Abstract
We sought to prospectively investigate the accuracy of an artificial intelligence (AI)-based tool for left ventricular ejection fraction (LVEF) assessment using a hand-held ultrasound device (HUD) in COVID-19 patients and to examine whether reduced LVEF predicts the composite endpoint of in-hospital death, advanced ventilatory support, shock, myocardial injury, and acute decompensated heart failure. COVID-19 patients were evaluated with a real-time LVEF assessment using an HUD equipped with an AI-based tool vs. assessment by a blinded fellowship-trained echocardiographer. Among 42 patients, those with LVEF < 50% were older with more comorbidities and unfavorable exam characteristics. An excellent correlation was demonstrated between the AI and the echocardiographer LVEF assessment (0.774, p < 0.001). Substantial agreement was demonstrated between the two assessments (kappa = 0.797, p < 0.001). The sensitivity, specificity, PPV, and NPV of the HUD for this threshold were 72.7% 100%, 100%, and 91.2%, respectively. AI-based LVEF < 50% was associated with worse composite endpoints; unadjusted OR = 11.11 (95% CI 2.25–54.94), p = 0.003; adjusted OR = 6.40 (95% CI 1.07–38.09, p = 0.041). An AI-based algorithm incorporated into an HUD can be utilized reliably as a decision support tool for automatic real-time LVEF assessment among COVID-19 patients and may identify patients at risk for unfavorable outcomes. Future larger cohorts should verify the association with outcomes.
1. Introduction
Given the well-established association between the cardiovascular system and COVID-19 disease, an echocardiogram may have an important role in patient evaluation [1,2,3]. Indeed, recently conducted large observational studies have reported the high prevalence of left and right ventricular dysfunction among almost one-third of a cohort of critically ill COVID-19 patients with different phenotypes of RV involvement [4,5]. Nonetheless, routine echocardiography for all COVID-19 patients is currently discouraged due to concerns of infection control, equipment contamination, and excessive workload in the setting of a pandemic [6,7]. A pragmatic approach to the increased demand for ultrasound usage for cardiovascular and pulmonary assessment is a hand-held ultrasound device (HUD), which is highly feasible with good quality and can be easily cleaned and dedicated to a specific ward [8,9]. The use of HUDs in COVID-19 cases was also found to lead to a significant reduction in the scanning time and the total duration of time spent in the patient’s room alongside good battery usage and reasonable operator-to-patient proximity [8,10,11]. An abnormal echocardiogram, including reduced left ventricular ejection fraction (LVEF) and moderate/severe valvular abnormalities, using HUDs in COVID-19 patients was associated with a higher proportion of comorbidities and independently predicted major adverse outcomes [12]. This may therefore be used as an important risk stratification tool among high-risk COVID-19 patients (oxygen saturation < 94%) [12]. However, HUDs have several limitations, including small screen sizes, limited image quality, a lack of advanced measurements, intermediate battery lives, and unclear findings which need to be confirmed by an official high-end device [13]. These challenges, as well as strict COVID-19 precautions, may affect the real-time diagnosis accuracy of left ventricular (LV) systolic function. Artificial intelligence (AI) is now increasingly used for imaging purposes, specifically including LV function assessment in the hands of experienced operators as well as novice users for clinical purposes [14]. As such, it may also be utilized as a decision support tool in the COVID-19 setting for ventricular assessment and for the potential screening of patients.
The objective of this study is to assess the diagnostic accuracy of an AI-based assessment tool using HUDs as compared with expert echocardiographers in evaluating the LVEF among COVID-19 patients and to examine whether AI-based reduced LVEF (<50%) is associated with patient outcomes.
2. Materials and Methods
2.1. Study Setting
This is a prospective study of real-time focused echocardiogram using an HUD conducted on PCR-confirmed non-selected COVID-19 patients hospitalized in designated wards at a tertiary medical center (Shaare Zedek Medical Center, Jerusalem, Israel) from 28 April through 26 July 2020. The study was approved by the hospital’s Institutional Review Board (IRB; 0138-20-SZMC, approved on 19 April 2020).
2.2. Study Endpoints
The primary endpoint of the study included the LVEF assessment accuracy by the AI-based tool as compared to a fellowship-trained echocardiographer of HUD clips among patients hospitalized with COVID-19. The secondary endpoint outcome measures included the composite of in-hospital death, advanced ventilatory support (high-flow nasal cannula, non-invasive positive airway pressure support, and invasive ventilation), shock, myocardial injury (defined as >3 times the upper normal limit of high-sensitivity cardiac troponin-I (hs-cTnI)), and acute decompensated heart failure (ADHF). Other secondary endpoint measures included venous thromboembolism, anti-COVID-19 drug use, sepsis, and length of hospital stay. The anti-COVID-19 drugs included dexamethasone (when used for this indication), azithromycin, hydroxychloroquine, remdesivir, opaganib, and COVID-19 convalescent plasma.
2.3. Study Protocol
All echocardiographic clips were acquired by cardiologists or intensivists with no prior dedicated fellowship training in echocardiography and with at least one year of routine echocardiogram acquisition as part of their practice. They wore personal protective equipment including a full gown, N-95 face mask, face screen, and two-to-three sets of gloves. Per the IRB approval, conscious patients provided informed consent verbally, and those not able to consent underwent an echocardiogram if clinically indicated. The echocardiogram was performed within 48 h of their hospitalization using an HUD (Vscan Extend with Dual Probe; General Electric, Boston, MA, USA) equipped with LVivo EF (DiA Imaging Analysis Ltd., Be’er Sheva, Israel; Figure 1), a program which uses AI to provide the automated calculation of LVEF from the apical 4-chamber (A4ch) view. This tool provides different LV parameters, including LVEF, LV end diastolic and systolic volume, and stroke volume.
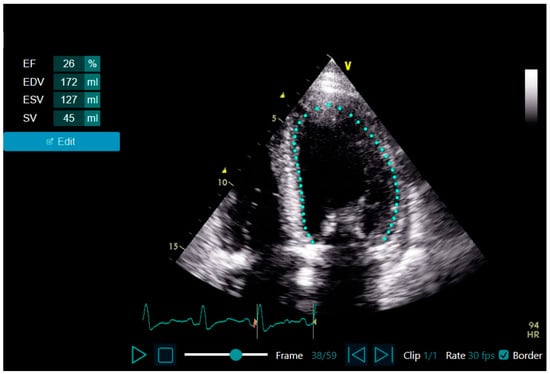
Figure 1.
The LVivo EF: AI-based tool for automated LVEF assessment from apical 4-chamber view echocardiographic clips. Abbreviations. AI, artificial intelligence; LVEF, left ventricular ejection fraction.
Upon exam completion, technical variables of the study were recorded, including heart rate (in beats per minute), the mean distance between the operator and the head of the patient (in centimeters; from the chin of the operator to the nose of the patient), length of study (in minutes), battery usage (in percentage change), and successful completion of all echocardiogram views. As was previously mentioned, COVID-19 can result in ventricular dysfunction, and as such, we included all patients with and without prior comorbidities in the study, including those with previously known heart failure with preserved ejection fraction.
2.4. Echocardiogram Acquisition and LVEF Assessment Using LVivo EF vs. Expert Assessment
The operators performed the focused echocardiography examination using the HUD and acquired the echocardiography clips from the following views: parasternal long-axis, parasternal short-axis, A4Ch, apical 2-chamber, and substernal views (including longitudinal and IVC views). If possible, an apical 3-chamber view was added as well. For the A4Ch views, the operators were instructed to focus on the LV, optimizing the clip and refraining from foreshortening. The interventricular septum was aligned parallel to the plane and a heart cycle of at least 2 beats was acquired. The depth was modified to ensure that the LV encompassed two-thirds of the view. The acquired clips were then assessed on-line for LVEF using the AI-based tool and sent wirelessly to a picture archiving platform (McKesson Cardiology™, version 14.0, San Diego, CA, USA). In the case of a failure of the AI tool to automatically calculate the LVEF or if the border tracings were incorrect, the image acquisition was repeated (up to four subsequent failures). Next, the acquired clips were assessed by a certified echocardiographic technician (equivalent to a Registered Diagnostic Cardiac Sonographer in the United States) for a second LVEF measurement using Simpson’s method, as well as being visually evaluated by an experienced cardiologist (A.B.), blinded to the AI-based tool’s results. The echocardiographer also assessed the clips for image quality according to a 3-categories scale (good—optimal visualization; fair—proper visualization of >50% of the segments; and poor—inappropriate visualization). Those who refused to participate as well as those with an unsuccessful AI automatic measurement were excluded from the study (as the accuracy of the AI-based tool and its association with outcomes cannot be addressed regarding these studies). Variables including demographics and past medical history were obtained from the electronic charts.
2.5. Sample Size Calculation
Sample size calculations were designed to meet the study primary endpoint and were performed using G*Power software (version 3.1.9.4, Heinrich Heine University Düsseldorf, Düsseldorf, Germany). We planned a paired study with LVEF comparison and a 1:1 ratio between the AI-based tool and the expert echocardiographer assessments. Based on previous data regarding LVivo EF accuracy [15], we assumed an effect size of 0.5 between the AI-based tool LVEF calculation and the echocardiographer assessment. Based on these assumptions, we calculated that data analyzed from 37 participants would suffice to reject the null hypothesis with a probability (power) of 0.9. The type I error was calculated as 0.05 and was two-sided.
2.6. Statistical Analyses
Descriptive statistics were used to analyze baseline and clinical characteristics as well as echocardiogram results and endpoints. Chi-square or Fisher’s exact tests were used for categorical variables, and the t-test or Mann–Whitney U test was used for continuous variables. Test selection was based on data distribution and normalcy. Study participants were divided into preserved or reduced LVEF (LVEF < 50%) based on their AI tool results. Continuous LVEF assessments were compared for linear correlation using Pearson’s correlation coefficient. For categorical variables, the inter-rater reliability using the Kappa coefficient was then calculated. The LVEF measurements were transformed into dichotomous variables to enable Kappa calculation using LVEF cutoffs of 50% (i.e., normal/preserved vs. reduced LVEF). The ability of the AI-based tool to identify patients with LVEF < 50% was then tested for sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively).
The unadjusted odds ratio (OR) and 95% confidence interval (CI) were calculated to test the associations between reduced LVEF and the composite and individual endpoints. The odds ratio was defined as the ratio of the odds of the endpoint in the presence of reduced LVEF and the odds of the endpoint in the absence of reduced LVEF (i.e., the presence or absence of LVEF < 50% and the endpoint).
Multivariate logistic regression analysis (OR and 95% CI) was calculated for the association between reduced LVEF and the composite endpoint, including age and combined pertinent comorbidities variable (baseline characteristics covariates with p values of less than 0.05). Statistical analysis was performed using SP Statistics for Windows version 21 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, with a p-value ≤ 0.05 considered statistically significant.
3. Results
A total of 56 patients were initially included in the study. Four patients refused to participate and thus were excluded. The AI automatic measurement was not successful in 10 patients, leaving 42 (75.0%) patients for inclusion in the analysis.
3.1. Baseline, Clinical, and Test Characteristics and in-Hospital Course: AI-Based Preserved vs. Reduced LVEF (Table 1)
Among 42 patients, 21 (50%) were males with a mean age of 53.3 ± 17.8 years and a mean BMI of 27.6 ± 5.1 kg/m2. Seven (16.7%) patients could not turn on their left side, and three (7.1%) were not proficient in maintaining effective communication (i.e., adhering to instructions and cooperating with the examination). The mean duration of the echocardiogram studies was 6.8 ± 2.2 min, battery usage was 13.1 ± 4.9%, and the mean operator-to-patient distance was 64.5 ± 9.3 cm.
As compared with patients with preserved LVEF, those with reduced LVEF (<50%) were significantly older (63.5 ± 16.3 vs. 49.7 ± 17.1 years), had a higher proportion of comorbidities (including a history of hypertension, hyperlipidemia, ischemic heart disease, past revascularization, and heart failure) and were more frequently treated with heart failure guideline-directed medical therapy, anti-platelets, and statins. Patients with reduced LVEF were more likely to present with shortness of breath; had a higher proportion of atrial fibrillation/flutter rhythm; and had higher levels of creatinine, hs-cTnI, C-reactive protein, and D-dimer but lower nadir levels of albumin. Concerning exam characteristics and technical aspects, patients with reduced LVEF had a longer exam as well as closer proximity to the examiner.

Table 1.
Baseline and clinical characteristics: preserved vs. reduced LVEF as per the AI-based tool.
Table 1.
Baseline and clinical characteristics: preserved vs. reduced LVEF as per the AI-based tool.
| Variable | All n = 42 | Preserved LVEF n = 31 | Reduced LVEF n = 11 | p-Value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, mean ± SD | 53.3 ± 17.8 | 49.7 ± 17.1 | 63.5 ± 16.3 | 0.026 |
| Male, n (%) | 21 (50.0) | 15 (48.4) | 6 (54.5) | 0.726 |
| Body mass index, mean ± SD | 27.6 ± 5.1 | 27.1 ± 4.3 | 28.7 ± 6.9 | 0.459 |
| Smoking, n (%) | 4 (9.5) | 3 (9.7) | 1 (9.1) | 1.000 |
| Diabetes mellitus, n (%) | 14 (33.3) | 8 (25.8) | 6 (54.5) | 0.082 |
| Hypertension, n (%) | 13 (31.0) | 5 (16.1) | 8 (72.7) | 0.001 |
| Hyperlipidemia, n(%) | 13 (31.0) | 5 (16.1) | 8 (72.7) | 0.001 |
| Ischemic heart disease, n (%) | 7 (16.7) | 2 (6.5) | 5 (45.5) | 0.009 |
| Cerebrovascular accident, n (%) | 1 (2.4) | 1 (3.2) | 0 | 1.000 |
| Revascularization, n (%) | 7 (16.7) | 2 (6.5) | 5 (45.5) | 0.009 |
| Heart failure, n (%) | 5 (11.9) | 1 (3.2) | 4 (36.4) | 0.013 |
| Valve replacement, n (%) | 2 (4.8) | 1 (3.2) | 1 (9.1) | 0.460 |
| CIED, n (%) | 1 (2.4) | 0 | 1 (9.1) | 0.262 |
| Cognitive decline, n (%) | 4 (9.5) | 2 (6.5) | 2 (18.2) | 0.277 |
| Debilitation, n (%) | 6 (14.3) | 3 (9.7) | 3 (27.3) | 0.314 |
| Chronic lung disease, n (%) | 3 (7.1) | 3 (9.7) | 0 | 0.554 |
| Liver disease, n (%) | 1 (2.4) | 1 (3.2) | 0 | 1.000 |
| Prior venous thromboembolism, n (%) | 3 (7.1) | 2 (6.5) | 1 (9.1) | 1.000 |
| Autoimmune disease, n (%) | 2 (4.8) | 1 (3.2) | 1 (9.1) | 0.460 |
| Hypothyroidism, n (%) | 3 (7.1) | 2 (6.5) | 1 (9.1) | 1.000 |
| Chronic medications | ||||
| ACE-I/ARB, n (%) | 10 (23.8) | 2 (6.5) | 8 (72.7) | <0.001 |
| β-blockers, n (%) | 11 (26.2) | 5 (16.1) | 6 (54.5) | 0.021 |
| Calcium channel blockers, n (%) | 3 (7.1) | 2 (6.5) | 1 (9.1) | 1.000 |
| Anti-platelets, n (%) | 10 (23.8) | 4 (12.9) | 6 (54.5) | 0.011 |
| Oral anticoagulation, n (%) | 5 (11.9) | 2 (6.5) | 3 (27.3) | 0.103 |
| Diuretics, n (%) | 2 (4.8) | 1 (3.2) | 1 (9.1) | 0.460 |
| Inhalations, n (%) | 4 (9.5) | 4 (12.9) | 0 | 0.558 |
| SGLT2 inhibitors, n (%) | 1 (2.4) | 0 | 1 (9.1) | 0.262 |
| Statins, n (%) | 13 (31.0) | 5 (16.1) | 8 (72.7) | 0.001 |
| COVID-19 presentation | ||||
| Chest pain, n (%) | 17 (40.5) | 10 (32.3) | 7 (63.6) | 0.086 |
| Shortness of breath, n (%) | 25 (59.5) | 15 (48.4) | 10 (90.9) | 0.016 |
| Heart rate (bpm), mean ± SD | 92.2 ± 21.1 | 91.8 ± 19.7 | 93.6 ± 35.0 | 0.632 |
| SBP (mmHg), mean ± SD | 123.0 ± 18.9 | 122.8 ± 18.6 | 123.3 ± 20.5 | 0.978 |
| DBP (mmHg), mean ± SD | 75.5 ± 12.7 | 75.1 ± 11.1 | 76.6 ± 17.1 | 0.800 |
| Oxygen saturation (%), mean ± SD | 90.3 ± 5.5 | 91.2 ± 5.1 | 87.7 ± 5.9 | 0.092 |
| In-hospital course | ||||
| Sinus tachycardia, n (%) | 5 (11.9) | 3 (9.7) | 2 (18.2) | 0.593 |
| Electrocardiogram changes | 0.078 | |||
| Normal, n (%) | 25 (59.5) | 20 (64.5) | 5 (45.5) | |
| Nonspecific changes, n (%) | 10 (23.8) | 8 (25.8) | 2 (18.2) | |
| TWI/ST-depression, n (%) | 5 (11.9) | 3 (9.7) | 2 (18.2) | |
| ST-elevation, n (%) | 2 (4.8) | 0 | 2 (18.2) | |
| Chest X-ray infiltrates, n (%) | 28 (66.7) | 18 (58.1) | 10 (90.9) | 0.067 |
| Atrial fibrillation/flutter, n (%) | 4 (9.5) | 0 | 4 (36.4) | 0.003 |
| Lab results | ||||
| White blood cells (peak), mean ± SD | 8.6 ± 3.5 | 8.1 ± 2.8 | 10.0 ± 4.9 | 0.322 |
| ANC/ALC (admission), median [IQR] | 4.2 [2.6–8.6] | 3.4 [2.4–6.9] | 6.5 [4.8–9.7] | 0.086 |
| Hemoglobin (admission), mean ± SD | 12.8 ± 2.3 | 13.2 ± 2.1 | 11.7 ± 2.5 | 0.110 |
| Platelets (admission), mean ± SD | 193.6 ± 55.7 | 192.7 ± 58.7 | 196.1 ± 49.0 | 0.591 |
| Creatinine (admission), mean ± SD | 1.0 ± 0.8 | 0.8 ± 0.3 | 1.6 ± 1.4 | 0.005 |
| Potassium (admission), mean ± SD | 4.0 ± 0.6 | 3.8 ± 0.4 | 4.4 ± 0.9 | 0.051 |
| Albumin (trough), mean ± SD | 3.4 ± 0.7 | 3.6 ± 0.6 | 2.8 ± 0.7 | 0.010 |
| Hs-cTnI (peak), median [IQR] | 6.5 [5.0–17.3] | 5 [5–8.5] | 40 [13.5–7467] | <0.001 |
| CRP (peak), median [IQR] | 6.5 [1.2–15.3] | 3.6 [0.7–9.8] | 21.4 [12.7–23.3] | <0.001 |
| D-dimer (peak), median [IQR] | 824 [549–1060] | 728 [402–977] | 1073 [921–1932] | 0.001 |
| Fibrinogen (admission), mean ± SD | 578.7 ± 163.6 | 555.9 ± 151.8 | 640.9 ± 185.4 | 0.226 |
| aPTT (peak), mean ± SD | 3.3 ± 4.9 | 33.3 ± 5.1 | 33.3 ± 4.3 | 0.717 |
| Exam characteristics and technical aspects | ||||
| Ability to turn left, n (%) | 35 (83.3) | 28 (90.3) | 7 (63.6) | 0.063 |
| Effective communication, n (%) | 39 (92.9) | 30 (96.8) | 9 (81.8) | 0.163 |
| Length of study (minutes), mean ± SD | 6.8 ± 2.2 | 6.4 ± 2.1 | 8.1 ± 2.0 | 0.007 |
| Full view successful completion, n (%) | 38 (90.5) | 29 (93.5) | 9 (81.8) | 0.277 |
| Study quality | 0.618 | |||
| Good, n (%) | 27 (64.3) | 19 (61.3) | 8 (72.7) | |
| Fair, n (%) | 8 (19.0) | 7 (22.6) | 1 (9.1) | |
| Poor, n (%) | 7 (16.7) | 5 (16.1) | 2 (18.2) | |
Abbreviations. ACE-I, angiotensin-converting enzyme; AI, artificial intelligence; ALC, absolute lymphocyte count; ANC, absolute neutrophile count; aPTT, activated partial thromboplastin time; ARB, angiotensin II receptor blocker; bpm, beats per minute; CIED, cardiovascular implantable electronic device; cm, centimeters; CRP, C-reactive protein; DBP, diastolic blood pressure; Hs-cTnI, high-sensitivity cardiac troponin I; IQR, interquartile range; mmHg, millimeter of mercury; LVEF, left ventricular ejection fraction; n, number; SD, standard deviation; SBP, systolic blood pressure; SGLT2, sodium-glucose transport protein 2; TWI, T-wave inversion.
3.2. LVEF Assessment Correlations and Agreement
Excellent correlation was demonstrated between the AI and the echocardiographer LVEF assessment (Pearson’s correlation of 0.774, p < 0.001, Figure 2A). Substantial agreement was demonstrated between the AI and the echocardiographer for LVEF using a threshold of 50% (kappa = 0.797, p < 0.001, Figure 2B).
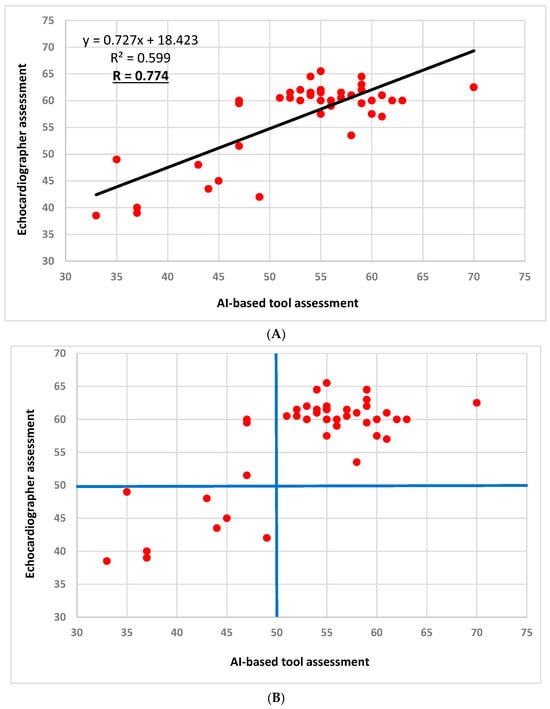
Figure 2.
LVEF assessment correlation and agreement between the AI-based tool and the echocardiographer (two patients had identical assessments that are superimposed: 60 vs. 55, respectively). (A): LVEF assessment correlation between the AI-based tool and the echocardiographer; Pearson’s correlation coefficient = 0.774 (p < 0.001). (B): LVEF assessment agreement between the AI-based tool and the echocardiographer using the LVEF cutoff of 50% (marked by the blue horizontal and longitudinal lines); Kappa coefficient = 0.797 (standard error of 0.110, p < 0.001). Abbreviations. AI, artificial intelligence; LVEF, left ventricular ejection fraction.
3.3. Sensitivity and Specificity Analyses
The sensitivity, specificity, PPV, and NPV of the HUD using the AI-based tool for the 50% LVEF threshold were 72.7%, 100%, 100%, and 91.2%, respectively.
3.4. Association between Reduced LVEF (<50%) Using the AI-Based Tool and Study Endpoints (Table 2 and Figure 3)
The AI-based diagnosis of reduced vs. preserved LVEF was associated with the endpoints of myocardial injury, ADHF, and acute kidney injury, and with the composite outcome, including in-hospital death, advanced ventilatory support, shock, myocardial injury, and ADHF (72.7% vs. 19.4%, respectively, p = 0.003, unadjusted OR = 11.11 with 95% CI 2.25–54.94). Multivariate analysis adjusting for age and combined pertinent comorbidities revealed that AI-based reduced LVEF was independently associated with the composite endpoint OR = 6.40 (95% CI 1.07–38.09, p = 0.041).
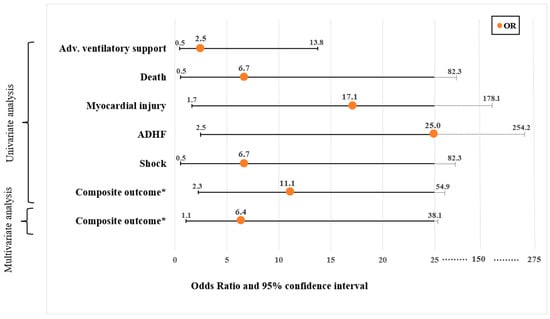
Figure 3.
Significant associations (odds ratio and 95% confidence interval+) between AI-based reduced left ventricular systolic function (LVEF < 50%) and serious adverse events (endpoints). The figure includes the univariate associations as well as multivariate logistic regression analysis results that were calculated to test the correlation between reduced LVEF and the composite and individual endpoints. The multivariate logistic regression analysis included age and combined pertinent comorbidities variables (baseline characteristic covariates with p values of less than 0.05). The odds ratios (ORs) are illustrated by the orange dots, while the line represents the 95% confidence interval (CI). The values in the x-axis are presented in a linear fashion up to 25. From this point on, the progression deviates from linearity. + Numeric results of OR and 95% CI and are detailed in Table 2. * The primary endpoint was defined as a composite endpoint of in-hospital death, advanced ventilatory support, shock, myocardial injury, and acute decompensated heart failure. Abbreviations. ADHF, acute decompensated heart failure; Adv., advanced; AI, artificial intelligence; LVEF, left ventricular ejection fraction.

Table 2.
The association between reduced LVEF as per the AI-based tool and serious adverse events (endpoints).
Table 2.
The association between reduced LVEF as per the AI-based tool and serious adverse events (endpoints).
| Variable | All n = 42 | Preserved LVEF n = 31 | Reduced LVEF n = 11 | p-Value | Unadjusted OR (95% CI) |
|---|---|---|---|---|---|
| Composite endpoint, n (%) | 14 (33.3) | 6 (19.4) | 8 (72.7) | 0.003 | 11.1 (2.25–54.94) |
| In-hospital death, n (%) | 2 (4.8) | 0 | 2 (18.2) | 0.064 | 6.7 (0.54–82.31) |
| Advanced VS, n (%) | 7 (16.7) | 4 (12.9) | 3 (27.3) | 0.282 | 2.5 (0.47–13.75) |
| Myocardial injury, n (%) | 5 (11.9) | 1 (3.2) | 4 (36.4) | 0.017 | 17.1 (1.65–178.08) |
| Shock, n (%) | 2 (4.8) | 0 | 2 (18.2) | 0.064 | 6.7 (0.54–82.31) |
| ADHF, n (%) | 6 (14.3) | 1 (3.2) | 5 (45.5) | 0.007 | 25 (2.46–254.15) |
| RRT, n (%) | 2 (4.8) | 0 | 2 (18.2) | 0.064 | 6.7 (0.54–82.31) |
| VTE, n (%) | 1 (2.4) | 1 (3.2) | 0 | 1.000 | 3.0 (0.17–52.53) |
| Anti-COVID drugs, n (%) | 18 (42.9) | 12 (38.7) | 6 (54.5) | 0.362 | 1.9 (0.47–7.63) |
| Sepsis, n (%) | 2 (4.8) | 0 | 2 (18.2) | 0.064 | 6.7 (0.54–82.31) |
| Acute kidney injury, n (%) | 6 (14.3) | 5 (16.1) | 1 (9.1) | 0.007 | 25.0 (2.46–254.15) |
| LOS (days), median [IQR] | 5.1 [2.7–10.4] | 4.8 [2.2–10.1] | 6 [4.3–12.4] | 0.201 | |
| LOS > median (5.1 days), n (%) | 21 (50.0) | 16 (51.6) | 5 (45.5) | 0.726 | 1.28 (0.32–5.01) |
Abbreviations. ADHF, acute decompensated heart failure; CI, confidence interval; IQR, interquartile range; LOS, length of stay; LVEF, left ventricular ejection fraction; n, number; OR, odds ratio; RRT, renal replacement therapy; VS, ventilatory support; VTE, venous thromboembolism.
4. Discussion
The current prospective study shows that among non-selected hospitalized COVID-19 patients, AI-based tool use on an HUD operated by clinicians is highly correlated to expert echocardiographers and provides a real-time accurate LVEF measurement over a range of cardiac functions. Moreover, the AI measurement can reach a high level of agreement with echocardiographers for the diagnosis of reduced LV systolic function (LVEF < 50%) with 100% sensitivity and PPV, very high NPV, and satisfactory specificity. Patients admitted with COVID-19 with reduced LVEF based on the AI tool assessment were older and presented with a higher burden of comorbidities, worse laboratory results, and a more prolonged exam and the closer proximity of the examiner to the patients. The study also reveals an association between AI-based reduced LV systolic function and worse composite endpoints.
HUD use has gained popularity and expanded across medical disciplines due to their many advantages, including small sizes, portability, low cost, and instantaneous assessment. These characteristics are useful in the COVID-19 setting, with a possible direct impact on immediate patient diagnosis and management [12]. Given the objective difficulties associated with the COVID-19 working environment for clinicians [16], real-time LVEF assessment can be challenging. In this context, this AI tool can provide the operator with rapid LVEF quantification, including the immediate visualization of its endocardial border tracking, as well as a tool for the risk stratification of these patients. Multiple software programs have incorporated automation to improve the accuracy and efficiency of manual tracings alongside different automated measurements [17]. A different automated tool for the evaluation of LV function has been validated by Asch et al., using echocardiographers, assuming the left ventricle contracts throughout the cardiac cycle along its long and short axis to produce an accurate LVEF from proportional changes in cardiac chamber size [18]. Unlike the current AI-based tool, their tool estimates LVEF without quantifying the end-systolic and diastolic volumes.
The accuracy of AI-based LVEF assessment has been recently evaluated by comparing AI-generated LVEF measurements vs. a sonographer’s tracings as compared to a final LVEF assessment by a cardiologist [19]. It was shown that the AI algorithm was non-inferior and even superior to the sonographer’s assessment. Also, and similar to the current study’s findings, Samtani et al. have validated this AI-based tool, LVivo EF, using clips acquired by a traditional echocardiogram device in a prospective study that included 242 patients, demonstrating that it can accurately quantify LVEF as compared with cardiac MRI without manual correction with a Pearson’s correlation of 0.89 [20]. Also, Filipiak-Strzecka et al. tested this AI tool on HUD-acquired clips (measurement was successful in 76 patients out of 112) by a trained cardiology resident, revealing an excellent correlation of 0.92 as compared with a high-end ultrasound device operated by an accredited echocardiographer [15]. The slightly higher correlation presented in these two aforementioned studies as compared with the present study may stem from the COVID-19 environment leading to a less convenient setting, resulting in lower exam quality.
With regard to measurement success, Samtani et al. have shown that this AI tool was unsuccessful in measuring the LVEF in 2.5% of the attempted tests (6/242 patients). Conversely, the rate of unsuccessful measurement in the present study was higher (~19%; 10/52 patients). This discrepancy may stem from the different ultrasound devices used for the echocardiogram clip acquisition and the study setting; whereas Samtani et al. used a high-end device on cardiac patients among non-COVID 19 patients, the present study used HUDs on patients admitted with COVID-19. Indeed, Filipiak-Strzecka et al. showed an unsuccessful measurement rate that was higher (32.1%; 36/112 exams) using an HUD, with a smaller number of attempts for each exam (three attempts).
The current study also shows that reduced LV systolic function based on AI assessment is associated with worse endpoints and is predictive of the composite endpoints of in-hospital death, advanced ventilatory support, shock, myocardial injury, and ADHF. To the best of our knowledge, no previous studies have investigated the association of AI automated echocardiogram indices with patient outcomes. An association between abnormal echocardiogram results, including valvular pathologies and reduced LVEF, and outcomes has been previously demonstrated by our group in a recent prospective study, revealing an independent association with the study composite endpoints, including death, mechanical ventilation, shock, and ADHF, supporting the current study’s findings [12].
Limitations
This prospective research was conducted at a single site and may be subjected to the relevant confounders. The populations and the quality of the exams may differ between hospitals. This may limit the generalizability of the findings. Also, while it is correlated with the study design and sample size calculation, the limited size of the cohort may expose it to biases. Furthermore, similar to other studies but with differing rates, the present research also reported a rate of unsuccessful measurements of 19% by the AI-based tool and also included up to four assessment attempts [15,20]. This limited generalizability is the “cost” of conducting a study in clinical settings, especially when it comes to a pandemic and the extreme precautions taken, including wearing a gown, mask, and gloves. Nonetheless, for patients in whom the AI-based tool was successful, we found reassuring accuracy as well as adjusted correlation with unfavorable outcomes.
5. Conclusions
This AI-based algorithm incorporated into an existing HUD can be reliably utilized as a decision support tool with high diagnostic performance for automatic real-time LVEF assessment among hospitalized COVID-19 patients. LVEF dysfunction based on this AI tool can identify patients with detrimental baseline and medical characteristics with more challenging echocardiogram exam features that may be at higher risk for unfavorable outcomes, thus providing a complementary tool to physicians to enhance patient care. Larger cohorts should be studied to examine whether AI-based reduced LVEF is associated with unfavorable outcomes.
Author Contributions
Conceptualization, Z.D., Y.S., N.L., M.G. and S.G.; methodology, Z.D., Y.S., N.L., A.B., and S.G.; software, Z.D., Y.S., N.L., A.O., D.B. and E.A.A.; validation, Z.D. and S.G.; formal analysis, Z.D.; investigation, Z.D., N.L. and S.G.; resources, Z.D.; data curation, Z.D.; writing—original draft preparation, Z.D., Y.S., N.L., E.A.A. and S.G.; writing—review and editing, Z.D., Y.S., N.L., A.O., D.B., A.B., S.C., M.G., E.A.A. and S.G.; visualization, Z.D., A.B. and S.G.; supervision, S.C., M.G., and S.G.; project administration, M.G. and S.G.; funding acquisition, Z.D. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Shaare Zedek Scientific Ltd. (Fund number: 18004712), Shaare Zedek Medical Center’s Technology Transfer Company.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Shaare Zedek Medical Center (0138-20-SZMC; approved on 19 April 2020).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study in accordance with the IRB approval.
Data Availability Statement
Data may be available for sharing.
Acknowledgments
The Vscan Extend™ devices used in the study were temporarily supplied by GE Healthcare for research purposes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of cardiac manifestations in COVID-19: A systematic echocardiographic study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terré, F.; De Backer, D.; Mekontso Dessap, A.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Task Force for the Management of COVID-19 of the European Society of Cardiology; Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; et al. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1—Epidemiology, pathophysiology, and diagnosis. Cardiovasc. Res. 2022, 118, 1385–1412. [Google Scholar] [PubMed]
- Kirkpatrick, J.N.; Mitchell, C.; Taub, C.; Kort, S.; Hung, J.; Swaminathan, M. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Dadon, Z.; Levi, N.; Alpert, E.A.; Orlev, A.; Belman, D.; Glikson, M.; Butnaru, A.; Gottlieb, S. The quality, safety, feasibility, and interpretive accuracy of echocardiographic and lung ultrasound assessment of COVID-19 patients using a hand-held ultrasound. Echocardiography 2022, 39, 886–894. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.R.; De Francis, G.; Schwartz, S.; Duvall, W.L.; Arora, B.; Silverman, D.I. Tablet-based limited echocardiography to reduce sonographer scan and decontamination time during the COVID-19 pandemic. J. Am. Soc. Echocardiogr. 2020, 33, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Dadon, Z.; Carasso, S.; Gottlieb, S. The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19. Biomedicines 2023, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Maheshwarappa, H.M.; Mishra, S.; Kulkarni, A.V.; Gunaseelan, V.; Kanchi, M. Use of handheld ultrasound device with artificial intelligence for evaluation of cardiorespiratory system in COVID-19. Indian J. Crit. Care Med. 2021, 25, 524. [Google Scholar] [PubMed]
- Dadon, Z.; Levi, N.; Orlev, A.; Belman, D.; Alpert, E.A.; Glikson, M.; Gottlieb, S.; Butnaru, A. The utility of handheld cardiac and lung ultrasound in predicting outcomes of hospitalised patients with COVID-19. Can. J. Cardiol. 2022, 38, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Savino, K.; Ambrosio, G. Handheld ultrasound and focused cardiovascular echography: Use and information. Medicina 2019, 55, 423. [Google Scholar] [CrossRef] [PubMed]
- Motazedian, P.; Marbach, J.A.; Prosperi-Porta, G.; Parlow, S.; Di Santo, P.; Abdel-Razek, O.; Jung, R.; Bradford, W.B.; Tsang, M.; Hyon, M.; et al. Diagnostic accuracy of point-of-care ultrasound with artificial intelligence-assisted assessment of left ventricular ejection fraction. NPJ Digit. Med. 2023, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Filipiak-Strzecka, D.; Kasprzak, J.D.; Wejner-Mik, P.; Szymczyk, E.; Wdowiak-Okrojek, K.; Lipiec, P. Artificial intelligence-powered measurement of left ventricular ejection fraction using a handheld ultrasound device. Ultrasound Med. Biol. 2021, 47, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Ladny, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Mosleh, W.; Shen, J.; Chow, C.M. Automation, machine learning, and artificial intelligence in echocardiography: A brave new world. Echocardiography 2018, 35, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Asch, F.M.; Mor-Avi, V.; Rubenson, D.; Goldstein, S.; Saric, M.; Mikati, I.; Surette, S.; Chaudhry, A.; Poilvert, N.; Hong, H.; et al. Deep learning–based automated echocardiographic quantification of left ventricular ejection fraction: A point-of-care solution. Circ. Cardiovasc. Imaging 2021, 14, e012293. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Kwan, A.C.; Cho, J.H.; Yuan, N.; Pollick, C.; Shiota, T.; Ebinger, J.; Bello, N.A.; Wei, J.; Josan, K.; et al. Blinded, randomized trial of sonographer versus AI cardiac function assessment. Nature 2023, 616, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Samtani, R.; Bienstock, S.; Lai, A.C.; Liao, S.; Baber, U.; Croft, L.; Stern, E.; Beerkens, F.; Ting, P.; Goldman, M.E. Assessment and validation of a novel fast fully automated artificial intelligence left ventricular ejection fraction quantification software. Echocardiography 2022, 39, 473–482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).