Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis
Abstract
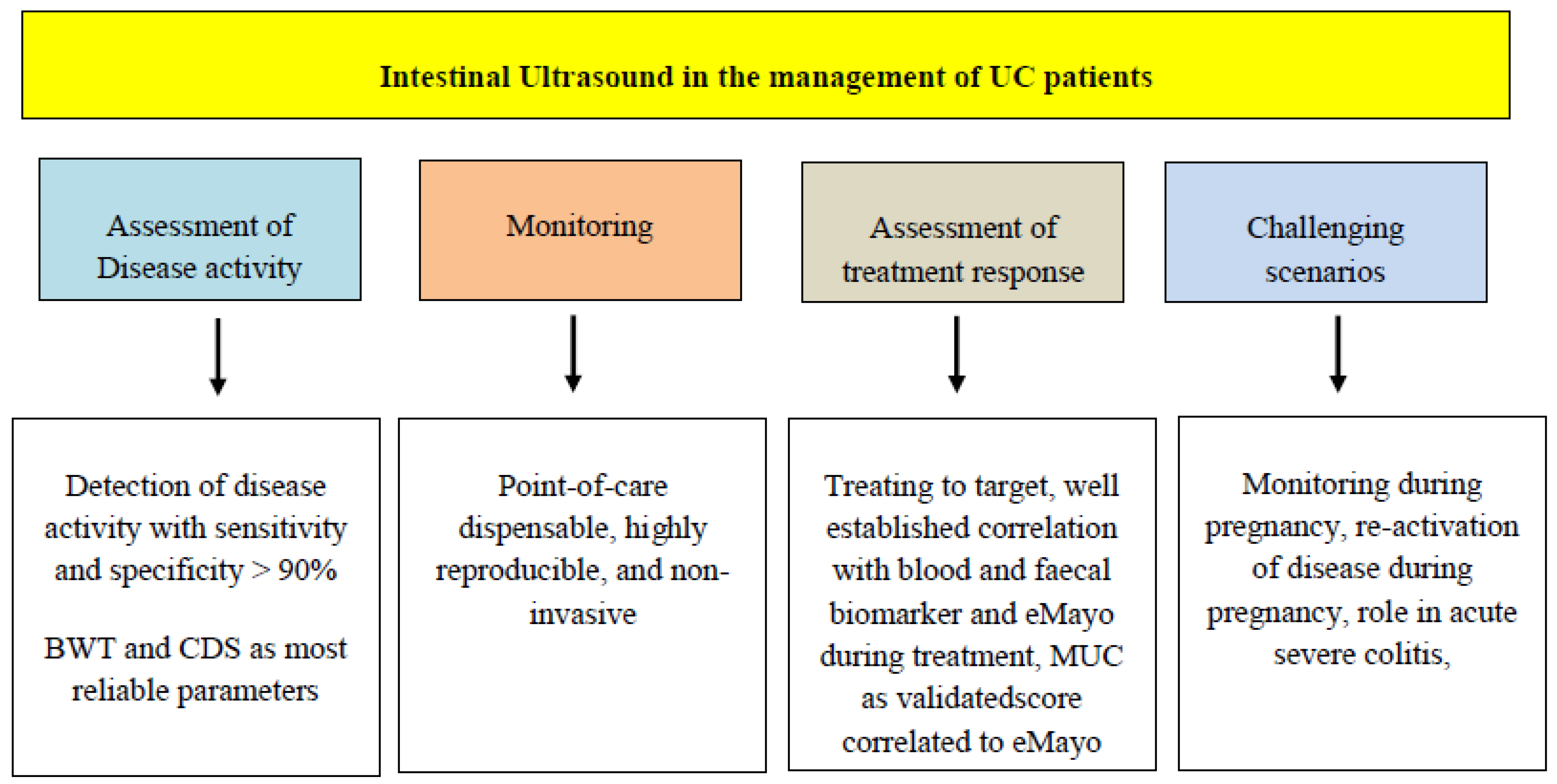
:1. Introduction
Review Criteria
2. IUS in UC Activity Assessment
2.1. Colonic Evaluation
2.2. The Challenge of Rectum Evaluation
| Study | Study Type | Cohort N. | Clinical Context | Reference Standard | Ultrasound Score | Major Findings | Reference |
|---|---|---|---|---|---|---|---|
| Allocca et al. (JCC 2018) | Prospective single center | 53 | Active and inactive UC | CS | MUC: 1.4 × BWT (mm) + 2 × CDS |
| [20] |
| Kinoshita et al. (J Gastroenterol 2019) | Prospective multicenter | 156 | Active and inactive UC | CS | NA |
| [18] |
| Bots et al. (JCC 2021) | Prospective single center | 60 | Active and inactive UC | CS | UC-IUS index: [BWT > 2 mm, 3 mm and 4 mm (1-2-3 pts)] + [CDS spots (1 pts), stretches (2 pts)] + (abnormal haustration 1 pts) + (i-fat 1 pts) |
| [19] |
| Sagami et al. (CGH 2021) | Metanalysis | 1101 | Active and inactive UC | CS | NA |
| [29] |
3. Bowel Ultrasound in UC Monitoring and Therapeutic Response Assessment
3.1. Tight Monitoring in UC: Preventing Colorectal Cancer (CRC) and Surgery Sparing
3.2. Treatment Response
3.3. The Role of Histology: Correlation with Transmural Activity?
4. Post-Operative Setting in UC: Pouch Assessment
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Wittig, B.M.; Helwig, U.; Börner, N.; Rössler, A.; Rath, S.; Maaser, C.; TRUST study group. Use of intestinal ultrasound to monitor crohn’s disease activity. Clin. Gastroenterol. Hepatol. 2017, 15, 535–542.e2. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; de Sio, I.; Cozzolino, A.; Rispo, A.; Manguso, F.; Del Vecchio Blanco, G.; Di Girolamo, E.; Castellano, L.; Ciacci, C.; Mazzacca, G. Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with Crohn’s disease. Am. J. Gastroenterol. 2004, 99, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Ilvemark, J.F.K.F.; Hansen, T.; Goodsall, T.M.; Seidelin, J.B.; Al-Farhan, H.; Allocca, M.; Begun, J.; Bryant, R.V.; Carter, D.; Christensen, B.; et al. Defining transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: Systematic review and expert consensus statement. J. Crohns Colitis 2022, 16, 554–580. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Maaser, C. The Use of Intestinal Ultrasound in Ulcerative Colitis-More Than a Mucosal Disease? Gastroenterology 2022, 163, 1485–1487. [Google Scholar] [CrossRef]
- Maconi, G.; Nylund, K.; Ripolles, T.; Calabrese, E.; Dirks, K.; Dietrich, C.F.; Hollerweger, A.; Sporea, I.; Saftoiu, A.; Maaser, C.; et al. EFSUMB recommendations and clinical guidelines for intestinal ultrasound (GIUS) in inflammatory bowel diseases. Ultraschall. Med. 2018, 39, 304–317. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Dubbins, P.A. Ultrasound demonstration of bowel wall thickness in inflammatory bowel disease. Clin. Radiol. 1984, 35, 227–231. [Google Scholar] [CrossRef]
- Limberg, B. Diagnosis of acute ulcerative colitis and colonic Crohn’s disease by colonic sonography. J. Clin. Ultrasound. 1989, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, F.; Dal Buono, A.; Allocca, M.; D’Amico, F.; Zilli, A.; Gabbiadini, R.; Danese, S. Bowel ultrasound in inflammatory bowel disease: How far in the grayscale? Life 2021, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Hollerweger, A.; Dirks, K.; Higginson, A.; Serra, C.; Calabrese, E.; Dong, Y.; Hausken, T.; Maconi, G.; Mihmanli, I.; et al. EFSUMB Gastrointestinal Ultrasound (GIUS) Task Force Group: Celiac sprue and other rare gastrointestinal diseases ultrasound features. Med. Ultrason. 2019, 21, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Fraquelli, M.; Castiglione, F.; Calabrese, E.; Maconi, G. Impact of intestinal ultrasound on the management of patients with inflammatory bowel disease: How to apply scientific evidence to clinical practice. Dig. Liver Dis. 2020, 52, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Limberg, B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z. Gastroenterol. 1999, 37, 495–508. [Google Scholar] [PubMed]
- Smith, R.L.; Taylor, K.M.; Friedman, A.B.; Gibson, R.N.; Gibson, P.R. Systematic review: Clinical utility of gastrointestinal ultrasound in the diagnosis, assessment and management of patients with ulcerative colitis. J. Crohns Colitis 2020, 14, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Parente, F.; Greco, S.; Molteni, M.; Cucino, C.; Maconi, G.; Sampietro, G.M.; Danelli, P.G.; Cristaldi, M.; Bianco, R.; Gallus, S.; et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment. Pharmacol. Ther. 2003, 18, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Katsurada, T.; Nishida, M.; Omotehara, S.; Onishi, R.; Mabe, K.; Onodera, A.; Sato, M.; Eto, K.; Suya, M.; et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: A prospective, multicenter study. J. Gastroenterol. 2019, 54, 521–529. [Google Scholar] [CrossRef]
- Bots, S.; Nylund, K.; Löwenberg, M.; Gecse, K.; D’Haens, G. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a novel UC-Ultrasound Index. J. Crohns Colitis 2021, 15, 1264–1271. [Google Scholar] [CrossRef]
- Allocca, M.; Fiorino, G.; Bonovas, S.; Furfaro, F.; Gilardi, D.; Argollo, M.; Magnoni, P.; Peyrin-Biroulet, L.; Danese, S. Accuracy of humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: A prospective study. J. Crohns Colitis 2018, 12, 1385–1391. [Google Scholar] [CrossRef]
- Shirahama, M.; Ishibashi, H.; Onohara, S.; Miyamoto, Y. Application of color Doppler ultrasonography to ulcerative colitis. J. Med. Ultrason. 2003, 30, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Ardizzone, S.; Parente, F.; Bianchi Porro, G. Ultrasonography in the evaluation of extension, activity, and follow-up of ulcerative colitis. Scand. J. Gastroenterol. 1999, 34, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Craviotto, V.; Dell’Avalle, C.; Furfaro, F.; Zilli, A.; D’Amico, F.; Bonovas, S.; Peyrin-Biroulet, L.; Fiorino, G.; Danese, S. Bowel ultrasound score is accurate in assessing response to therapy in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2022, 55, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Pascu, M.; Roznowski, A.B.; Müller, H.P.; Adler, A.; Wiedenmann, B.; Dignass, A.U. Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel. Inflamm. Bowel. Dis. 2004, 10, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Parente, F.; Molteni, M.; Marino, B.; Colli, A.; Ardizzone, S.; Greco, S.; Sampietro, G.; Foschi, D.; Gallus, S. Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: A prospective study. Am. J. Gastroenterol. 2010, 105, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Arienti, V.; Campieri, M.; Boriani, L.; Gionchetti, P.; Califano, C.; Giancane, S.; Furno, A.; Gasbarrini, G. Management of severe ulcerative colitis with the help of high resolution ultrasonography. Am. J. Gastroenterol. 1996, 91, 2163–2169. [Google Scholar] [PubMed]
- Rispo, A.; Calabrese, G.; Testa, A.; Imperatore, N.; Patturelli, M.; Allocca, M.; Guarino, A.D.; Cantisani, N.M.; Toro, B.; Castiglione, F. Hocus pocus: The role of handheld ultrasonography in predicting disease extension and endoscopic activity in ulcerative colitis. J. Crohns Colitis 2023, 17, 1089–1096. [Google Scholar] [CrossRef]
- Christian, M.; Giovanni, M.; Torsten, K.; Mariangela, A. Ultrasonography in inflammatory bowel disease—So far we are? United. Eur. Gastroenterol. J. 2022, 10, 225–232. [Google Scholar] [CrossRef]
- 29. Sagami, S.; Kobayashi, T.; Miyatani, Y.; Okabayashi, S.; Yamazaki, H.; Takada, T.; Kinoshita, K.; Allocca, M.; Kunisaki, R.; Ramaswamy, P.K.; et al. Accuracy of Ultrasound for Evaluation of Colorectal Segments in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol 2021, 19, 908–921.e6. [Google Scholar] [CrossRef]
- Rimola, J.; Torres, J.; Kumar, S.; Taylor, S.A.; Kucharzik, T. Recent advances in clinical practice: Advances in cross-sectional imaging in inflammatory bowel disease. Gut 2022, 71, 2587–2597. [Google Scholar] [CrossRef]
- Mallouhi, A.; Bonatti, H.; Peer, S.; Lugger, P.; Conrad, F.; Bodner, G. Detection and characterization of perianal inflammatory disease: Accuracy of transperineal combined gray scale and color Doppler sonography. J. Ultrasound. Med. 2004, 23, 19–27. [Google Scholar] [CrossRef]
- Sagami, S.; Kobayashi, T.; Aihara, K.; Umeda, M.; Morikubo, H.; Matsubayashi, M.; Kiyohara, H.; Nakano, M.; Ohbu, M.; Hibi, T. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 1373–1383. [Google Scholar] [CrossRef]
- 33. Tokushima, K.; Jimbo, K.; Suzuki, M.; Endo, Y.; Hibio, M.; Maruyama, K.; Kashiwagi, K.; Arai, N.; Sato, M.; Kudo, T.; et al. Differentiation of active ulcerative colitis vs noninflammatory bowel disease proctitis by transperineal superb microvascular imaging. Inflamm. Bowel. Dis. 2023, izad186. [Google Scholar] [CrossRef]
- Allocca, M.; Furfaro, F.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Point-of-Care Ultrasound in Inflammatory Bowel Disease. J. Crohns. Colitis. 2021, 15, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Kucharzik, T.; Rubin, D.T. Intestinal ultrasound in the assessment and management of inflammatory bowel disease: Is it ready for standard practice? Gastroenterology 2023, 164, 851–855. [Google Scholar] [CrossRef]
- Parente, F.; Molteni, M.; Marino, B.; Colli, A.; Ardizzone, S.; Greco, S.; Sampietro, G.; Gallus, S. Bowel ultrasound and mucosal healing in ulcerative colitis. Dig. Dis. 2009, 27, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yoshida, S.; Tanaka, S.; Takemura, Y.; Oka, S.; Yoshihara, M.; Yamada, H.; Chayama, K. Predicting the clinical response to cytapheresis in steroid-refractory or -dependent ulcerative colitis using contrast-enhanced ultrasonography. Scand. J. Gastroenterol. 2009, 44, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Dell’Avalle, C.; Craviotto, V.; Furfaro, F.; Zilli, A.; D’Amico, F.; Bonovas, S.; Peyrin-Biroulet, L.; Fiorino, G.; Danese, S. Predictive value of Milan ultrasound criteria in ulcerative colitis: A prospective observational cohort study. United Eur. Gastroenterol. J. 2022, 10, 190–197. [Google Scholar] [CrossRef]
- Allocca, M.; Filippi, E.; Costantino, A.; Bonovas, S.; Fiorino, G.; Furfaro, F.; Peyrin-Biroulet, L.; Fraquelli, M.; Caprioli, F.; Danese, S. Milan ultrasound criteria are accurate in assessing disease activity in ulcerative colitis: External validation. United Eur. Gastroenterol. J. 2021, 9, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Piazza OSed, N.; Noviello, D.; Filippi, E.; Conforti, F.; Furfaro, F.; Fraquelli, M.; Costantino, A.; Danese, S.; Vecchi, M.; Fiorino, G.; et al. Superior predictive value of transmural over endoscopic severity for colectomy risk in ulcerative colitis: A multicenter prospective cohort study. J. Crohns Colitis 2023, jjad152. [Google Scholar] [CrossRef]
- Garcia, N.M.; Cohen, N.A.; Rubin, D.T. Treat-to-target and sequencing therapies in Crohn’s disease. United Eur. Gastroenterol. J. 2022, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Petersen, F.; Helwig, U.; Fischer, I.; Roessler, A.; Rath, S.; Lang, D.; Kucharzik, T.; German IBD Study Group and the TRUST&UC study group. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: Results from the TRUST&UC study. Gut 2020, 69, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Dell’Avalle, C.; Furfaro, F.; Zilli, A.; D’Amico, F.; Peyrin-Biroulet, L.; Fiorino, G.; Danese, S. Early intestinal ultrasound predicts long-term endoscopic response to biologics in ulcerative colitis. J. Crohns Colitis 2023, 17, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- de Voogd, F.; van Wassenaer, E.A.; Mookhoek, A.; Bots, S.; van Gennep, S.; Löwenberg, M.; D’Haens, G.R.; Gecse, K.B. Intestinal ultrasound is accurate to determine endoscopic response and remission in patients with moderate to severe ulcerative colitis: A longitudinal prospective cohort study. Gastroenterology 2022, 163, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Su, C.; Sands, B.E. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Dinesen, L.C.; Walsh, A.J.; Protic, M.N.; Heap, G.; Cummings, F.; Warren, B.F.; George, B.; Mortensen, N.J.; Travis, S.P. The pattern and outcome of acute severe colitis. J. Crohns Colitis 2010, 4, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Øresland, T.; Bemelman, W.A.; Sampietro, G.M.; Spinelli, A.; Windsor, A.; Ferrante, M.; Marteau, P.; Zmora, O.; Kotze, P.G.; Espin-Basany, E.; et al. European evidence based consensus on surgery for ulcerative colitis. J. Crohns Colitis 2015, 9, 4–25. [Google Scholar] [CrossRef]
- Scarallo, L.; Maniscalco, V.; Paci, M.; Renzo, S.; Naldini, S.; Barp, J.; Tasciotti, L.; Lionetti, P. Bowel ultrasound scan predicts corticosteroid failure in children with acute severe colitis. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 46–51. [Google Scholar] [CrossRef]
- Ilvemark, J.F.K.F.; Wilkens, R.; Thielsen, P.; Dige, A.; Boysen, T.; Brynskov, J.; Bjerrum, J.T.; Seidelin, J.B. Early intestinal ultrasound predicts intravenous corticosteroid response in hospitalised patients with severe ulcerative colitis. J. Crohns Colitis 2022, 16, 1725–1734. [Google Scholar] [CrossRef]
- Bryant, R.V.; Friedman, A.B.; Wright, E.K.; Taylor, K.M.; Begun, J.; Maconi, G.; Maaser, C.; Novak, K.L.; Kucharzik, T.; Atkinson, N.S.S.; et al. Gastrointestinal ultrasound in inflammatory bowel disease: An underused resource with potential paradigm-changing application. Gut 2018, 67, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Z.S.; Dolinger, M.; Kayal, M.; Rao, B.B.; Bhattacharya, A.; Dubinsky, M.C.; Ungaro, R.C. Clinical Challenge: Proactive Precise Management of Active Ulcerative Colitis During Pregnancy-Advantages of Point-of-Care Intestinal Ultrasound and Therapeutic Drug Monitoring. Dig. Dis. Sci. 2022, 67, 3557–3561. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.; Wright, E.K.; Begun, J.; Bryant, R.V.; An, Y.K.; Ross, A.L.; Kiburg, K.V.; Bell, S.J. Monitoring inflammatory bowel disease in pregnancy using gastrointestinal ultrasonography. J. Crohns Colitis 2020, 14, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- De Voogd, F.; Joshi, H.; Van Wassenaer, E.; Bots, S.; D’Haens, G.; Gecse, K. Intestinal ultrasound to evaluate treatment response during pregnancy in patients with inflammatory bowel disease. Inflamm. Bowel. Dis. 2022, 28, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Abdi, T.; Gentry, M.; Laine, L. Histological Disease Activity as a Predictor of Clinical Relapse Among Patients With Ulcerative Colitis: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2016, 111, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.A.; Mani, V.; Goodman, M.J.; Dutt, S.; Herd, M.E. Microscopic activity in ulcerative colitis: What does it mean? Gut 1991, 32, 174–178. [Google Scholar] [CrossRef]
- Bessissow, T.; Kron, C.M.; Marcus, V.; Lemieux, C.; Laneuville, J.; Afif, W.; Wild, G.; Lakatos, P.L.; Brassard, P.; Bitton, A. Impact of endoscopic and histologic activity on disease relapse in ulcerative colitis. Am. J. Gastroenterol. 2022, 117, 1632–1638. [Google Scholar] [CrossRef]
- Mosli, M.H.; Feagan, B.G.; Zou, G.; Sandborn, W.J.; D’Haens, G.; Khanna, R.; Shackelton, L.M.; Walker, C.W.; Nelson, S.; Vandervoort, M.K.; et al. Development and validation of a histological index for UC. Gut 2017, 66, 50–58. [Google Scholar] [CrossRef]
- Singh, S.; Al-Darmaki, A.; Frolkis, A.D.; Seow, C.H.; Leung, Y.; Novak, K.L.; Ghosh, S.; Eksteen, B.; Panaccione, R.; Kaplan, G.G. Postoperative Mortality Among Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis of Population-Based Studies. Gastroenterology 2015, 149, 928–937. [Google Scholar] [CrossRef]
- Bemelman, W.A. S-ECCO collaborators. Evolving role of IBD surgery. J. Crohns Colitis 2018, 12, 1005–1007. [Google Scholar] [CrossRef]
- Kayal, M.; Ungaro, R.C.; Riggs, A.; Kamal, K.; Agrawal, M.; Cohen-Mekelburg, S.; Axelrad, J.; Faye, A.; Scherl, E.; Lawlor, G.; et al. Ileal pouch anal anastomosis for the management of ulcerative colitis is associated with significant disability. Clin. Gastroenterol. Hepatol. 2022, 20, e761–e769. [Google Scholar] [CrossRef] [PubMed]
- Fazio, V.W.; Kiran, R.P.; Remzi, F.H.; Coffey, J.C.; Heneghan, H.M.; Kirat, H.T.; Manilich, E.; Shen, B.; Martin, S.T. Ileal pouch anal anastomosis: Analysis of outcome and quality of life in 3707 patients. Ann. Surg. 2013, 257, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Fazio, V.W.; Remzi, F.H.; Delaney, C.P.; Bennett, A.E.; Achkar, J.P.; Brzezinski, A.; Khandwala, F.; Liu, W.; Bambrick, M.L.; et al. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am. J. Gastroenterol. 2005, 100, 93–101. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Tremaine, W.J.; Batts, K.P.; Pemberton, J.H.; Phillips, S.F. Pouchitis after ileal pouch-anal anastomosis: A Pouchitis Disease Activity Index. Mayo. Clin. Proc. 1994, 69, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, Z.S.; Friedman, A.B.; Con, D.; Chandran, S.; Gibson, D.; Pham, A.; De Cruz, P.; Tay, K.; Bell, S.; Rosella, O.; et al. Accuracy of Gastrointestinal Ultrasound and Calprotectin in the Assessment of Inflammation and its Location in Patients with an Ileoanal Pouch. J. Crohns Colitis 2022, 16, 79–90. [Google Scholar] [CrossRef]
- Geyl, S.; Guillo, L.; Laurent, V.; D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Transmural healing as a therapeutic goal in Crohn’s disease: A systematic review. Lancet Gastroenterol. Hepatol. 2021, 6, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Pecere, S.; Holleran, G.; Ainora, M.E.; Garcovich, M.; Scaldaferri, F.; Gasbarrini, A.; Zocco, M.A. Usefulness of contrast-enhanced ultrasound (CEUS) in Inflammatory Bowel Disease (IBD). Dig. Liver Dis. 2018, 50, 761–767. [Google Scholar] [CrossRef]
- Dal Buono, A.; Faita, F.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Ultrasound Elastography in Inflammatory Bowel Diseases: A Systematic Review of Accuracy Compared with Histopathological Assessment. J. Crohns Colitis 2022, 16, 1637–1646. [Google Scholar] [CrossRef]



| Study | Study Type | Cohort N. | Clinical Context | Definition | Type of Treatment | Primary Outcome | Major Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| TRUST&UC study-Maaser et al. (Gut 2020) | Multicenter, prospective | 253 | Active UC | SCCAI ≥ 5 | SCS, AZA/6MP, anti-integrin, anti-TNF | % of pts with normalization of BWT in clinical responders (↓ SCCAI by ≥3 points) |
| [42] |
| De Voogd et al. (Gastro 2022) | Multicenter prospective | 30 | Active UC | EMS ≥ 2 in at least 1 segment | TOF | BWT in SC endoscopic responders versus non-responders at 8 wks |
| [44] |
| IIlvemark et al. (JCC 2022) | Multicenter prospective | 56 | Active severe UC | Full Mayo score ≥ 8 | SCS | Outcome I: ↓ pMayo score ≥ 30% and ≥3 points, (rectal bleeding subscore of 1 or 0, or a decrease in rectal bleeding subscore ≥1 point) Outcome II: % pts avoiding RT or surgery |
| [50] |
| Allocca et al. (JCC 2023) | Single center, prospective | 49 | Active UC | EMS > 1 | IFX, VDZ, ADA, UST | MUC ≤ 6.2 at week 12 predicting EMS ≤ 1 at reassessment |
| [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchi, A.; Dal Buono, A.; D’Amico, F.; Furfaro, F.; Zilli, A.; Fiorino, G.; Parigi, T.L.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis. J. Clin. Med. 2023, 12, 7569. https://doi.org/10.3390/jcm12247569
Barchi A, Dal Buono A, D’Amico F, Furfaro F, Zilli A, Fiorino G, Parigi TL, Peyrin-Biroulet L, Danese S, Allocca M. Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis. Journal of Clinical Medicine. 2023; 12(24):7569. https://doi.org/10.3390/jcm12247569
Chicago/Turabian StyleBarchi, Alberto, Arianna Dal Buono, Ferdinando D’Amico, Federica Furfaro, Alessandra Zilli, Gionata Fiorino, Tommaso Lorenzo Parigi, Laurent Peyrin-Biroulet, Silvio Danese, and Mariangela Allocca. 2023. "Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis" Journal of Clinical Medicine 12, no. 24: 7569. https://doi.org/10.3390/jcm12247569
APA StyleBarchi, A., Dal Buono, A., D’Amico, F., Furfaro, F., Zilli, A., Fiorino, G., Parigi, T. L., Peyrin-Biroulet, L., Danese, S., & Allocca, M. (2023). Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis. Journal of Clinical Medicine, 12(24), 7569. https://doi.org/10.3390/jcm12247569








