High-Flow Tracheal Oxygen for Tracheostomy Tube Removal in Lung Transplant Recipients
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFNO | High-Flow Nasal Oxygen |
| HFTO | High-Flow Tracheal Oxygen |
| IRCU | Intermediate Respiratory Care Unit |
References
- Hadem, J.; Gottlieb, J.; Seifert, D.; Fegbeutel, C.; Sommer, W.; Greer, M.; Wiesner, O.; Kielstein, J.T.; Schneider, A.S.; Ius, F.; et al. Prolonged mechanical ventilation after lung transplantation—A single-center study. Am. J. Transplant. 2016, 16, 1579–1587. [Google Scholar] [CrossRef]
- Gao, P.; Li, C.; Wu, J.; Zhang, P.; Liu, X.; Li, Y.; Ding, J.; Su, Y.; Zhu, Y.; He, W.; et al. Establishment of a risk prediction model for prolonged mechanical ventilation after lung transplantation: A retrospective cohort study. BMC Pulm. Med. 2023, 23, 11. [Google Scholar] [CrossRef]
- Epstein, S.K. Late complications of tracheostomy. Respir. Care 2005, 50, 542–549. [Google Scholar] [PubMed]
- Ceriana, P.; Carlucci, A.; Navalesi, P.; Rampulla, C.; Delmastro, M.; Piaggi, G.; De Mattia, E.; Nava, S. Weaning from tracheotomy in long-term mechanically ventilated patients: Feasibility of a decisional flowchart and clinical outcome. Intensive Care Med. 2003, 29, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Twose, P.; Thomas, C.; Morgan, M.; Broad, M.A. Comparison of high-flow oxygen therapy with standard oxygen therapy for prevention of postoperative pulmonary complications after major head and neck surgery involving insertion of a tracheostomy: A feasibility study. Br. J. Oral Maxillofac. Surg. 2019, 57, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, C.; Odoh, M.; Satoh, D.; Hashiguchi, T.; Inada, E. High-flow oxygen via tracheostomy facilitates weaning from prolonged mechanical ventilation in patients with restrictive pulmonary dysfunction: Two case reports. J. Med. Case Rep. 2018, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Corley, A.; Edwards, M.; Spooner, A.J.; Dunster, K.R.; Anstey, C.; Fraser, J.F. High-flow oxygen via tracheostomy improves oxygenation in patients weaning from mechanical ventilation: A randomized crossover study. Intensive Care Med. 2017, 43, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Natalini, D.; Grieco, D.L.; Santantonio, M.T.; Mincione, L.; Toni, F.; Anzellotti, G.M.; Eleuteri, D.; Di Giannatale, P.; Antonelli, M.; Maggiore, S.M. Physiological effects of high-flow oxygen in tracheostomized patients. Ann. Intensive Care 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, E.; Rankin, N.; Ryan, S.; Pack, R. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. 2004, 30, 1491–1494. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Intensive Care 2015, 3, 15. [Google Scholar] [CrossRef]
- McIntyre, N.R.; Epstein, S.K.; Carson, S.; Scheinhorn, D.; Christopher, K.; Muldoon, S. Management of patients requiring prolonged mechanical ventilation—Report of a NAMDRC consensus conference. Chest 2005, 128, 3937–3954. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.D.; Kiely, J.L.; McNicholas, W.T. Controlled oxygen therapy and carbon dioxide retention during exacerbations of chronic obstructive pulmonary disease. Lancet 2001, 357, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Corrado, A.; Arcaro, G.; Gallan, F.; Ori, C.; Minuzzo, M.; Bevilacqua, M. Mechanical insufflation-exsufflation improves outcomes for neuromuscular disease patients with respiratory tract infections. Am. J. Phys. Med. Rehabil. 2005, 84, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Raskin, J.; Vanstapel, A.; Verbeken, E.K.; Beeckmans, H.; Vanaudenaerde, B.M.; Verleden, S.E.; Neyrinck, A.P.; Ceulemans, L.J.; Van Raemdonck, D.E.; Verleden, G.M.; et al. Mortality after lung transplantation: A single-centre cohort analysis. Transpl. Int. 2020, 33, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.; Sacanell, J.; Riera, J.; Masclans, J.R.; Rello, J. Early postoperative management of lung transplantation. Minerva Anestesiol. 2014, 80, 1234–1245. [Google Scholar] [PubMed]
- Feltracco, P.; Milevoj, M.; Alberti, V.; Carollo, C.; Michieletto, E.; Rea, F.; Loy, M.; Marulli, G.; Ori, C. Early tracheostomy following lung transplantation. Transplant. Proc. 2011, 43, 1151–1155. [Google Scholar] [CrossRef]
- Masclans, J.-R.; Zapatero, A.; Sacanell, J. High Flow Therapy in Post-Lung Transplant Patients. Arch. Bronconeumol. 2017, 53, 182–183. [Google Scholar] [CrossRef]
- Durbin, C.G., Jr. Tracheostomy: Why, when, and how? Respir. Care 2010, 55, 1056–1068. [Google Scholar]
- Cheung, N.H.; Napolitano, L.M. Tracheostomy: Epidemiology, indications, timing, technique, and outcomes. Respir. Care 2014, 59, 895–915. [Google Scholar] [CrossRef]
- Chen, X.; Tan, C.; Jiang, H. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy in intensive care unit patients after extubation. Am. J. Transl. Res. 2023, 15, 1239–1246. [Google Scholar]
- Williams, R.; Rankin, N.; Smith, T.; Galler, D.; Seakins, P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit. Care Med. 1996, 24, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Granton, D.; Chaudhuri, D.; Wang, D.H.; Einav, S.; Helviz, Y.; Mauri, T.; Mancebo, J.; Frat, J.-P.; Jog, S.; Hernandez, G.; et al. High-flow nasal cannula compared with conventional oxygen therapy or noninvasive ventilation immediately postextubation: A systematic review and meta-analysis. Crit. Care Med. 2020, 48, e1129–e1136. [Google Scholar] [CrossRef] [PubMed]
- Chanques, G.; Constantin, J.-M.; Sauter, M.; Jung, B.; Sebbane, M.; Verzilli, D.; Lefrant, J.-Y.; Jaber, S. Discomfort associated with underhumidified high-flow oxygen therapy in critically ill patients. Intensive Care Med. 2009, 35, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Stelfox, H.; Crimi, C.; Berra, L.; Noto, A.; Schmidt, U.; Bigatello, L.M.; Hess, D. Determinants of tracheostomy decannulation: An international survey. Crit. Care 2008, 12, R26. [Google Scholar] [CrossRef]
- Lersritwimanmaen, P.; Rittayamai, N.; Tscheikuna, J.; Brochard, L.J. High-flow oxygen therapy in tracheostomized subjects with prolonged mechanical ventilation: A randomized crossover physiologic study. Respir. Care 2021, 66, 806–813. [Google Scholar] [CrossRef]
- Padiyar, J. Critical care considerations in the post-operative period for the lung transplant patient. J. Thorac. Dis. 2021, 13, 6747–6753. [Google Scholar] [CrossRef]
- Kuerner, T. Essential rules and requirements for global clinical trials in rare lung diseases: A sponsor’s standpoint. Respir. Investig. 2015, 53, 2–6. [Google Scholar] [CrossRef]
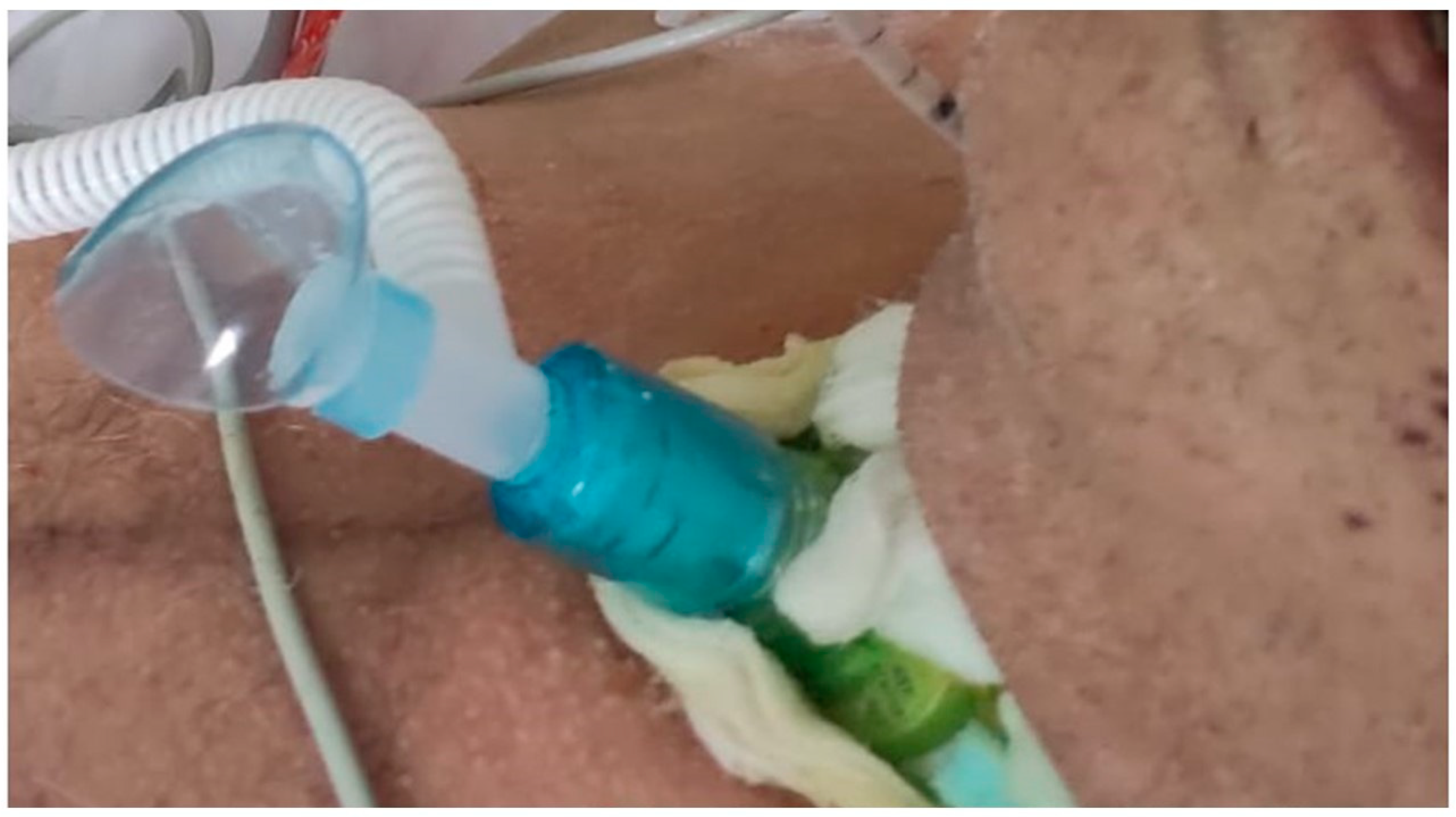
Stable clinical condition, in particular the following:
|
Absence of respiratory distress and stable ABG, in particular the following:
|
Absence of tracheal mucous encumbrance, in particular the following:
|
| Absence of upper-airway abnormalities evaluated through fiberoptic endoscopic examination. |
| Adequate swallowing evaluated using gag reflex, blue dye, and video fluoroscopy. |
| Overall (n = 27) | HFTO Group (n = 14) | COT Group (n = 13) | p-Value | |
|---|---|---|---|---|
| Baseline Demographic and Clinical Data | ||||
| Age, years | 53 (33–64) | 51.5 (33–62) | 55 (44–64) | 0.1815 |
| Female, n (%) | 9 (33.3) | 5 (35.7) | 4 (30.8) | 0.9999 |
| Smokers, n (%) | 18 (85.7) | 9 (75) | 9 (100) | 0.2285 |
| Body mass index, kg/m2 | 23.5 (16.4–31.2) | 23.9 (16.4–31.2) | 23.4 (22.1–29.1) | 0.8852 |
Baseline disease, n (%)
| ||||
| 17 (63) | 8 (57.1) | 9 (69.2) | 0.6945 | |
| 8 (29.6) | 4 (28.5) | 4 (30.8) | 0.9999 | |
| 2 (7.4) | 2 (14.3) | 0 (0) | 0.4815 | |
Pts with comorbidities, n (%)
| ||||
| 21 (77.8) | 10 (71.4) | 11 (84.6) | 0.6483 | |
| 2 (7.4) | 1 (7.1) | 1 (7.1) | 0.9999 | |
| 2 (7.4) | 2 (14.3) | 0 (0) | 0.4814 | |
| 2 (7.4) | 1 (7.1) | 1 (7.1) | 0.9999 | |
| 1 (3.7) | 0(0) | 1 (7.7) | 0.4814 | |
| 4 (14.8) | 1 (7.1) | 3 (23.1) | 0.3259 | |
| Pre-operative NIV, n (%) | 4 (14.8) | 1 (7.1) | 3 (23.1) | 0.3259 |
Type of immunosuppressive therapy, n (%)
| ||||
| 16 (59.3) | 5 (35.7) | 11 (84.6) | 0.0183 | |
| 11 (40.7) | 9 (64.3) | 2 (15.4) | 0.0183 | |
| Type of tracheostomy, (surgical/percutaneous) | 25/2 | 13/1 | 12/1 | 0.9999 |
| Clinical, laboratory and BGA data on ventilator disconnection | ||||
| Duration of the weaning protocol, days | 3–63 | 7 (3–29) | 12 (3–63) | 0.09 |
| Heart rate, beats/min | 89.5 (72–113) | 88.5 (80–106) | 89.5 (72–113) | 0.9794 |
| Respiratory rate, breaths/min | 17(13–33) | 21.5 (17–33) | 17 (13–20) | 0.0055 |
| Body temperature, (°C) | 36.5 (35.6–37.7) | 36.5 (35.6–37.7) | 36.6 (35.6–37.5) | 0.9607 |
| Pts with positive BAL for MDRO, n (%) | 17 (63) | 7 (50) | 10 (76.9) | 0.2364 |
| White blood cell count, × 109/L | 11.43 (4.98–20.74) | 12.77 (4.98–20.74) | 9.08 (5.27–18.57) | 0.0652 |
| Haemoglobin, g/L | 89 (83–106) | 89.5 (84–106) | 89 (83–98) | 0.8840 |
| D-dimer, μg/L | 842 (282–4041) | 1286 (503–4041) | 737 (282–2443) | 0.0345 |
| Serum C-reactive protein, mg/dL | 30 (2.9–320.0) | 31 (3–130) | 30 (13–320) | 0.6976 |
| Creatinine, μmol/L | 70 (25–158) | 64 (25–99) | 77 (32–158) | 0.2641 |
| proBNP, ng/L | 463 (45–3359) | 405 (199–611) | 463 (45–3359) | 0.9999 |
| Troponin, ng/L | 103 (28–687) | 101 (28–251) | 375 (62–687) | 0.2827 |
| PaO2 (O2 suppl), mmHg | 99.9 (68.7–313.4) | 104.0 (68.7–313.4) | 91.1 (75.6–195.0) | 0.5935 |
| PaCO2, mmHg | 39.5 (28.6–56.1) | 39.5 (28.6–56.0) | 40.0 (28.9–56.1) | 0.7158 |
| Arterial pH | 7.44 (7.36–7.52) | 7.44 (7.37–7.50) | 7.43 (7.36–7.52) | 0.6609 |
| SaO2, % | 97.1 (93.4–99.3) | 97.1 (93.4–99.3) | 97.3 (95.3–98.7) | 0.9806 |
| PaO2/FiO2, mmHg | 287 (98–522) | 269.5 (98–522) | 287 (178–487) | 0.7709 |
| APACHE II score | 10 (6–15) | 9.5 (6–14) | 10 (8–15) | 0.3175 |
| Overall (n = 27) | HFTO Group (n = 14) | COT Group (n = 13) | p-Value | |
|---|---|---|---|---|
| Patients who underwent decannulation, n (%) | 19 (70.4) | 13 (92.8) | 6 (46.1) | 0.0128 |
| Patients who developed URTI, n (%) | 4 (14.8) | 2 (14.3) | 2 (15.4) | 0.999 |
Patients who required BAA
| 12 (44.4) | 4 (28.6) | 8 (61.5) | 0.1283 |
| 6 (22.2) | 3 (21.4) | 3 (23.1) | 0.999 | |
| Length of IRCU stay, days | 21 (3–67) | 16 (3–67) | 25 (14–59) | 0.1565 |
| Death during hospitalization, n (%) | 0 (0) | 0 (0) | 0 (0) | 0.999 |
Treatment-related complications, n (%)
| ||||
| 0 (0) | 0 (0) | 0 (0) | 0.999 | |
| 1 (3.7) | 1 (7.1) | 0 (0) | 0.999 | |
| 6 (22.2) | 3 (21.4) | 3 (21.4) | 0.999 | |
| 2 (7.4) | 1 (7.1) | 1 (7.1) | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionello, F.; Guarnieri, G.; Arcaro, G.; Bertagna De Marchi, L.; Molena, B.; Contessa, C.; Boscolo, A.; Rea, F.; Navalesi, P.; Vianello, A. High-Flow Tracheal Oxygen for Tracheostomy Tube Removal in Lung Transplant Recipients. J. Clin. Med. 2023, 12, 7566. https://doi.org/10.3390/jcm12247566
Lionello F, Guarnieri G, Arcaro G, Bertagna De Marchi L, Molena B, Contessa C, Boscolo A, Rea F, Navalesi P, Vianello A. High-Flow Tracheal Oxygen for Tracheostomy Tube Removal in Lung Transplant Recipients. Journal of Clinical Medicine. 2023; 12(24):7566. https://doi.org/10.3390/jcm12247566
Chicago/Turabian StyleLionello, Federico, Gabriella Guarnieri, Giovanna Arcaro, Leonardo Bertagna De Marchi, Beatrice Molena, Cristina Contessa, Annalisa Boscolo, Federico Rea, Paolo Navalesi, and Andrea Vianello. 2023. "High-Flow Tracheal Oxygen for Tracheostomy Tube Removal in Lung Transplant Recipients" Journal of Clinical Medicine 12, no. 24: 7566. https://doi.org/10.3390/jcm12247566
APA StyleLionello, F., Guarnieri, G., Arcaro, G., Bertagna De Marchi, L., Molena, B., Contessa, C., Boscolo, A., Rea, F., Navalesi, P., & Vianello, A. (2023). High-Flow Tracheal Oxygen for Tracheostomy Tube Removal in Lung Transplant Recipients. Journal of Clinical Medicine, 12(24), 7566. https://doi.org/10.3390/jcm12247566






