Risk Factors for Spinal Cord Injury during Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score
Abstract
1. Introduction
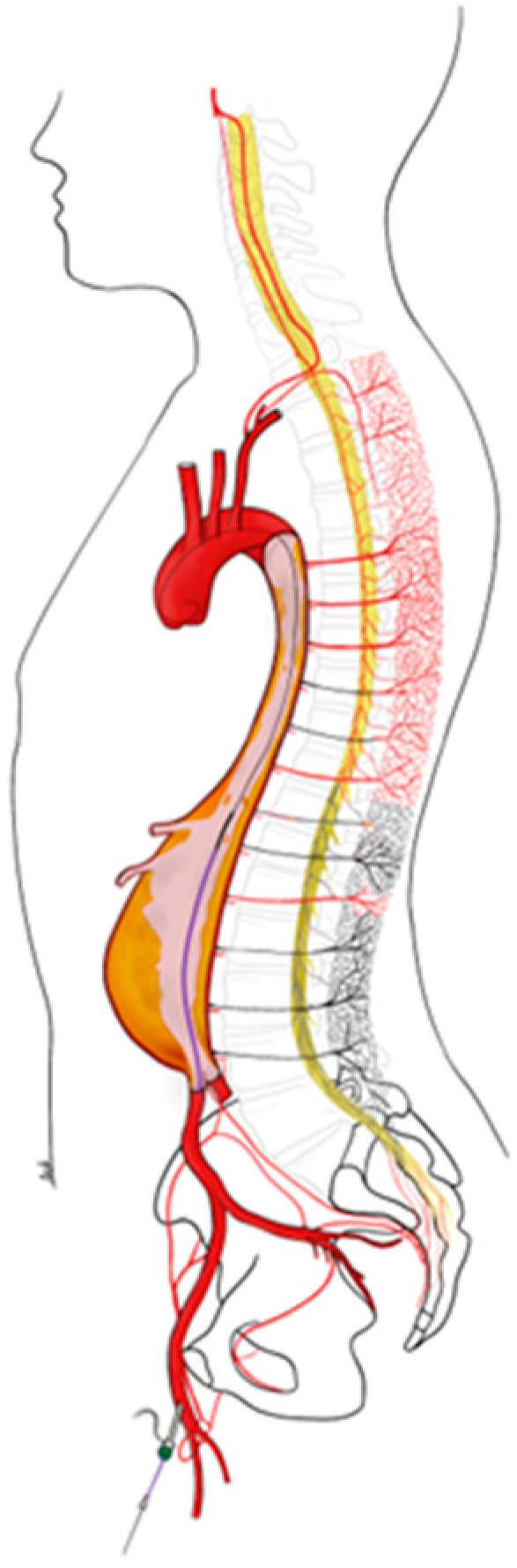
2. Pathogenesis of SCI after Extensive Aortic Endovascular Repair
3. Risk Factors for Paraplegia
3.1. Patient-Related Factors
- Patient condition
- In 1681 patients who underwent complex endovascular aortic repair between 2005 and 2020 as part of the US Aortic Research Consortium, age (age ≥70 years (odd ratio (OR) 1.64; 95% confidence interval (CI) 1.63–1.64; p = 0.029)) and a history of peripheral vascular disease (OR 1.65; 95% CI 1.64–1.65; p = 0.034) were significantly associated with SCI [3]. Impaired preoperative renal function (glomerular filtration rate < 60 mL/min/1.73 m2) is also described as a risk factor (OR 2.43; 95% CI 1.18–4.99; p = 0.016) among 243 patients [15].
- Prior history of aortic repair
- Several single center studies suggest that prior open infrarenal aortic repair is associated with a higher risk of SCI in the case of proximal thoracic (TEVAR) or thoracoabdominal (TAA) endovascular repair. However, a retrospective review of the Vascular Quality Initiative database found a comparable rate of SCI in 9506 patients treated for TEVAR or TAA and with or without prior repair [16]. The role of prior aortic repair is still under controversy.
- Atherosclerosis
- Cumulative cardiovascular risk factors and associated diseases (chronic obstructive pulmonary disease (COPD), obesity, chronic renal insufficiency) have been postulated as markers of widespread peripheral atherosclerotic disease, suggesting that patients may have a compromised collateral network of blood supply to the spinal cord preoperatively. In the end, patients may have a shaggy aorta with a high risk of atheroembolization triggered by intravascular manipulations during surgery. In this case, the Shaggy Aorta Scoring System is a useful method for predicting postoperative embolic complications after TEVAR [17].
- Patient anatomical factors
- In the same retrospective review of the Vascular Quality Initiative database of 9506 patients who had undergone extensive endovascular repair, multivariate regression revealed that aortic dissection was an independent factor for postoperative SCI (OR 1.65; 95% CI 1.26–2.16; p < 0.001). However, the main parameter associated with SCI is the extent of the aneurysm and consequently the length of aortic coverage [3,18]. In addition, preoperative occlusion of one or both hypogastric or subclavian arteries contributes to reducing alternative inflow routes to the spinal collateral network. Occlusion of a single collateral bed has long been associated with an increased risk of immediate SCI and poor recovery [8]. Preventive revascularization of the left subclavian artery is recommended (level B, class IIa) to reduce the risk of neurological complications such as stroke and SCI [19].
3.2. Procedure-Related Factors
- Urgent case
- Urgent repairs are more likely to lead to hemodynamic instability if the collateral arterial network is still underdeveloped. In fact, in the large DeBakey Medical Center cohort, emergency repairs were found to more than double the risk of SCI (RRR, 2.31; p = 0.002) [20]. An urgent repair in the acute phase of an aortic syndrome appears to be the most dangerous, and its postponement after the 15th day should always be considered when feasible.
- Length of aortic coverage
- A large extent of aortic coverage is significantly associated with an increased risk of paraplegia, as shown in a recent large analysis by the US Aortic Consortium (n = 1681 patients; OR 4.79; 95% CI 4.77–4.81; p < 0.001) [3] and a multicenter Italian cohort treated for thoracoabdominal aneurysms of Crawford extents I–III (n = 351 patients; OR 20.90; 95% CI 2.69–162.57; p < 0.004) [18]. Feezor et al. concluded that the risk of SCI is increased by 30% for every 2 cm of additional thoracic aortic coverage [21]. Similarly, Bisdas et al. concluded that each percent of aortic coverage above the superior mesenteric artery leads to a 1.03-fold increase in the risk of SCI [22].
- Hypotension and hemodynamic instability
- Prolonged hypotension or hemodynamic instability has been shown to contribute to the risk of immediate or delayed SCI [4,20,23,24]. This risk exists at least in the first few weeks after extensive TEVAR. In a large report that focused on the results of 1114 open type II repairs, SCI occurred in 13.6% of cases, although it should be noted that approximately half of the cases of persistent SCI did not occur immediately after the procedure. Delayed SCI was consistently preceded by hemodynamic instability (hypotension, bleeding, tachyarrhythmia, or heart failure), and hypotension was associated with delayed SCI in 43% [20].
3.3. Prognostic Scores Available
4. Bundled Protocol for Spinal Cord Protection
4.1. Surgical Factor: Staging
4.2. Anatomical Approach: Preservation of the Spinal Network
4.3. Medical Approach: Optimization of Spinal Cord Oxygenation
- Permissive hypertension and spinal cord perfusion pressure (SCPP)
- Since the irreversible damage to the spinal cord occurs 12 to 48 h after surgery, an immediate response is imperative to try to reverse spinal cord suffering and permanent impairment. Thus, increasing mean arterial pressure (MAP) appears to be the first immediate action that should be taken without further delay given the close relationship between systemic blood pressure and spinal cord perfusion [51]. In general, vasopressor agents such as noradrenaline are administered to maintain a target MAP of 80–100 mmHg and to ensure an SCPP of at least 70 mmHg [52].
- SCPP: spinal cord perfusion pressure; MAPd: distal mean aortic pressure; CSFP: cerebrospinal fluid pressure; CVP: central venous pressure.
- Failure to maintain a patient’s preoperative baseline arterial pressure in the early postoperative period is strongly associated with delayed postoperative SCI [53]. MAP can be further increased in 5–10 mmHg steps in case of persisting SCI.
- Active CSFD can also rapidly improve PPM by lowering SCPP and must be considered in urgent cases.
- Secondary spinal cord injuries (SSCIs)
- Irreversible, systemic secondary SCI is a major prognostic factor in the prevention of neurological sequelae following a lesion process. It refers to the changes that develop over a period of time (from hours to days) after the primary spinal injury. This includes a whole cascade of cellular, chemical, tissue, or vascular changes in the spine that contribute to further destruction of the spinal tissue [54]. This concept was originally developed for traumatic brain injury, but can be extended to any central neurological damage, including SCI in aortic endovascular surgery. These lesions, termed “secondary,” may be intraspinal in origin, a consequence of metabolic and inflammatory disturbances associated with the primary ischemia, or they may be systemic in origin, when failure of vital cardiorespiratory functions leads to spinal ischemia [55]. This is referred to as secondary spinal injury of systemic origin (Table 3). This concept explains the higher proportion of SCI in patients with COPD (hypoxemia) or transfusion of packed red blood cells (anemia):
- -
- Oxygen
- Any hypoxemia should be considered potentially dangerous. Maintaining a PaO2 of at least 60 mmHg (SpO2 > 95%) is therefore a primary goal.
- -
- Hemoglobin
- Evidence-based recommendations for patient blood management have recently been published [56]. A formal program must be implemented for every anemic patient prior to complex endovascular aortic repair. A hemoglobin concentration of 7–8 g/dL is the transfusion threshold that applies in intensive care units for cardiac surgery or critically ill patients. However, for patients with acute central nervous system injury, there is a lack of high-quality published data. In the absence of a clear recommendation, it is usual to consider a transfusion threshold of 10 g/dL in case of SCI [24].
4.4. Neuromonitoring
- Intraoperative neuromonitoring
- This technology, when available, is part of the strategy to reduce SCI real-time identification of motor or sensory neurological dysfunction so that immediate action can be taken to improve cord perfusion. In case of peroperative alteration of the motor (anterior cord) or sensory (dorsal part of the evoked potential of the spinal cord (MEP)) function, the first strategy is to increase the mean arterial pressure to reverse the alteration and return to reference values. If hemodynamic optimization is not sufficient, revascularization of the pelvis and lower limbs may be proposed if technically feasible, and finally early interruption of the procedure if possible.
- Biomarkers
- For early detection of SCI, real-time biomarkers may be of utmost importance, particularly in the perioperative setting when the patient is under general anesthesia and cannot be examined clinically. Cerebrospinal fluid (CSF) appears to be the fluid of choice for this purpose, as it continuously interacts with the spinal cord tissue and is therefore predestined to reflect metabolic changes. Research about proteomic profiling from CSF has shown that these markers are released too long after the onset of acute SCI and therefore cannot be used. Studies on serum markers have also found a slow and delayed release into the bloodstream [58].
- In a more recent work, changes in sensitive anaerobic metabolites with early release were studied for the first time. The authors used microdialysis of CSF during intraoperative procedures to detect severe disturbances in neurological energy metabolism in real time: lactate, lactate/pyruvate ratio, and glucose and glycerol levels. The authors reported a correlation between an increase in the lactate/pyruvate ratio, indicating the onset of anaerobic metabolism, and >50% change in motor evoked potential (MEP) in two patients. This could be a promising tool for the early detection of SCI in the future [57].
4.5. Pharmacologic Adjuncts
4.6. Basic Research on Early Detection of SCI
- Neuromonitoring
- Due to the risk of false-positive results in neuromonitoring, experimental research is being conducted to improve the technique. Transesophageal MEP has been reported to provide a shorter response time in animal models [60]. Research into the possibility of stimulating and recording intercostal nerve activity has also been published [61].
- Biomarkers
- The focus of current experiments on biomarkers is on structural proteins that are released into the cerebrospinal fluid and bloodstream as a result of damage to nerve tissue. However, despite the extensive literature on this topic, the diagnostic possibilities are currently limited. It has been proposed that in the future a multimodal approach be adopted to optimize spinal cord protection, combining near-infrared spectroscopy of the collateral network, neuromonitoring, and a combination of biomarkers [11].
5. Cerebrospinal Fluid Drainage (CSFD)
5.1. Rational
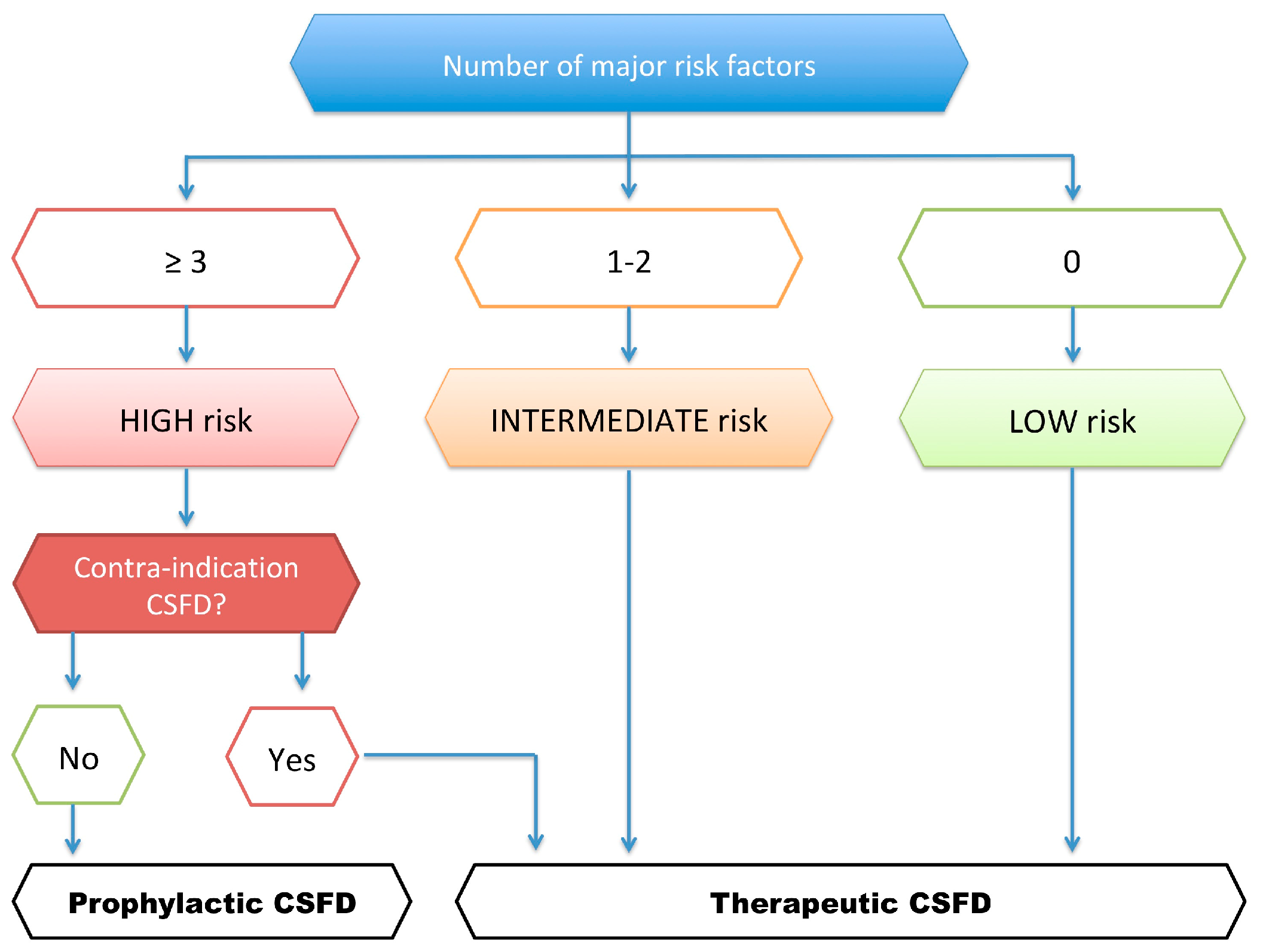
5.2. Risk of CSFD and Contraindications
5.3. Nantes University Protocol for CSFD Use
5.4. Our Initial Experience after the Implementation of This Protective Protocol
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. International Perspectives on Spinal Cord Injury. Available online: https://www.who.int/publications-detail-redirect/international-perspectives-on-spinal-cord-injury (accessed on 5 February 2023).
- Adams, H.D.; van Geertruyden, H.H. Neurologic Complications of Aortic Surgery. Ann. Surg. 1956, 144, 574. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, V.J.; Motyl, C.M.; Novak, Z.; Eagleton, M.J.; Farber, M.A.; Gasper, W.; Oderich, G.S.; Mendes, B.; Schanzer, A.; Tenorio, E.; et al. Predictors and Outcomes of Spinal Cord Injury Following Complex Branched/Fenestrated Endovascular Aortic Repair in the US Aortic Research Consortium. J. Vasc. Surg. 2023, 77, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Scali, S.T.; Giles, K.A.; Wang, G.J.; Kubilis, P.; Neal, D.; Huber, T.S.; Upchurch, G.R.; Siracuse, J.J.; Shutze, W.P.; Beck, A.W. National Incidence, Mortality Outcomes, and Predictors of Spinal Cord Ischemia after Thoracic Endovascular Aortic Repair. J. Vasc. Surg. 2020, 72, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Kitpanit, N.; Ellozy, S.H.; Connolly, P.H.; Agrusa, C.J.; Lichtman, A.D.; Schneider, D.B. Risk Factors for Spinal Cord Injury and Complications of Cerebrospinal Fluid Drainage in Patients Undergoing Fenestrated and Branched Endovascular Aneurysm Repair. J. Vasc. Surg. 2021, 73, 399–409.e1. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, V.J.; Eagleton, M.J.; Farber, M.A.; Oderich, G.S.; Schanzer, A.; Timaran, C.H.; Schneider, D.B.; Sweet, M.P.; Beck, A.W. Spinal Cord Protection Practices Used during Endovascular Repair of Complex Aortic Aneurysms by the U.S. Aortic Research Consortium. J. Vasc. Surg. 2021, 73, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Dias-Neto, M.; Tenorio, E.R.; Huang, Y.; Jakimowicz, T.; Mendes, B.C.; Kölbel, T.; Sobocinski, J.; Bertoglio, L.; Mees, B.; Gargiulo, M.; et al. Comparison of Single- and Multistage Strategies during Fenestrated-Branched Endovascular Aortic Repair of Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2023, 77, 1588–1597.e4. [Google Scholar] [CrossRef] [PubMed]
- Eagleton, M.J.; Shah, S.; Petkosevek, D.; Mastracci, T.M.; Greenberg, R.K. Hypogastric and Subclavian Artery Patency Affects Onset and Recovery of Spinal Cord Ischemia Associated with Aortic Endografting. J. Vasc. Surg. 2014, 59, 89–95. [Google Scholar] [CrossRef]
- Arora, L.; Hosn, M.A. Spinal Cord Perfusion Protection for Thoraco-Abdominal Aortic Aneurysm Surgery. Curr. Opin. Anesthesiol. 2019, 32, 72. [Google Scholar] [CrossRef]
- Awad, H.; Ramadan, M.E.; El Sayed, H.F.; Tolpin, D.A.; Tili, E.; Collard, C.D. Spinal Cord Injury after Thoracic Endovascular Aortic Aneurysm Repair. Can. J. Anesth. 2017, 64, 1218–1235. [Google Scholar] [CrossRef]
- Khachatryan, Z.; Haunschild, J.; von Aspern, K.; Borger, M.A.; Etz, C.D. Ischemic Spinal Cord Injury—Experimental Evidence and Evolution of Protective Measures. Ann. Thorac. Surg. 2022, 113, 1692–1702. [Google Scholar] [CrossRef]
- Melissano, G.; Bertoglio, L.; Civelli, V.; Moraes Amato, A.C.; Coppi, G.; Civilini, E.; Calori, G.; De Cobelli, F.; Del Maschio, A.; Chiesa, R. Demonstration of the Adamkiewicz Artery by Multidetector Computed Tomography Angiography Analysed with the Open-Source Software OsiriX. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Minatoya, K.; Matsuda, H.; Sasaki, H.; Iba, Y.; Oda, T.; Kobayashi, J. Embolism Is Emerging as a Major Cause of Spinal Cord Injury after Descending and Thoracoabdominal Aortic Repair with a Contemporary Approach: Magnetic Resonance Findings of Spinal Cord Injury. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.I.; Gariani, J.; Sztajzel, R.; Barnaure-Nachbar, I.; Delattre, B.M.; Lovblad, K.O.; Dietemann, J.-L. Spinal Cord Ischemia: Practical Imaging Tips, Pearls, and Pitfalls. Am. J. Neuroradiol. 2015, 36, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Kölbel, T.; Kubitz, J.C.; Wipper, S.; Konstantinou, N.; Heidemann, F.; Rohlffs, F.; Debus, S.E.; Tsilimparis, N. Risk of Spinal Cord Ischemia after Fenestrated or Branched Endovascular Repair of Complex Aortic Aneurysms. J. Vasc. Surg. 2019, 69, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rokosh, R.S.; Smith, D.E.; Maldonado, T.S.; Cayne, N.S.; Jacobowitz, G.R.; Rockman, C.B.; Patel, V.I.; Veith, F.J.; Galloway, A.C.; et al. Prior Infrarenal Aortic Surgery Is Not Associated with Increased Risk of Spinal Cord Ischemia after Thoracic Endovascular Aortic Repair and Complex Endovascular Aortic Repair. J. Vasc. Surg. 2022, 75, 1152–1162.e6. [Google Scholar] [CrossRef]
- Maeda, K.; Ohki, T.; Kanaoka, Y.; Shukuzawa, K.; Baba, T.; Momose, M. A Novel Shaggy Aorta Scoring System to Predict Embolic Complications Following Thoracic Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, E.; Melloni, A.; Gallitto, E.; Fargion, A.; Isernia, G.; Kahlberg, A.; Bertoglio, L.; Faggioli, G.; Lenti, M.; Pratesi, C.; et al. Spinal Cord Ischemia After Thoracoabdominal Aortic Aneurysms Endovascular Repair: From the Italian Multicenter Fenestrated/Branched Endovascular Aneurysm Repair Registry. J. Endovasc. Ther. 2023, 30, 281–288. [Google Scholar] [CrossRef]
- Piazza, M.; Zavatta, M.; Lamaina, M.; Taglialavoro, J.; Squizzato, F.; Grego, F.; Antonello, M. Early Outcomes of Routine Delayed Shunting in Carotid Endarterectomy for Asymptomatic Patients. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 334–341. [Google Scholar] [CrossRef]
- Coselli, J.S.; Green, S.Y.; Price, M.D.; Zhang, Q.; Preventza, O.; de la Cruz, K.I.; Whitlock, R.; Amarasekara, H.S.; Woodside, S.J.; Perez-Orozco, A.; et al. Spinal Cord Deficit after 1114 Extent II Open Thoracoabdominal Aortic Aneurysm Repairs. J. Thorac. Cardiovasc. Surg. 2020, 159, 1–13. [Google Scholar] [CrossRef]
- Feezor, R.J.; Martin, T.D.; Hess, P.J.; Daniels, M.J.; Beaver, T.M.; Klodell, C.T.; Lee, W.A. Extent of Aortic Coverage and Incidence of Spinal Cord Ischemia After Thoracic Endovascular Aneurysm Repair. Ann. Thorac. Surg. 2008, 86, 1809–1814. [Google Scholar] [CrossRef]
- Bisdas, T.; Panuccio, G.; Sugimoto, M.; Torsello, G.; Austermann, M. Risk Factors for Spinal Cord Ischemia after Endovascular Repair of Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2015, 61, 1408–1416. [Google Scholar] [CrossRef]
- Chang, C.K.; Chuter, T.A.M.; Reilly, L.M.; Ota, M.K.; Furtado, A.; Bucci, M.; Wintermark, M.; Hiramoto, J.S. Spinal Arterial Anatomy and Risk Factors for Lower Extremity Weakness Following Endovascular Thoracoabdominal Aortic Aneurysm Repair with Branched Stent-Grafts. J. Endovasc. Ther. 2008, 15, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Weigang, E.; Hartert, M.; Lonn, L.; Mestres, C.A.; Di Bartolomeo, R.; Bachet, J.E.; Carrel, T.P.; Grabenwöger, M.; Schepens, M.A.A.M.; et al. Contemporary Spinal Cord Protection during Thoracic and Thoracoabdominal Aortic Surgery and Endovascular Aortic Repair: A Position Paper of the Vascular Domain of the European Association for Cardio-Thoracic Surgery†. Eur. J. Cardiothorac. Surg. 2015, 47, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.Y.; Morcos, R.; Broce, M.; Bates, M.C.; AbuRahma, A.F. New Preoperative Spinal Cord Ischemia Risk Stratification Model for Patients Undergoing Thoracic Endovascular Aortic Repair. Vasc. Endovascular Surg. 2020, 54, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Khemlani, K.H.; Schurink, G.W.; Buhre, W.; Schreiber, J.U. Cerebrospinal Fluid Drainage in Thoracic and Thoracoabdominal Endovascular Aortic Repair: A Survey of Current Clinical Practice in European Medical Centers. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Naraba, H.; Hashimoto, H.; Mochizuki, M.; Takahashi, Y.; Sonoo, T.; Ogawa, Y.; Matsuishi, Y.; Shimojo, N.; Inoue, Y.; et al. Novel Protocol Combining Physical and Nutrition Therapies, Intensive Goal-Directed REhabilitation with Electrical Muscle Stimulation and Nutrition (IGREEN) Care Bundle. Crit. Care 2021, 25, 415. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Li, L.; Kleinman, K.; Szumita, P.M.; Massaro, A.F. Associations Between Ventilator Bundle Components and Outcomes. JAMA Intern. Med. 2016, 176, 1277. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Pandharipande, P.P. The Pain, Agitation, and Delirium Care Bundle: Synergistic Benefits of Implementing the 2013 Pain, Agitation, and Delirium Guidelines in an Integrated and Interdisciplinary Fashion. Crit. Care Med. 2013, 41, S99–S115. [Google Scholar] [CrossRef]
- Roquilly, A.; Cinotti, R.; Jaber, S.; Vourc’h, M.; Pengam, F.; Mahe, P.J.; Lakhal, K.; Demeure Dit Latte, D.; Rondeau, N.; Loutrel, O.; et al. Implementation of an Evidence-Based Extubation Readiness Bundle in 499 Brain-Injured Patients. a before-after Evaluation of a Quality Improvement Project. Am. J. Respir. Crit. Care Med. 2013, 188, 958–966. [Google Scholar] [CrossRef]
- Scali, S.T.; Kim, M.; Kubilis, P.; Feezor, R.J.; Giles, K.A.; Miller, B.; Fatima, J.; Huber, T.S.; Berceli, S.A.; Back, M.; et al. Implementation of a Bundled Protocol Significantly Reduces Risk of Spinal Cord Ischemia after Branched or Fenestrated Endovascular Aortic Repair. J. Vasc. Surg. 2018, 67, 409–423.e4. [Google Scholar] [CrossRef]
- Dijkstra, M.L.; Vainas, T.; Zeebregts, C.J.; Hooft, L.; van der Laan, M.J. Editor’s Choice—Spinal Cord Ischaemia in Endovascular Thoracic and Thoraco-Abdominal Aortic Repair: Review of Preventive Strategies. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.K.; Patel, V.I.; Wagener, G. Spinal Cord Protection for Thoracoabdominal Aortic Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Parotto, M.; Ouzounian, M.; Djaiani, G. Spinal Cord Protection in Elective Thoracoabdominal Aortic Procedures. J. Cardiothorac. Vasc. Anesth. 2019, 33, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Editor’s Choice—Current Options and Recommendations for the Treatment of Thoracic Aortic Pathologies Involving the Aortic Arch: An Expert Consensus Document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2019, 57, 165–198. [Google Scholar] [CrossRef]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the Diagnosis and Treatment of Aortic Diseases—Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the adult the Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Etz, C.D.; Kari, F.A.; Mueller, C.S.; Silovitz, D.; Brenner, R.M.; Lin, H.-M.; Griepp, R.B. The Collateral Network Concept: A Reassessment of the Anatomy of Spinal Cord Perfusion. J. Thorac. Cardiovasc. Surg. 2011, 141, 1020–1028. [Google Scholar] [CrossRef]
- Etz, C.D.; Kari, F.A.; Mueller, C.S.; Brenner, R.M.; Lin, H.-M.; Griepp, R.B. The Collateral Network Concept: Remodeling of the Arterial Collateral Network after Experimental Segmental Artery Sacrifice. J. Thorac. Cardiovasc. Surg. 2011, 141, 1029–1036. [Google Scholar] [CrossRef]
- Bischoff, M.S.; Scheumann, J.; Brenner, R.M.; Ladage, D.; Bodian, C.A.; Kleinman, G.; Ellozy, S.H.; Luozzo, G.D.; Etz, C.D.; Griepp, R.B. Staged Approach Prevents Spinal Cord Injury in Hybrid Surgical-Endovascular Thoracoabdominal Aortic Aneurysm Repair: An Experimental Model. Ann. Thorac. Surg. 2011, 92, 138–146. [Google Scholar] [CrossRef]
- Zoli, S.; Etz, C.D.; Roder, F.; Brenner, R.M.; Bodian, C.A.; Kleinman, G.; Luozzo, G.D.; Griepp, R.B. Experimental Two-Stage Simulated Repair of Extensive Thoracoabdominal Aneurysms Reduces Paraplegia Risk. Ann. Thorac. Surg. 2010, 90, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Zoli, S.; Mueller, C.S.; Bodian, C.A.; Luozzo, G.D.; Lazala, R.; Plestis, K.A.; Griepp, R.B. Staged Repair Significantly Reduces Paraplegia Rate after Extensive Thoracoabdominal Aortic Aneurysm Repair. J. Thorac. Cardiovasc. Surg. 2010, 139, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Homann, T.M.; Plestis, K.A.; Zhang, N.; Luehr, M.; Weisz, D.J.; Kleinman, G.; Griepp, R.B. Spinal Cord Perfusion after Extensive Segmental Artery Sacrifice: Can Paraplegia Be Prevented? Eur. J. Cardiothorac. Surg. 2007, 31, 643–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tenorio, E.R.; Eagleton, M.J.; Kärkkäinen, J.M.; Oderich, G.S. Prevention of Spinal Cord Injury during Endovascular Thoracoabdominal Repair. J. Cardiovasc. Surg. 2019, 60, 1629. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Debus, E.S.; Mohr, F.-W.; Kölbel, T. First-in-Man Endovascular Preconditioning of the Paraspinal Collateral Network by Segmental Artery Coil Embolization to Prevent Ischemic Spinal Cord Injury. J. Thorac. Cardiovasc. Surg. 2015, 149, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Petroff, D.; Czerny, M.; Kölbel, T.; Melissano, G.; Lonn, L.; Haunschild, J.; Aspern, K.; Neuhaus, P.; Pelz, J.; Epstein, D.M.; et al. Paraplegia Prevention in Aortic Aneurysm Repair by Thoracoabdominal Staging with ‘Minimally Invasive Staged Segmental Artery Coil Embolisation’ (MIS2ACE): Trial Protocol for a Randomised Controlled Multicentre Trial. BMJ Open 2019, 9, e025488. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.; Elenbaas, T.W.; Schurink, G.W.H.; Mess, W.H.; Mochtar, B. Assessment of Spinal Cord Integrity during Thoracoabdominal Aortic Aneurysm Repair. Ann. Thorac. Surg. 2002, 74, S1864–S1866. [Google Scholar] [CrossRef]
- Czerny, M.; Eggebrecht, H.; Sodeck, G.; Verzini, F.; Cao, P.; Maritati, G.; Riambau, V.; Beyersdorf, F.; Rylski, B.; Funovics, M.; et al. Mechanisms of Symptomatic Spinal Cord Ischemia After TEVAR: Insights from the European Registry of Endovascular Aortic Repair Complications (EuREC). J. Endovasc. Ther. 2012, 19, 37–43. [Google Scholar] [CrossRef]
- Rizvi, A.Z.; Murad, M.H.; Fairman, R.M.; Erwin, P.J.; Montori, V.M. The Effect of Left Subclavian Artery Coverage on Morbidity and Mortality in Patients Undergoing Endovascular Thoracic Aortic Interventions: A Systematic Review and Meta-Analysis. J. Vasc. Surg. 2009, 50, 1159–1169. [Google Scholar] [CrossRef]
- Kise, Y.; Kuniyoshi, Y.; Inafuku, H.; Nagano, T.; Hirayasu, T.; Yamashiro, S. Directly Measuring Spinal Cord Blood Flow and Spinal Cord Perfusion Pressure via the Collateral Network: Correlations with Changes in Systemic Blood Pressure. J. Thorac. Cardiovasc. Surg. 2015, 149, 360–366. [Google Scholar] [CrossRef]
- Estrera, A.L.; Miller, C.C.; Huynh, T.T.T.; Porat, E.; Safi, H.J. Neurologic Outcome after Thoracic and Thoracoabdominal Aortic Aneurysm Repair. Ann. Thorac. Surg. 2001, 72, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Luehr, M.; Kari, F.A.; Bodian, C.A.; Smego, D.; Plestis, K.A.; Griepp, R.B. Paraplegia after Extensive Thoracic and Thoracoabdominal Aortic Aneurysm Repair: Does Critical Spinal Cord Ischemia Occur Postoperatively? J. Thorac. Cardiovasc. Surg. 2008, 135, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef]
- Jones, P.A.; Andrews, P.J.; Midgley, S.; Anderson, S.I.; Piper, I.R.; Tocher, J.L.; Housley, A.M.; Corrie, J.A.; Slattery, J.; Dearden, N.M. Measuring the Burden of Secondary Insults in Head-Injured Patients during Intensive Care. J. Neurosurg. Anesthesiol. 1994, 6, 4–14. [Google Scholar] [CrossRef]
- Mueller, M.M.; Remoortel, H.V.; Meybohm, P.; Aranko, K.; Aubron, C.; Burger, R.; Carson, J.L.; Cichutek, K.; Buck, E.D.; Devine, D.; et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA J. Am. Med. Assoc. 2019, 321, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Simoniuk, U.D.; Haunschild, J.; von Aspern, K.; Boschmann, M.; Klug, L.; Khachatryan, Z.; Bianchi, E.; Ossmann, S.; Oo, A.Y.; Borger, M.A.; et al. Near Real-Time Bedside Detection of Spinal Cord Ischaemia during Aortic Repair by Microdialysis of the Cerebrospinal Fluid. Eur. J. Cardiothorac. Surg. 2020, 58, 629–637. [Google Scholar] [CrossRef]
- Püschel, A.; Ebel, R.; Fuchs, P.; Hofmann, J.; Schubert, J.K.; Roesner, J.P.; Bergt, S.; Wree, A.; Vollmar, B.; Klar, E.; et al. Can Recognition of Spinal Ischemia Be Improved? Application of Motor-Evoked Potentials, Serum Markers, and Breath Gas Analysis in an Acutely Instrumented Pig Model. Ann. Vasc. Surg. 2018, 49, 191–205. [Google Scholar] [CrossRef]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef]
- Yamanaka, K.; Tsuda, K.; Takahashi, D.; Washiyama, N.; Yamashita, K.; Shiiya, N. Bipolar Transesophageal Thoracic Spinal Cord Stimulation: A Novel Clinically Relevant Method for Motor-Evoked Potentials. JTCVS Tech. 2020, 4, 28–35. [Google Scholar] [CrossRef]
- Takahashi, S.; Orihashi, K.; Imai, K.; Mizukami, T.; Takasaki, T.; Sueda, T. Transintercostal-Evoked Spinal Cord Potential in Thoracic Aortic Surgery. Ann. Vasc. Surg. 2014, 28, 1775–1781. [Google Scholar] [CrossRef]
- Alqaim, M.; Cosar, E.; Crawford, A.S.; Robichaud, D.I.; Walz, J.M.; Schanzer, A.; Simons, J.P. Lumbar Drain Complications in Patients Undergoing Fenestrated or Branched Endovascular Aortic Aneurysm Repair: Development of an Institutional Protocol for Lumbar Drain Management. J. Vasc. Surg. 2020, 72, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Bertoglio, L.; Bignamini, A.A.; Mani, K.; Kölbel, T.; Oderich, G.; Chiesa, R.; Lepidi, S.; Abisi, S.; Adam, D.; et al. Editor’s Choice—PRINciples of Optimal antithrombotiC Therapy and Coagulation managEment during Elective Fenestrated and Branched EndovaScular Aortic repairS (PRINCE2SS): An International Expert Based Delphi Consensus Study. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, J.M.; Cirillo-Penn, N.C.; Sen, I.; Tenorio, E.R.; Mauermann, W.J.; Gilkey, G.D.; Kaufmann, T.J.; Oderich, G.S. Cerebrospinal Fluid Drainage Complications during First Stage and Completion Fenestrated-Branched Endovascular Aortic Repair. J. Vasc. Surg. 2020, 71, 1109–1118.e2. [Google Scholar] [CrossRef] [PubMed]
- Borghese, O.; Brisard, L.; Le Corvec, T.; Hauguel, A.; Guimbretière, G.; Maurel, B. Spinal Cord Protection during Thoracic and Thoracoabdominal Endovascular Aortic Repair: 5-Year Results of a Preventive Protocol Including Prophylactic Cerebrospinal Fluid Drainage in High-Risk Patients. J. Endovasc. Ther. 2024, in press. [Google Scholar]


| Age (by decade) | 0.5 |
| Celiac coverage | 1 |
| Current smoker | 1 |
| Dialysis | 1.5 |
| 3 or more aortic devices | 1 |
| Emergent or urgent surgery | 1 |
| Adjunct procedures aorta-related | 1.5 |
| Adjunct procedures not aorta-related | 1.5 |
| Total device length 19–31 cm | 1.5 |
| Total device length ≥ 32 cm | 3 |
| ASA class 4 or class 5 | 1 |
| Total procedure time > 154 min | 1 |
| High-volume center (tertiary referral center performing ≥ 50 procedures annually [26]) | −1 |
| eGFR > 60% | −1 |
| Estimated risk for spinal cord injury | Total number of points |
| Low risk | 0–4 |
| Medium risk | 4.5–6.5 |
| High risk | ≥7 |
| Intervention | Rational | Potential Risks | Evidence | Class of Recommendations |
|---|---|---|---|---|
| Segmental artery occlusion for staged procedures | Triggers arterial collateralization and stabilizes blood supply to the spinal cord from alternate inflow sources | Extended duration of treatment, risk of aneurysm rupture, spinal cord ischemia | C | EACTS 2015: II-B |
| LSA revascularization | Preserves perfusion of vertebral artery | Laryngeal nerve injury, vocal cord paralysis, thoracic duct injury | Preventive | |
| B–NR B C C | ACC-AHA 2022: I EACTS-ESVS 2019: II-A ESVS 2017: II-A EACTS 2015: II-A | |||
| Curative | ||||
| C–LD | ACC-AHA 2022: II-B | |||
| Permissive hypertension | Increases MAP to preserve blood flow in spinal cord collateral network | Cardiac dysfunction, hemorrhage | C | EACTS 2015: II-A |
| High hemoglobin threshold | Increases oxygen delivery to spinal cord | Transfusion-associated complications, immunization, cost | C | EACTS 2015: II-A |
| Perioperative neuromonitoring | Detects early impaired spinal cord function | Cost of medical devices, expertise required, false-positive rate | C C | ESVS 2017: II-B EACTS 2015: II-B |
| Pharmacological adjuncts | Reduce spinal cord edema or excitatory amino acids | Hyperglycemia, infection and gastrointestinal bleeding (steroids), postoperative pain (naloxone), hypovolemia (mannitol) | C | EACTS 2015: II-B |
| Selective CSFD drainage | Reduces CSFP and optimizes SCPP | Intracranial and neuraxial bleeding, infection, mechanical complication | Early | |
| A C C C | ACC-AHA 2022: I ESVS 2017: II-A EACTS 2015: II-A ESC 2014: II-A | |||
| Delayed | ||||
| C C | EACTS-ESVS 2019: I EACTS 2015: II-A | |||
| Extra-Neurologic (Systemic) | Neurologic |
|---|---|
| Hypoxemia | Spinal cord compression (hematoma, tumor) |
| Low blood pressure | Vasospasm |
| Hypercapnia | Seizure |
| Anemia | Infection |
| Hyperthermia | |
| Hyperglycemia | |
| Hypocapnia | |
| Hyponatremia/Hypernatremia |
| Total aortic coverage > 200 mm |
| Coverage of the area Th9–Th12 |
| Supra-celiac coverage > 40 mm |
| LSA or hypogastric coverage without immediate revascularization strategy |
| Prior aortic repair (abdominal and/or thoracic descending—endovascular and/or open surgery) |
| Prior spinal cord injury episode during TEVAR procedure or recent AAS (<15 days) |
| Procedure in the first 15 days following AAS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brisard, L.; El Batti, S.; Borghese, O.; Maurel, B. Risk Factors for Spinal Cord Injury during Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score. J. Clin. Med. 2023, 12, 7520. https://doi.org/10.3390/jcm12247520
Brisard L, El Batti S, Borghese O, Maurel B. Risk Factors for Spinal Cord Injury during Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score. Journal of Clinical Medicine. 2023; 12(24):7520. https://doi.org/10.3390/jcm12247520
Chicago/Turabian StyleBrisard, Laurent, Salma El Batti, Ottavia Borghese, and Blandine Maurel. 2023. "Risk Factors for Spinal Cord Injury during Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score" Journal of Clinical Medicine 12, no. 24: 7520. https://doi.org/10.3390/jcm12247520
APA StyleBrisard, L., El Batti, S., Borghese, O., & Maurel, B. (2023). Risk Factors for Spinal Cord Injury during Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score. Journal of Clinical Medicine, 12(24), 7520. https://doi.org/10.3390/jcm12247520






