Variability during Hemodialysis: A Cross-Sectional Study. Cardiovasc. Diabetol. 2016, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Min, K.-B.; Min, J.-Y.; Paek, D.; Cho, S.-I. The Impact of the Components of Metabolic Syndrome on Heart Rate Variability: Using the NCEP-ATP III and IDF Definitions. PACE-PACING Clin. Electrophysiol. 2008, 31, 584–591. [Google Scholar] [CrossRef]
- Min, J.-Y.; Paek, D.; Cho, S.-I.; Min, K.-B. Exposure to Environmental Carbon Monoxide May Have a Greater Negative Effect on Cardiac Autonomic Function in People with Metabolic Syndrome. Sci. Total Environ. 2009, 407, 4807–4811. [Google Scholar] [CrossRef]
- Kang, M.G.; Koh, S.B.; Cha, B.S.; Park, J.K.; Woo, J.M.; Chang, S.J. Association between Job Stress on Heart Rate Variability and Metabolic Syndrome in Shipyard Male Workers. YONSEI Med. J. 2004, 45, 838–846. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Z.-H.; Zeng, F.; Zhou, L. Associations between the Severity of Metabolic Syndrome and Cardiovascular Autonomic Function in a Chinese Population. J. Endocrinol. Investig. 2013, 36, 993–999. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, L.; Zhou, Y.; Xiao, L.; Zhang, X.; Yuan, J.; Chen, W. Cardiometabolic Traits Mediated the Relationship from Urinary Polycyclic Aromatic Hydrocarbons Metabolites to Heart Rate Variability Reduction: A Community-Based Study. Environ. Pollut. 2018, 243, 28–36. [Google Scholar] [CrossRef]
- Tyagi, A.; Cohen, M.; Reece, J.; Telles, S.; Jones, L. Heart Rate Variability, Flow, Mood and Mental Stress During Yoga Practices in Yoga Practitioners, Non-Yoga Practitioners and People with Metabolic Syndrome. Appl. Psychophysiol. Biofeedback 2016, 41, 381–393. [Google Scholar] [CrossRef]
- Endukuru, C.K.; Gaur, G.S.; Yerrabelli, D.; Sahoo, J.; Vairappan, B. Impaired Baroreflex Sensitivity and Cardiac Autonomic Functions Are Associated with Cardiovascular Disease Risk Factors among Patients with Metabolic Syndrome in a Tertiary Care Teaching Hospital of South-India. DIABETES Metab. Syndr. Res. Rev. 2020, 14, 2043–2051. [Google Scholar] [CrossRef]
- Saito, I.; Maruyama, K.; Eguchi, E.; Kato, T.; Kawamura, R.; Takata, Y.; Onuma, H.; Osawa, H.; Tanigawa, T. Low Heart Rate Variability and Sympathetic Dominance Modifies the Association between Insulin Resistance and Metabolic Syndrome:—The Toon Health Study. Circ. J. 2017, 81, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- MacAgnan, F.E.; Pandolfo Feoli, A.M.; Russomano, T.; Feoli, A.M.P.; Russomano, T. Acute Physical Effort Increases Sympathovagal Balance Responses to Autonomic Stimulation in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2019, 17, 67–74. [Google Scholar] [CrossRef]
- Supriya, R.; Li, F.-F.F.-F.; Yang, Y.-D.Y.-D.; Liang, W.; Baker, J.S. Association between Metabolic Syndrome Components and Cardiac Autonomic Modulation among Children and Adolescents: A Systematic Review and Meta-Analysis. Biology 2021, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Yang, T.; Chu, C.H.; Hsieh, P.C.; Hsu, C.H.; Chou, Y.C.; Yang, S.H.; Bai, C.H.; You, S.L.; Hwang, L.C.; Chung, T.C.; et al. C-Reactive Protein Concentration as a Significant Correlate for Metabolic Syndrome: A Chinese Population-Based Study. Endocrine 2013, 43, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.W.; Jason Sims, J.; Canavan, A.; Hsu, T.; Ujhelyi, M.R. Impaired Vagal Reflex Activity in Insulin-Resistant Rats. J. Cardiovasc. Pharmacol. 1999, 33, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Jacobs, D.R.; Sidney, S.; Liu, K. Influence of Autonomic Nervous System Dysfunction on the Development of Type 2 Diabetes: The CARDIA Study. Diabetes Care 2003, 26, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, Y.; Nakane, H.; Uehara, C.; Nagasawa, T.; Mitsuyama, S.; Ohkawa, K.; Kario, K.; Ozawa, S. Temporal Relationships among Changes in the RR-Interval and the Powers of the Low- and High-Frequency Components of Heart Rate Variability in Normal Subjects. Physiol. Rep. 2023, 11, e15557. [Google Scholar] [CrossRef]
- Porto, A.A.; Benjamim, C.J.R.; Gonzaga, L.A.; Luciano de Almeida, M.; Bueno Júnior, C.R.; Garner, D.M.; Valenti, V.E. Caffeine Intake and Its Influences on Heart Rate Variability Recovery in Healthy Active Adults after Exercise: A Systematic Review and Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1071–1082. [Google Scholar] [CrossRef]
- Jarczok, M.N.; Weimer, K.; Braun, C.; Williams, D.P.; Thayer, J.F.; Gündel, H.O.; Balint, E.M. Heart Rate Variability in the Prediction of Mortality: A Systematic Review and Meta-Analysis of Healthy and Patient Populations. Neurosci. Biobehav. Rev. 2022, 143, 104907. [Google Scholar] [CrossRef]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A Quantitative Systematic Review of Normal Values for Short-Term Heart Rate Variability in Healthy Adults. PACE-Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Empana, J.P.; Estrugo, N.; Escriou, G.; Ketthab, H.; Pruny, J.F.; Castellino, P.; Laude, D.; Thomas, F.; Pannier, B.; et al. The Neural Baroreflex Pathway in Subjects with Metabolic Syndrome: A Sub-Study of the Paris Prospective Study III. Medicine 2016, 95, e2472. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF Ratio Does Not Accurately Measure Cardiac Sympatho-Vagal Balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Stefanaki, C.; Michos, A.; Latsios, G.; Tousoulis, D.; Peppa, M.; Zosi, P.; Boschiero, D.; Bacopoulou, F. Sexual Dimorphism of Heart Rate Variability in Adolescence: A Case-Control Study on Depression, Anxiety, Stress Levels, Body Composition, and Heart Rate Variability in Adolescents with Impaired Fasting Glucose. Int. J. Environ. Res. Public Health 2020, 17, 2688. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, R.A.; Pastre, C.M.; Vanderlei, L.C.M.; Godoy, M.F. Poincaré Plot Indexes of Heart Rate Variability: Relationships with Other Nonlinear Variables. Auton. Neurosci. Basic Clin. 2013, 177, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Lomas, G.; Alejandro, D.L.O.; Jurado-Fasoli, L.; Castillo, M.J.; Femia, P.; Amaro-Gahete, F.J. Assessment of Autonomous Nerve System through Non-Linear Heart Rate Variability Outcomes in Sedentary Healthy Adults. PeerJ 2020, 8, e10178. [Google Scholar] [CrossRef] [PubMed]
- Maestri, R.; Pinna, G.D.; Porta, A.; Balocchi, R.; Sassi, R.; Signorini, M.G.; Dudziak, M.; Raczak, G. Assessing Nonlinear Properties of Heart Rate Variability from Short-Term Recordings: Are These Measurements Reliable? Physiol. Meas. 2007, 28, 1067–1077. [Google Scholar] [CrossRef]
- Young, L.H.; Wackers, F.J.T.; Chyun, D.A.; Davey, J.A.; Barrett, E.J.; Taillefer, R.; Heller, G.V.; Iskandrian, A.E.; Wittlin, S.D.; Filipchuk, N.; et al. Cardiac Outcomes after Screening for Asymptomatic Coronary Artery Disease in Patients with Type 2 Diabetes the DIAD Study: A Randomized Controlled Trial. JAMA 2009, 301, 1547–1555. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Evans, G.W.; Gerstein, H.C.; Fonseca, V.; Fleg, J.L.; Hoogwerf, B.J.; Genuth, S.; Grimm, R.H.; Corson, M.A.; Prineas, R. Effects of Cardiac Autonomic Dysfunction on Mortality Risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes Care 2010, 33, 1578–1584. [Google Scholar] [CrossRef]
- Balcioglu, A.S.; Akinci, S.; Cicek, D.; Eldem, H.O.; Coner, A.; Bal, U.A.; Muderrisoglu, H. Which Is Responsible for Cardiac Autonomic Dysfunction in Non-Diabetic Patients with Metabolic Syndrome: Prediabetes or the Syndrome Itself? Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S13–S20. [Google Scholar] [CrossRef]
- Howorka, K.; Pumprla, J.; Haber, P.; Koller-Strametz, J.; Mondrzyk, J.; Schabmann, A. Effects of Physical Training on Heart Rate Variability in Diabetic Patients with Various Degrees of Cardiovascular Autonomic Neuropathy. Cardiovasc. Res. 1997, 34, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Guzmán, J.E.; Mollà-Casanova, S.; Arias-Mutis, Ó.J.; Bizy, A.; Calvo, C.; Alberola, A.; Chorro, F.J.; Zarzoso, M. Differences in Long-Term Heart Rate Variability between Subjects with and without Metabolic Syndrome: A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 203. [Google Scholar] [CrossRef] [PubMed]


| Database | Search Equation |
|---|---|
| PubMed | ((“heart rate variability” [Title/Abstract] or “autonomic control” [Title/Abstract] or “HRV” [Title/Abstract] or “cardiac autonomic control” [Title/Abstract] or “cardiac autonomic function” [Title/Abstract] or “cardiac autonomic modulation” [Title/Abstract]) AND (“metabolic syndrome” [Title/Abstract])) |
| Web of Science | (“heart rate variability” or “autonomic control” or “HRV” or “cardiac autonomic control” or “cardiac autonomic function” or “cardiac autonomic modulation”) and (“metabolic syndrome”) |
| Scopus | (TITLE-ABS-KEY (“metabolic syndrome”) and TITLE-ABS-KEY (“heart rate variability”) or TITLE-ABS-KEY (“autonomic control”) or TITLE-ABS-KEY (“HRV”) or TITLE-ABS-KEY (“cardiac autonomic control”) or TITLE-ABS-KEY (“cardiac autonomic function”) or TITLE-ABS-KEY (“cardiac autonomic modulation”)) |
| Reference | Methodological Evaluation (%) | n | Age (Years) | Gender | MS Definition | Recording Characteristics | Analyzed HRV Variables | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recording Time (min) | Body Position | Time | Frequency | Spectral Methods | Non-Linear | ||||||
| Liao et al., 1998 [18] | 81% | 2359 | 45–64 | Both | HTA, DM-2, dislipidemia | 2 | Supine | SDNN | HF, LF, LF/HF | FFT | No |
| Brunner et al., 2002 [25] | 63% | 183 | 45–63 | Men | NCEP-ATP III | 5 | Supine | SDNN | TP, LF, HF | Blackman-Tukey | No |
| Kang et al., 2004 [38] | 69% | 156 | 41–55 | Men | ≥3 risk factors | 5 | Sitting | SDNN, rMSSD | HF, LF, LF/HF | NR | No |
| Park et al., 2006 [19] | 88% | 413 | 64–79 | Men | NCEP-ATP III | 7 | Sitting | SDNN | HF, LF, LF/HF | FFT | No |
| K.-B. Min et al., 2008 [36] | 88% | 1041 | 20–87 | Both | NCEP-ATP III, IDF | 5 | Sitting | SDNN | LF, HF | NR | No |
| J.-Y. Min et al., 2009 [37] | 69% | 986 | 20–87 | Both | NCEP-ATP III | 5 | Sitting | SDNN | LF, HF | FFT | No |
| Koskinen et al., 2009 [26] | 88% | 1889 | 24–39 | Both | NCEP-ATP III, IDF, EGIR | 3 | Supine | No | LH, HF, TP, LF/HF | FFT | No |
| Assoumou et al., 2010 [29] | 94% | 1010 | 64–66 | Both | NCEP-ATP III | 5 | Supine | No | TP, HF, LF, VLF, ULF, LF/HF | FFT | No |
| C.-J. Chang et al., 2010 [32] | 75% | 1289 | 36–48 | Both | NCEP-ATP III | 5 | Supine | SDNN | HF, LF, LF/HF | FFT | No |
| Rasic-Milutinovic et al., 2010 [31] | 88% | 47 | 45–65 | Both | NCEP ATP III | NR | NR | SDNN rMSSD | HF, LF, LF/HF, TP, VLF | FFT | No |
| Y.-W. Chang et al., 2012 [33] | 88% | 129 | 19–62 | Both | NCEP-ATP III | 5 | Supine | No | LH, HF, TP, LF/HF | FFT | No |
| Soares-Miranda et al., 2012 [30] | 81% | 163 | 19–21 | Both | N/A | 5 | Supine | rMSSD, SDNN, NN50, pNN50 | HF, LF/HF | FFT | SD1, SD2 |
| Tibana et al., 2013 [22] | 88% | 19 | 30–40 | Women | NCEP-ATP III | 5 | NR | R-R, SDNN, rMSSD | HF, LF, LF/HF | FFT | No |
| Li et al., 2013 [39] | 94% | 2119 | 50–70 | Both | NCEP-ATP III | 15 | Supine | No | LH, HF, TP, LF/HF | NR | No |
| Stuckey et al., 2015 [21] | 88% | 220 | 23–70 | Both | NCEP-ATP III | 5 | Supine | SDNN, rMSSD | LF, HF | FFT | SD1, SD2, α1, Aprox. Entropy |
| Chen et al., 2016 [34] | 88% | 345 | 20–65 | Both | ¤ | 5 | NR | SDNN, rMSSD | VLF, LF, HF, TP | FFT | No |
| Tyagi et al., 2016 [41] | 56% | 30 | 40–50 | Both | IDF | 5 | NR | rMSSD, pNN50, R-R | HF, LF, LF/HF | FFT | No |
| Y.-M. Chang et al., 2016 [35] | 88% | 175 | 50–80 | Both | IDF | 5 | Supine | No | LF, HF, LF/HF, TP, VLF | FFT | No |
| Silva et al., 2017 [9] | 94% | 36 | 40–50 | Women | § | 12 | Sitting | SDNN, rMSSD | HF, LF, LF/HF | FFT | Shannon Entropy |
| Feriani et al., 2017 [23] | 94% | 28 | 65–75 | Women | NCEP-ATP III | 20 | NR | SDNN, rMSSD, pNN50 | HF, LF, LF/HF | FFT | No |
| Saito et al., 2017 [43] | 94% | 2016 | 30–79 | Both | ≥3 risk factors | 5 | NR | SDNN, rMSSD | HF, LF, LF/HF | NR | No |
| Pennathur et al., 2017 [20] | 94% | 50 | 40–60 | Both | NCEP-ATP III | 5 | Supine | No | HF, LF, LF/HF | Wavelet transform | No |
| Guo et al., 2018 [40] | 100% | 2476 | 45–70 | Both | NCEP-ATP III | 5 | Sitting | No | LF, HF, LF/HF, VLF, TP | NR | No |
| Carvalho et al., 2018 [10] | 88% | 66 | 30–40 | Both | NCEP-ATP III | 300 consecutives R-R intervals | Supine | R-R, rMSSD, pNN50, RRtri, TINN | No | N/A | SD1, SD2, α1, Shannon Entropy |
| MacAgnan et al., 2019 [44] | 88% | 14 | 40–60 | Both | NCEP ATP III | 250–350 consecutives R-R intervals | Supine | R-R | HF, LF, LF/HF | Autoregressive algorithm | No |
| Kangas et al., 2019 [28] | 88% | 572 | 40–60 | Both | IC | 5 | Supine | No | TP, LF, HF, LF/HF | FFT | No |
| Leppanen et al., 2020 [27] | 94% | 443 | 6–8 | Both | NR | 5 | Supine | R-R, rMSSD | HF, LF, LF/HF | NR | No |
| Endukuru et al., 2020 [42] | 94% | 176 | 40–55 | Both | NCEP ATP III | 5 | Supine | R-R, SDNN, pNN50, NN50, rMSSD | LF, HF, LF/HF, VLF, TP | NR | No |
| Reference | SDNN | rMSSD | R-R | pNN50 |
|---|---|---|---|---|
| Liao et al., 1998 [18] | ↓ | |||
| Brunner et al., 2002 [25] | ↓ | ↓ | ||
| Kang et al., 2004 [38] | ↓ | = | ||
| Park et al., 2006 [19] | = | |||
| K.-B. Min et al., 2008 [36] | ↓ | |||
| J.-Y. Min et al., 2009 [37] | ↓ | |||
| C.-J. Chang et al., 2010 [32] | ↓ | |||
| Tibana et al., 2013 [22] | ↓ | ↓ | ↓ | |
| Stuckey et al., 2015 [21] | ↓w | = | ↓w | |
| Chen et al., 2016 [34] | ↓ | ↓ | ||
| Tyagi et al., 2016 [41] | ↓ | ↓ | ↓ | |
| Silva et al., 2017 [9] | ↓ | ↓ | ||
| Feriani et al., 2017 [23] | ↓ | ↓ | ↓ | |
| Saito et al., 2017 [43] | = | ↓ | ||
| Carvalho et al., 2018 [10] | = | ↓ | ↓ | = |
| MacAgnan et al., 2019 [44] | ↓ | |||
| Endukuru et al., 2020 [42] | ↓ | ↓ | ↓ | ↓ |
| Reference | HF | LF | LF/HF |
|---|---|---|---|
| Liao et al., 1998 [18] | ↓ | ↓ | = |
| Brunner et al., 2002 [25] | ↓ | ↓ | |
| Kang et al., 2004 [38] | = | = | = |
| Park et al., 2006 [19] | = | = | = |
| K.-B. Min et al., 2008 [36] | ↓ | ↓ | |
| J.-Y. Min et al., 2009 [37] | ↓ | ↓ | |
| Koskinen et al., 2009 [26] | ↓ | ↓ | ↑w |
| Assoumou et al., 2010 [29] | = | ↓ | ↓ |
| C.-J. Chang et al., 2010 [32] | ↓ | = | ↑ |
| Rasic-Milutinovic et al., 2010 [31] | ↓ | ||
| Y.-W. Chang et al., 2012 [33] | = | = | = |
| Tibana et al., 2013 [22] | ↓ | ↑ | ↑ |
| Li et al., 2013 [39] | ↓ | ↓ | ↓ |
| Stuckey et al., 2015 [21] | = | ↑w | = |
| Chen et al., 2016 [34] | ↓ | ↓ | |
| Tyagi et al., 2016 [41] | ↓ | ↑ | ↑ |
| Y.-M. Chang et al., 2016 [35] | = | = | = |
| Silva et al., 2017 [9] | ↓ | = | ↑ |
| Feriani et al., 2017 [23] | ↓ | ↑ | ↑ |
| Saito et al., 2017 [43] | ↓ | = | ↑ |
| Pennathur et al., 2017 [20] | = | = | ↑ |
| Guo et al., 2018 [40] | ↓ | ↓ | = |
| MacAgnan et al., 2019 [44] | ↓ | ↑ | ↑ |
| Kangas et al., 2019 [28] | ↓ | ↓m | = |
| Endukuru et al., 2020 [42] | ↓ | ↓ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Guzmán, J.E.; Mollà-Casanova, S.; Serra-Añó, P.; Arias-Mutis, Ó.J.; Calvo, C.; Bizy, A.; Alberola, A.; Chorro, F.J.; Zarzoso, M. Short-Term Heart Rate Variability in Metabolic Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6051. https://doi.org/10.3390/jcm12186051
Ortiz-Guzmán JE, Mollà-Casanova S, Serra-Añó P, Arias-Mutis ÓJ, Calvo C, Bizy A, Alberola A, Chorro FJ, Zarzoso M. Short-Term Heart Rate Variability in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(18):6051. https://doi.org/10.3390/jcm12186051
Chicago/Turabian StyleOrtiz-Guzmán, Johan E., Sara Mollà-Casanova, Pilar Serra-Añó, Óscar J. Arias-Mutis, Conrado Calvo, Alexandra Bizy, Antonio Alberola, Francisco J. Chorro, and Manuel Zarzoso. 2023. "Short-Term Heart Rate Variability in Metabolic Syndrome: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 18: 6051. https://doi.org/10.3390/jcm12186051
APA StyleOrtiz-Guzmán, J. E., Mollà-Casanova, S., Serra-Añó, P., Arias-Mutis, Ó. J., Calvo, C., Bizy, A., Alberola, A., Chorro, F. J., & Zarzoso, M. (2023). Short-Term Heart Rate Variability in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(18), 6051. https://doi.org/10.3390/jcm12186051






