Integrating Virtual Surgical Planning and 3D-Printed Tools with Iliac Bone Grafts for Orbital and Zygomatic Reconstruction in Hemifacial Microsomia Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion of Patients
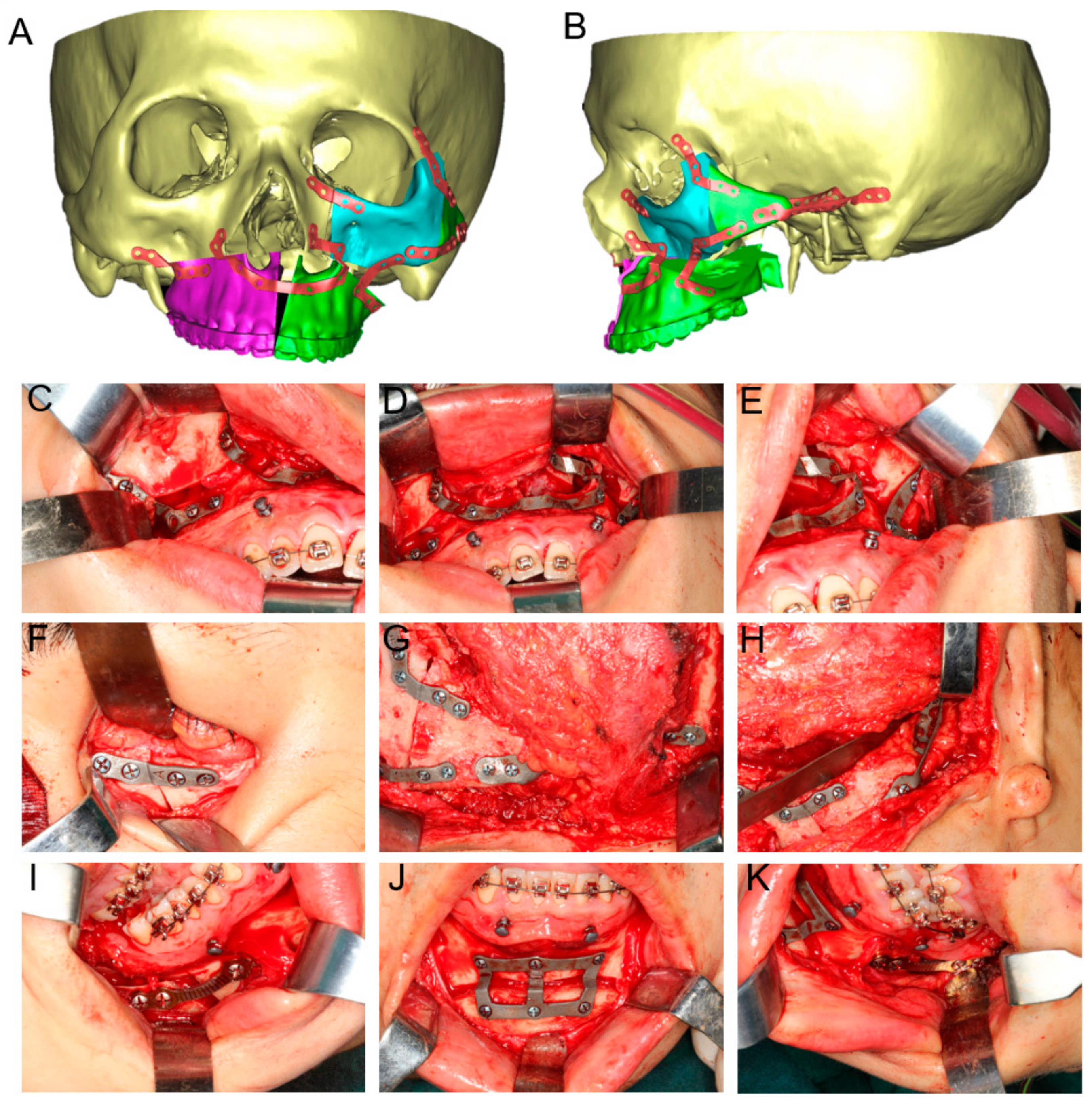
2.2. Virtual Surgical Planning
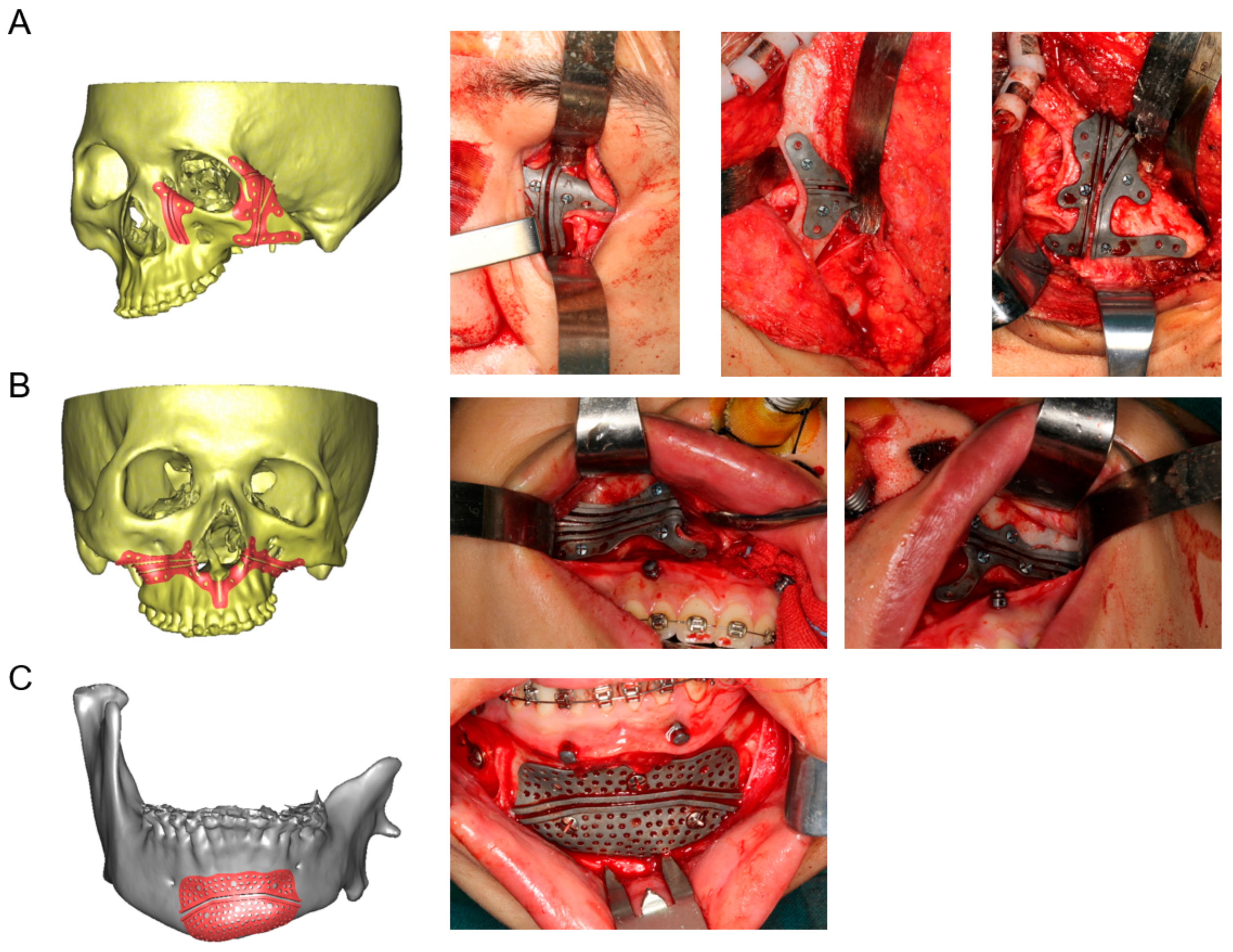
2.3. Design and Fabrication of 3D-Printed Cutting Guides, Fixation Plates, and Titanium Mesh
2.4. Surgical Procedure
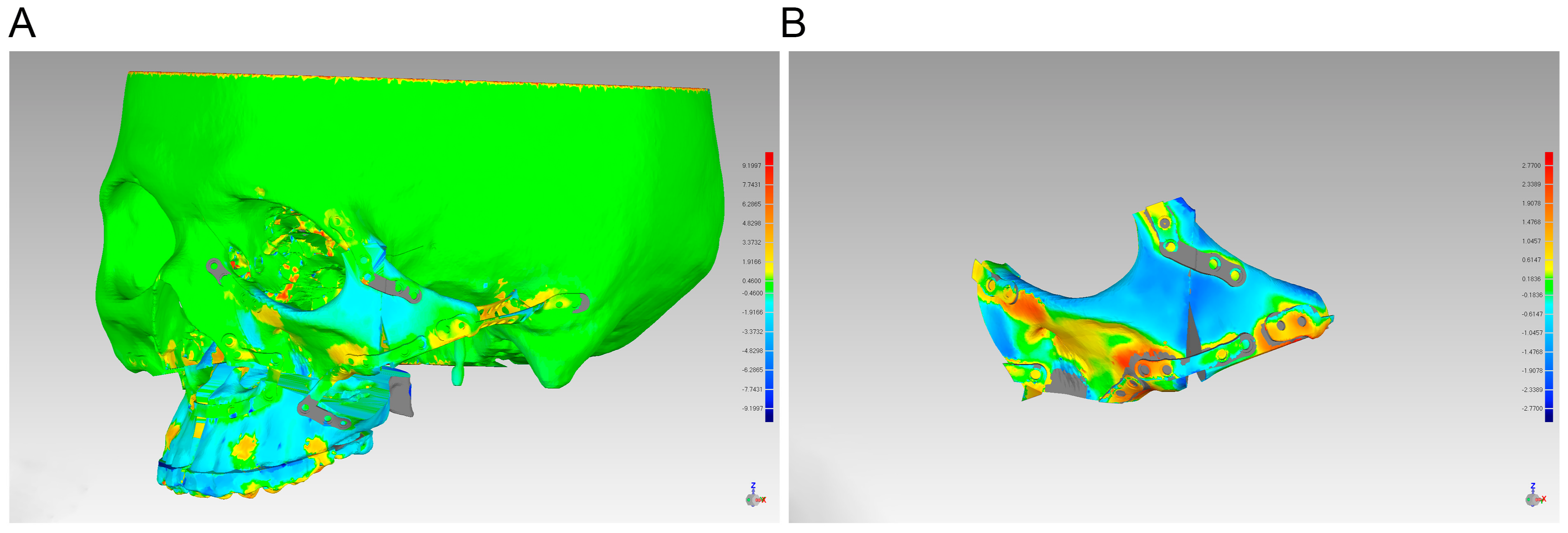
2.5. Evaluation
2.6. Statistical Analysis
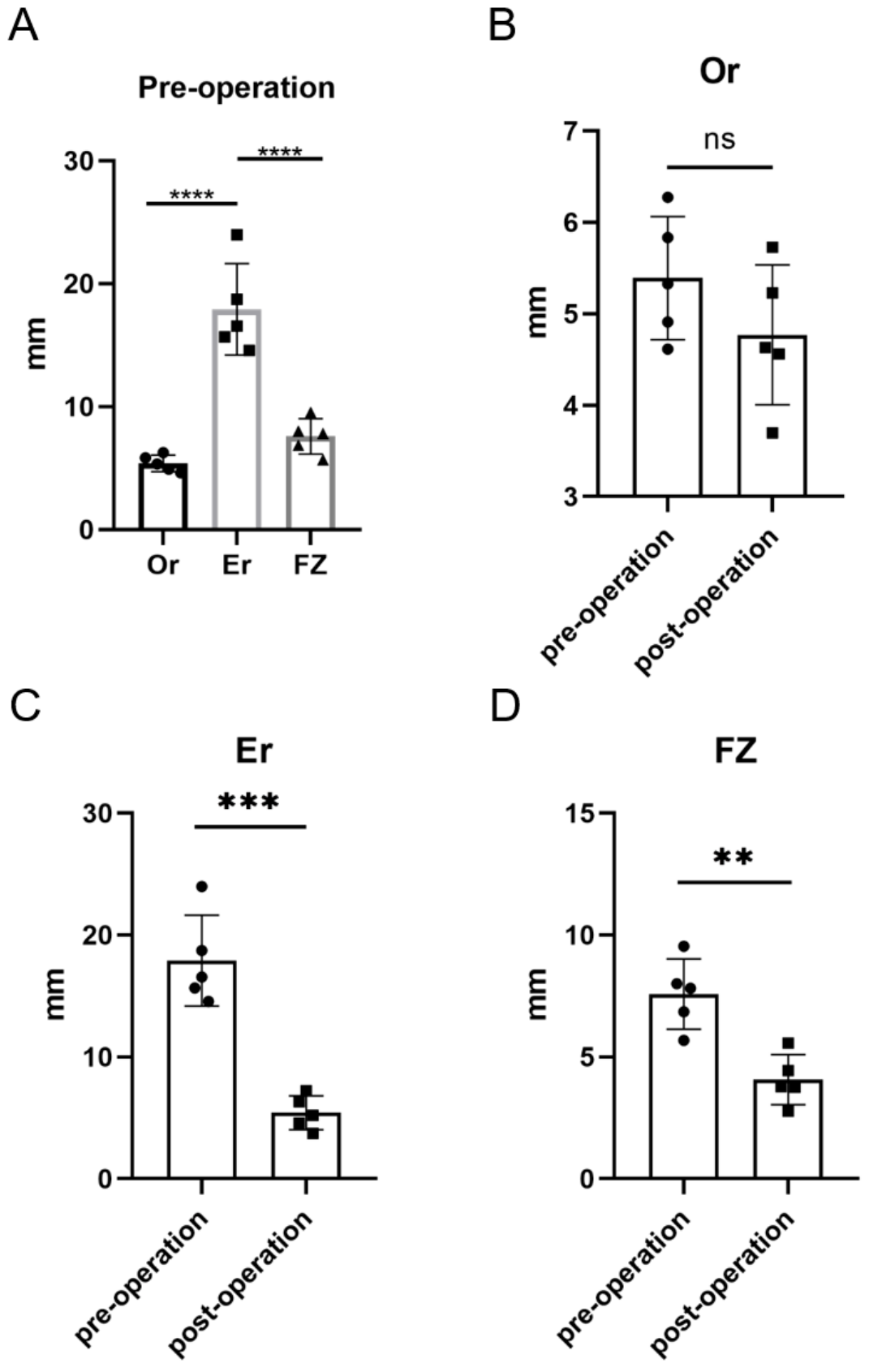
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuin, A.J.; Tahiri, Y.; Paine, K.M.; Paliga, J.T.; Taylor, J.A.; Bartlett, S.P. Clarifying the Relationships among the Different Features of the OMENS plus Classification in Craniofacial Microsomia. Plast. Reconstr. Surg. 2015, 135, 149e–156e. [Google Scholar] [CrossRef]
- Paul, M.A.; Opyrchal, J.; Knakiewicz, M.; Jaremkow, P.; Bajtek, J.; Chrapusta, A. Hemifacial Microsomia Review: Recent Advancements in Understanding the Disease. J. Craniofac. Surg. 2020, 31, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Tingaud-Sequeira, A.; Trimouille, A.; Sagardoy, T.; Lacombe, D.; Rooryck-Thambo, C. Oculo-auriculo-vertebral spectrum: New genes and literature review on a complex disease. J. Med. Genet. 2022, 59, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Sun, H.; Bian, Q.; Liu, Z.; Wang, X. The etiology, clinical features, and treatment options of hemifacial microsomia. Oral Dis. 2023, 29, 2449–2462. [Google Scholar] [CrossRef]
- Apostolopoulos, K.; Bous, R.M.; ElNaghy, R.; Kumar, A.R.; Valiathan, M. Examining the variability of bone and soft tissue morphology in Hemifacial Microsomia: A case series of 8 patients. J. Craniomaxillofac. Surg. 2021, 49, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Z.; Wang, Y.; Li, X.; Ye, B.; Li, J. The accuracy of virtual-surgical-planning-assisted treatment of hemifacial microsomia in adult patients: Distraction osteogenesis vs. orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2019, 48, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, Y.; Chang, C.S.; Tuin, J.; Paliga, J.T.; Lowe, K.M.; Taylor, J.A.; Bartlett, S.P. Costochondral grafting in craniofacial microsomia. Plast. Reconstr. Surg. 2015, 135, 530–541. [Google Scholar] [CrossRef]
- Arif, H.; Ashraf, R.; Khan, F.; Khattak, Y.R.; Nisar, H.; Ahmad, I. Total temporomandibular joint reconstruction prosthesis in hemifacial microsomia: A systematic review. Orthod. Craniofac. Res. 2023. [Google Scholar] [CrossRef]
- Maas, B.; Pluijmers, B.I.; Knoops, P.G.M.; Ruff, C.; Koudstaal, M.J.; Dunaway, D. Using principal component analysis to describe the midfacial deformities in patients with craniofacial microsomia. J. Craniomaxillofac. Surg. 2018, 46, 2032–2041. [Google Scholar] [CrossRef]
- Roy, T.; Steinbacher, D.M. Virtual Planning and 3D Printing in Contemporary Orthognathic Surgery. Semin. Plast. Surg. 2022, 36, 169–182. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, S.; Fan, X.; You, Y.; Ye, G.; Zhou, N. A Meta-analysis and Systematic Review Comparing the Effectiveness of Traditional and Virtual Surgical Planning for Orthognathic Surgery: Based on Randomized Clinical Trials. J. Oral Maxillofac. Surg. 2021, 79, 471.e1–471.e19. [Google Scholar] [CrossRef]
- Di Blasio, C.; Di Blasio, M.; Vaienti, B.; Di Francesco, F.; Lanza, A.; Minervini, G.; Segù, M.; Di Blasio, A. Planning the Aesthetics of the Mandibular Angles in Orthognathic Surgery: Traditional vs. Virtual 3D Articulators. Appl. Sci. 2022, 12, 12064. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Y.; Abdelrehem, A.; Jiang, T.; Wang, X. Application of Virtual Planning for Three-Dimensional Guided Maxillofacial Reconstruction of Pruzansky-Kaban III Hemifacial Microsomia Using Custom Made Fixation Plate. J. Craniofac. Surg. 2021, 32, 896–901. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Zhang, Z.; Li, X.; Ye, B.; Li, J. Comprehensive consideration and design with the virtual surgical planning-assisted treatment for hemifacial microsomia in adult patients. J. Craniomaxillofac. Surg. 2018, 46, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Miyamoto, I.; Hoshi, I.; Kawamata, S.; Takahashi, N.; Shimazaki, N.; Kondo, H.; Yamada, H. Towards Optimum Mandibular Reconstruction for Dental Occlusal Rehabilitation: From Preoperative Virtual Surgery to Autogenous Particulate Cancellous Bone and Marrow Graft with Custom-Made Titanium Mesh-A Retrospective Study. J. Clin. Med. 2023, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Kaban, L.B.; Moses, M.H.; Mulliken, J.B. Surgical correction of hemifacial microsomia in the growing child. Plast. Reconstr. Surg. 1988, 82, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Horgan, J.E.; Padwa, B.L.; LaBrie, R.A.; Mulliken, J.B. OMENS-Plus: Analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate Craniofac. J. 1995, 32, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chi, Y.; Huang, H.; Su, S.; Xue, H.; Hou, J. Combined use of 3D printing and computer-assisted navigation in the clinical treatment of multiple maxillofacial fractures. Asian J. Surg. 2023, 46, 2284–2292. [Google Scholar] [CrossRef]
- Park, J.; Baumrind, S.; Curry, S.; Carlson, S.K.; Boyd, R.L.; Oh, H. Reliability of 3D dental and skeletal landmarks on CBCT images. Angle Orthod. 2019, 89, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Chai, Y.; Mooi, W.; Chen, X.; Xu, H.; Zin, M.A.; Lin, L.; Zhang, Y.; Yang, X.; Chai, G. Computer-assisted surgery in therapeutic strategy distraction osteogenesis of hemifacial microsomia: Accuracy and predictability. J. Craniomaxillofac. Surg. 2019, 47, 204–218. [Google Scholar] [CrossRef]
- Zuo, K.J.; Heinelt, M.; Ho, E.S.; Forrest, C.R.; Zuker, R.M.; Borschel, G.H. Dynamic Reconstruction of Facial Paralysis in Craniofacial Microsomia. Plast. Reconstr. Surg. 2022, 149, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.; Kim, B.S.; Han, W.; Yan, Y.; Wang, X.; Li, X.; Zhang, Y.; Chai, G. Quantitative structural analysis of hemifacial microsomia mandibles in different age groups. Front. Pediatr. 2023, 11, 1157607. [Google Scholar] [CrossRef]
- Xu, X.; Tang, X.-J.; Zhang, Z.-Y.; Li, B.-H.; Yin, L.; Feng, S.; Liu, W. Association of Mandibular Dysplasia with Maxillary Volumetric and Linear Measurements in Children with Hemifacial Microsomia. J. Craniofac. Surg. 2020, 31, 2204–2207. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhang, C.; Tian, M.; Jiang, T.; Zhang, L.; Yu, H.; Shi, J.; Wang, X. Intraoral Condylectomy with 3D-Printed Cutting Guide versus with Surgical Navigation: An Accuracy and Effectiveness Comparison. J. Clin. Med. 2023, 12, 3816. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Liu, J.; Mao, L.; Xia, L.; Fang, B. Hemifacial microsomia treated with a hybrid technique combining distraction osteogenesis and a mandible-guided functional appliance: Pilot study. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, X.; Gu, S.; Li, H.; Zin, M.A.; Mooi, W.J.; Han, W.; Zhang, Y.; Chai, G. Early hemi-mandibular lengthening by distraction osteogenesis contributes to compensatory maxillary growth. J. Craniomaxillofac. Surg. 2020, 48, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Schmetz, A.; Amiel, J.; Wieczorek, D. Genetics of craniofacial malformations. Semin. Fetal Neonatal Med. 2021, 26, 101290. [Google Scholar] [CrossRef]
- Smartt, J.M., Jr.; Campbell, C.; Hallac, R.; Alford, J.; Derderian, C.A. A Three-Dimensional Study of Midfacial Changes Following Le Fort II Distraction with Zygomatic Repositioning in Syndromic Patients. J. Craniofac. Surg. 2017, 28, e728–e731. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, F.; Zhou, C.; Lin, L.; Zhang, Y.; Chai, G.; Xie, L.; Qi, F.; Li, Q. Does intraoperative navigation improve the accuracy of mandibular angle osteotomy: Comparison between augmented reality navigation, individualised templates and free-hand techniques. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1188–1195. [Google Scholar] [CrossRef]
- Lim, H.K.; Choi, Y.J.; Choi, W.C.; Song, I.S.; Lee, U.L. Three-dimensional soft tissue changes after reduction malarplasty in female patients. Int. J. Oral Maxillofac. Surg. 2022, 51, 1556–1561. [Google Scholar] [CrossRef]
- Han, M.D.; Kwon, T.G. Zygoma and Mandibular Angle Reduction: Contouring Surgery to Correct the Square Face in Asians. Oral Maxillofac. Surg. Clin. N. Am. 2023, 35, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, W.; Tang, X.; Li, C.; Zhang, Z. Three-dimensional Analysis of the Temporal Bone Morphology in Patients with Craniofacial Microsomia. Cleft Palate Craniofac. J. 2023, 10556656221149250. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Hou, M.; Yang Lin, Y.; Yuan Wang, Y. Digital localization of osteotomy position in prominent zygomatic arch. J. Craniomaxillofac. Surg. 2021, 49, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, P.W.; Al-Nawas, B. Bone reconstruction of extensive maxillomandibular defects in adults. Periodontology 2000 2023. [Google Scholar] [CrossRef]
- Omara, M.; Raafat, L.; Elfaramawi, T. Secondary alveolar cleft grafting using autogenous mineralized plasmatic matrix (MPM) versus cancellous bone particles derived from anterior iliac crest. Clin. Oral Investig. 2023, 27, 4259–4270. [Google Scholar] [CrossRef]



| Landmark | Definition | |
|---|---|---|
| Na | Nasion | Midpoint of the frontonasal suture |
| Po * | Porion | Most superior point of the external acoustic meatus |
| Or * | Orbitale | Most inferior point of the infraorbital rim |
| FZ * | Frontozygomatic suture | Midpoint point of the frontozygomatic suture at the level of the lateral orbital rim |
| Er * | Zygomaticotemporal suture | Midpoint of the zygomaticotemporal suture |
| Se | Sella | Midpoint of the pituitary fossa |
| Ba | Basion | The most inferior point on the anterior margin of the foramen magnum in the middle |
| Sample | Gender | Age (years) | Mean 3D Deviation (mm) | Max 3D Deviation (mm) | Bone Resorption Rates |
|---|---|---|---|---|---|
| 1 | F | 24 | 0.98 | 2.84 | 40.64% |
| 2 | F | 19 | 1.00 | 2.87 | 45.29% |
| 3 | F | 21 | 1.33 | 3.82 | 43.28% |
| 4 | M | 26 | 0.97 | 3.26 | 49.73% |
| 5 | M | 24 | 1.05 | 2.77 | 47.24% |
| Mean ± SD | 22.8 ± 2.77 | 1.07 ± 0.15 | 3.11 ± 0.44 | 45.24% ± 3.13% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Bao, J.; Shen, G.; Cai, M.; Yu, H. Integrating Virtual Surgical Planning and 3D-Printed Tools with Iliac Bone Grafts for Orbital and Zygomatic Reconstruction in Hemifacial Microsomia Patients. J. Clin. Med. 2023, 12, 7538. https://doi.org/10.3390/jcm12247538
Zhao Z, Bao J, Shen G, Cai M, Yu H. Integrating Virtual Surgical Planning and 3D-Printed Tools with Iliac Bone Grafts for Orbital and Zygomatic Reconstruction in Hemifacial Microsomia Patients. Journal of Clinical Medicine. 2023; 12(24):7538. https://doi.org/10.3390/jcm12247538
Chicago/Turabian StyleZhao, Zhiyang, Jiahao Bao, Guofang Shen, Ming Cai, and Hongbo Yu. 2023. "Integrating Virtual Surgical Planning and 3D-Printed Tools with Iliac Bone Grafts for Orbital and Zygomatic Reconstruction in Hemifacial Microsomia Patients" Journal of Clinical Medicine 12, no. 24: 7538. https://doi.org/10.3390/jcm12247538
APA StyleZhao, Z., Bao, J., Shen, G., Cai, M., & Yu, H. (2023). Integrating Virtual Surgical Planning and 3D-Printed Tools with Iliac Bone Grafts for Orbital and Zygomatic Reconstruction in Hemifacial Microsomia Patients. Journal of Clinical Medicine, 12(24), 7538. https://doi.org/10.3390/jcm12247538






