Objectivizing Measures of Post-Stroke Hand Rehabilitation through Multi-Disciplinary Scales
Abstract
1. Introduction
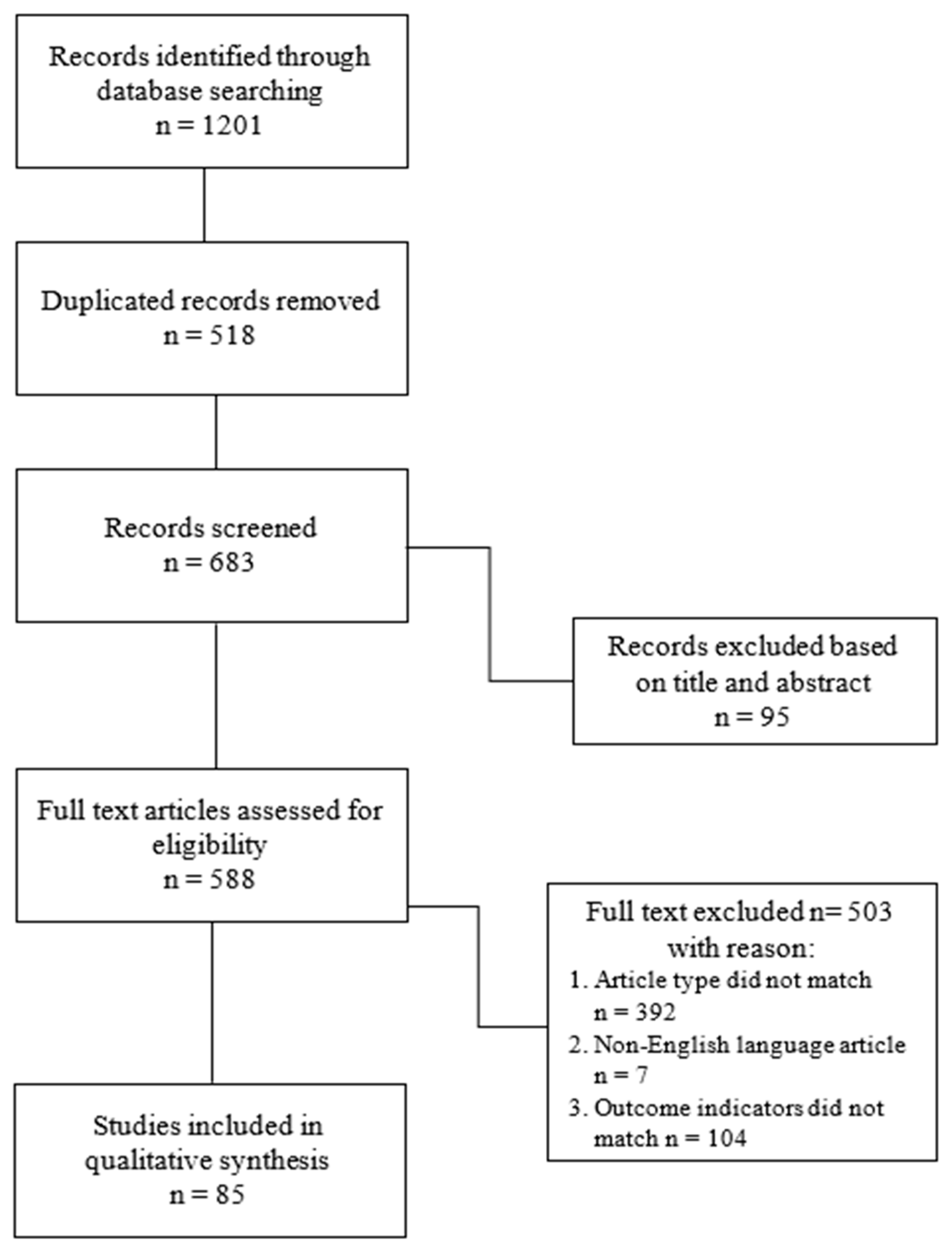
2. Materials and Methods
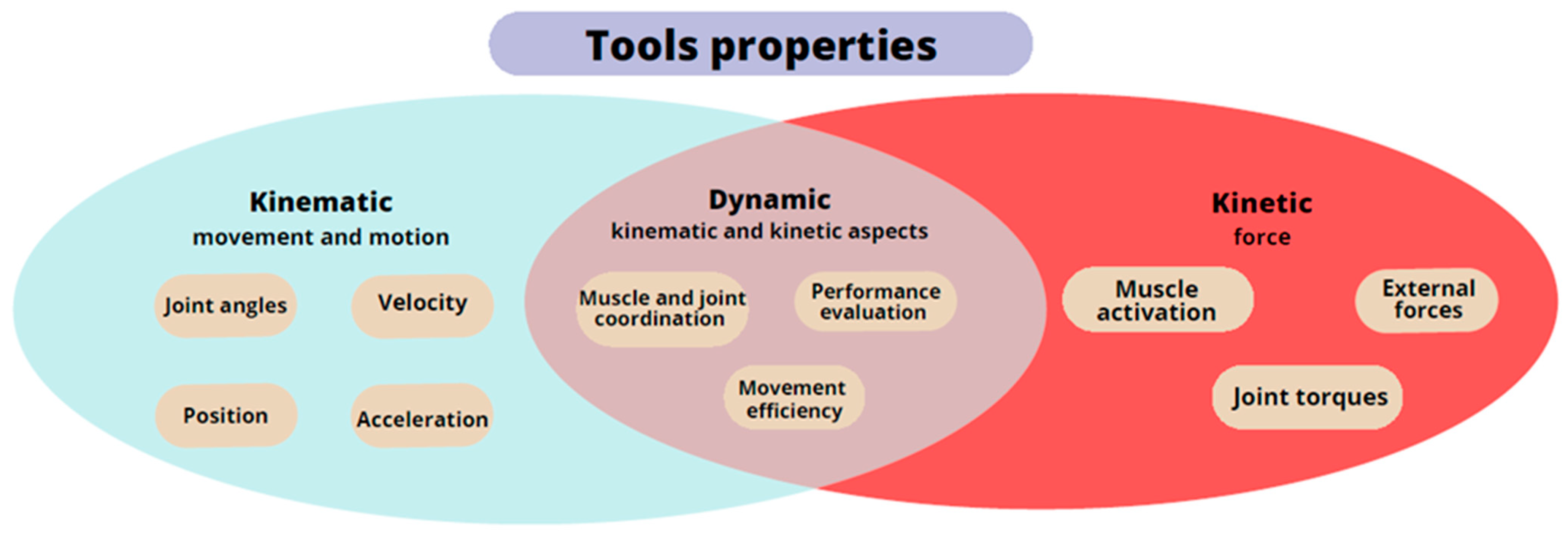
3. Diagnosis, Hand Measurements, and Instruments Support: Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alt Murphy, M.; Resteghini, C.; Feys, P.; Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015, 15, 29. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil. Neural Repair. 2017, 31, 793–799. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 142. [Google Scholar] [CrossRef]
- Rabelo, M.; Nunes, G.S.; da Costa Amante, N.M.; de Noronha, M.; Fachin-Martins, E. Reliability of muscle strength assessment in chronic post-stroke hemiparesis: A systematic review and meta-analysis. Top. Stroke Rehabil. 2016, 23, 26–36. [Google Scholar] [CrossRef]
- Lim, H.; Madhavan, S. Effects of Cross-Education on Neural Adaptations Following Non-Paretic Limb Training in Stroke: A Scoping Review with Implications for Neurorehabilitation. J. Mot. Behav. 2023, 55, 111–124. [Google Scholar] [CrossRef]
- Owen, M.; Ingo, C.; Dewald, J.P.A. Upper Extremity Motor Impairments and Microstructural Changes in Bulbospinal Pathways in Chronic Hemiparetic Stroke. Front. Neurol. 2017, 8, 257. [Google Scholar] [CrossRef]
- Hu, X.; Suresh, A.K.; Rymer, W.Z.; Suresh, N.L. Altered motor unit discharge patterns in paretic muscles of stroke survivors assessed using surface electromyography. J. Neural Eng. 2016, 13, 046025. [Google Scholar] [CrossRef]
- Huang, P.C.; Hsieh, Y.W.; Wang, C.M.; Wu, C.Y.; Huang, S.C.; Lin, K.C. Predictors of motor, daily function, and quality-of-life improvements after upper-extremity robot-assisted rehabilitation in stroke. Am. J. Occup. Ther. 2014, 68, 325–333. [Google Scholar] [CrossRef]
- Sunderland, A. Recovery of ipsilateral dexterity after stroke. Stroke 2000, 31, 430–433. [Google Scholar] [CrossRef]
- Ekstrand, E.; Rylander, L.; Lexell, J.; Brogardh, C. Perceived ability to perform daily hand activities after stroke and associated factors: A cross-sectional study. BMC Neurol. 2016, 16, 208. [Google Scholar] [CrossRef]
- Bertrand, A.M.; Fournier, K.; Wick Brasey, M.G.; Kaiser, M.L.; Frischknecht, R.; Diserens, K. Reliability of maximal grip strength measurements and grip strength recovery following a stroke. J. Hand Ther. 2015, 28, 356–362; quiz 363. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Parikh, R.J.; Sutaria, J.M.; Ahsan, M.; Nuhmani, S.; Alghadir, A.H.; Khan, M. Effects of myofascial release with tennis ball on spasticity and motor functions of upper limb in patients with chronic stroke: A randomized controlled trial. Medicine 2022, 101, e29926. [Google Scholar] [CrossRef]
- Plantin, J.; Pennati, G.V.; Roca, P.; Baron, J.C.; Laurencikas, E.; Weber, K.; Godbolt, A.K.; Borg, J.; Lindberg, P.G. Quantitative Assessment of Hand Spasticity After Stroke: Imaging Correlates and Impact on Motor Recovery. Front. Neurol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Dewald, J.P.; Beer, R.F. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 2001, 24, 273–283. [Google Scholar] [CrossRef]
- Reisman, D.S.; Scholz, J.P. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain 2003, 126, 2510–2527. [Google Scholar] [CrossRef]
- Reissner, L.; Fischer, G.; List, R.; Taylor, W.R.; Giovanoli, P.; Calcagni, M. Minimal detectable difference of the finger and wrist range of motion: Comparison of goniometry and 3D motion analysis. J. Orthop. Surg. Res. 2019, 14, 173. [Google Scholar] [CrossRef]
- Du, W.Y.; Huang, T.S.; Hsu, K.C.; Lin, J.J. Measurement of scapular medial border and inferior angle prominence using a novel scapulometer: A reliability and validity study. Musculoskelet. Sci. Pract. 2017, 32, 120–126. [Google Scholar] [CrossRef]
- Mullins, J.; Mawson, C.; Nahavandi, S. Haptic handwriting aid for training and rehabilitation. In Proceedings of the 2005 IEEE International Conference on Systems, Man and Cybernetics, Waikoloa, HI, USA, 12 October 2005; pp. 2690–2694. [Google Scholar]
- Koter, K.; Samowicz, M.; Redlicka, J.; Zubrycki, I. Hand Measurement System Based on Haptic and Vision Devices towards Post-Stroke Patients. Sensors 2022, 22, 2060. [Google Scholar] [CrossRef]
- Tran, T.Q.B.; du Toit, C.; Padmanabhan, S. Artificial intelligence in healthcare-the road to precision medicine. J. Hosp. Manag. Health Policy 2021, 5, 29. [Google Scholar] [CrossRef]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef]
- Imura, T.; Nagasawa, Y.; Fukuyama, H.; Imada, N.; Oki, S.; Araki, O. Effect of early and intensive rehabilitation in acute stroke patients: Retrospective pre-/post-comparison in Japanese hospital. Disabil. Rehabil. 2018, 40, 1452–1455. [Google Scholar] [CrossRef]
- Bushnell, C.; Bettger, J.P.; Cockroft, K.M.; Cramer, S.C.; Edelen, M.O.; Hanley, D.; Katzan, I.L.; Mattke, S.; Nilsen, D.M.; Piquado, T.; et al. Chronic Stroke Outcome Measures for Motor Function Intervention Trials: Expert Panel Recommendations. Circ. Cardiovasc. Qual. Outcomes 2015, 8 (Suppl. S3), S163–S169. [Google Scholar] [CrossRef]
- Alt Murphy, M.; Bjorkdahl, A.; Forsberg-Warleby, G.; Persson, C.U. Implementation of evidence-based assessment of upper extremity in stroke rehabilitation: From evidence to clinical practice. J. Rehabil. Med. 2021, 53, jrm00148. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.X.; Zhong, M.H.; Zhou, R.Z.; Liu, X.Q.; Tang, N.S.; Feng, X.C.; Gao, C.F. Effects of corticospinal tract integrity on upper limb motor function recovery in stroke patients treated with repetitive transcranial magnetic stimulation. J. Integr. Neurosci. 2022, 21, 50. [Google Scholar] [CrossRef]
- Xu, Q.; Li, C.; Pan, Y.; Li, W.; Jia, T.; Li, Z.; Ma, D.; Pang, X.; Ji, L. Impact of smart force feedback rehabilitation robot training on upper limb motor function in the subacute stage of stroke. NeuroRehabilitation 2020, 47, 209–215. [Google Scholar] [CrossRef]
- Huang, W.H.; Dou, Z.L.; Jin, H.M.; Cui, Y.; Li, X.; Zeng, Q. The Effectiveness of Music Therapy on Hand Function in Patients With Stroke: A Systematic Review of Randomized Controlled Trials. Front. Neurol. 2021, 12, 641023. [Google Scholar] [CrossRef]
- Krakauer, J.W. Arm function after stroke: From physiology to recovery. Semin. Neurol. 2005, 25, 384–395. [Google Scholar] [CrossRef]
- Chien, W.T.; Chong, Y.Y.; Tse, M.K.; Chien, C.W.; Cheng, H.Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wu, C.Y.; Wang, W.E.; Lin, K.C.; Chang, K.C.; Chen, C.C.; Liu, C.T. Bilateral robotic priming before task-oriented approach in subacute stroke rehabilitation: A pilot randomized controlled trial. Clin. Rehabil. 2017, 31, 225–233. [Google Scholar] [CrossRef]
- Yarossi, M.; Patel, J.; Qiu, Q.; Massood, S.; Fluet, G.; Merians, A.; Adamovich, S.; Tunik, E. The Association Between Reorganization of Bilateral M1 Topography and Function in Response to Early Intensive Hand Focused Upper Limb Rehabilitation Following Stroke Is Dependent on Ipsilesional Corticospinal Tract Integrity. Front. Neurol. 2019, 10, 258. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Zarahn, E.; Riley, C.; Speizer, A.; Chong, J.Y.; Lazar, R.M.; Marshall, R.S.; Krakauer, J.W. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil. Neural Repair. 2008, 22, 64–71. [Google Scholar] [CrossRef]
- Teremetz, M.; Colle, F.; Hamdoun, S.; Maier, M.A.; Lindberg, P.G. A novel method for the quantification of key components of manual dexterity after stroke. J. Neuroeng. Rehabil. 2015, 12, 64. [Google Scholar] [CrossRef]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J. Predicting daily use of the affected upper extremity 1 year after stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 274–283. [Google Scholar] [CrossRef]
- Hlustik, P.; Solodkin, A.; Gullapalli, R.P.; Noll, D.C.; Small, S.L. Somatotopy in human primary motor and somatosensory hand representations revisited. Cereb. Cortex 2001, 11, 312–321. [Google Scholar] [CrossRef]
- Hamzei, F.; Dettmers, C.; Rijntjes, M.; Weiller, C. The effect of cortico-spinal tract damage on primary sensorimotor cortex activation after rehabilitation therapy. Exp. Brain Res. 2008, 190, 329–336. [Google Scholar] [CrossRef]
- Wang, H.; Arceo, R.; Chen, S.; Ding, L.; Jia, J.; Yao, J. Effectiveness of interventions to improve hand motor function in individuals with moderate to severe stroke: A systematic review protocol. BMJ Open 2019, 9, e032413. [Google Scholar] [CrossRef]
- Gowda, A.S.; Memon, A.N.; Bidika, E.; Salib, M.; Rallabhandi, B.; Fayyaz, H. Investigating the viability of motor imagery as a physical rehabilitation treatment for patients with stroke-induced motor cortical damage. Cureus 2021, 13, e14001. [Google Scholar] [CrossRef]
- Wilkins, K.B.; Owen, M.; Ingo, C.; Carmona, C.; Dewald, J.P.A.; Yao, J. Neural Plasticity in Moderate to Severe Chronic Stroke Following a Device-Assisted Task-Specific Arm/Hand Intervention. Front. Neurol. 2017, 8, 284. [Google Scholar] [CrossRef]
- Lan, Y.; Yao, J.; Dewald, J.P.A. The Impact of Shoulder Abduction Loading on Volitional Hand Opening and Grasping in Chronic Hemiparetic Stroke. Neurorehabil. Neural Repair. 2017, 31, 521–529. [Google Scholar] [CrossRef]
- Smania, N.; Paolucci, S.; Tinazzi, M.; Borghero, A.; Manganotti, P.; Fiaschi, A.; Moretto, G.; Bovi, P.; Gambarin, M. Active finger extension: A simple movement predicting recovery of arm function in patients with acute stroke. Stroke 2007, 38, 1088–1090. [Google Scholar] [CrossRef]
- Nijland, R.H.; van Wegen, E.E.; Harmeling-van der Wel, B.C.; Kwakkel, G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: Early prediction of functional outcome after stroke: The EPOS cohort study. Stroke 2010, 41, 745–750. [Google Scholar] [CrossRef]
- Boake, C.; Noser, E.A.; Ro, T.; Baraniuk, S.; Gaber, M.; Johnson, R.; Salmeron, E.T.; Tran, T.M.; Lai, J.M.; Taub, E.; et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil. Neural Repair. 2007, 21, 14–24. [Google Scholar] [CrossRef]
- Lang, C.E.; DeJong, S.L.; Beebe, J.A. Recovery of thumb and finger extension and its relation to grasp performance after stroke. J. Neurophysiol. 2009, 102, 451–459. [Google Scholar] [CrossRef]
- Stinear, C.M.; Barber, P.A.; Petoe, M.; Anwar, S.; Byblow, W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012, 135 Pt 8, 2527–2535. [Google Scholar] [CrossRef]
- Carpinella, I.; Jonsdottir, J.; Ferrarin, M. Multi-finger coordination in healthy subjects and stroke patients: A mathematical modelling approach. J. Neuroeng. Rehabil. 2011, 8, 19. [Google Scholar] [CrossRef]
- Wenzelburger, R.; Kopper, F.; Frenzel, A.; Stolze, H.; Klebe, S.; Brossmann, A.; Kuhtz-Buschbeck, J.; Golge, M.; Illert, M.; Deuschl, G. Hand coordination following capsular stroke. Brain 2005, 128 Pt 1, 64–74. [Google Scholar] [CrossRef][Green Version]
- Ewoldt, J.K.; Raghavan, P.; Suresh, N.L. Quantitative Measurement of Resistance to Passive Joint Motion in Chronic Stroke Survivors. In Spasticity and Muscle Stiffness: Restoring Form and Function; Springer: Berlin/Heidelberg, Germany, 2022; pp. 47–62. [Google Scholar]
- Hu, X.L.; Tong, K.Y.; Li, L. The mechanomyography of persons after stroke during isometric voluntary contractions. J. Electromyogr. Kinesiol. 2007, 17, 473–483. [Google Scholar] [CrossRef]
- O’Dwyer, N.J.; Ada, L.; Neilson, P.D. Spasticity and muscle contracture following stroke. Brain 1996, 119 Pt 5, 1737–1749. [Google Scholar] [CrossRef]
- Kamper, D.G.; Harvey, R.L.; Suresh, S.; Rymer, W.Z. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve 2003, 28, 309–318. [Google Scholar] [CrossRef]
- Kamper, D.G.; Fischer, H.C.; Cruz, E.G.; Rymer, W.Z. Weakness is the primary contributor to finger impairment in chronic stroke. Arch. Phys. Med. Rehabil. 2006, 87, 1262–1269. [Google Scholar] [CrossRef]
- Singh, N.; Saini, M.; Kumar, N.; Srivastava, M.V.P.; Mehndiratta, A. Evidence of neuroplasticity with robotic hand exoskeleton for post-stroke rehabilitation: A randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 76. [Google Scholar] [CrossRef]
- Gündüz, O.H.; Toprak, C.Ş. Hand function in stroke. In Hand Function: A Practical Guide to Assessment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 125–135. [Google Scholar]
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef]
- Alfano, C.M.; Zucker, D.S.; Pergolotti, M.; Ness, K.K.; Jones, L.W.; Price, N.D.; Schmitz, K.H.; Ligibel, J.A. A precision medicine approach to improve cancer rehabilitation’s impact and integration with cancer care and optimize patient wellness. Curr. Phys. Med. Rehabil. Rep. 2017, 5, 64–73. [Google Scholar] [CrossRef]
- Oh, B.M. A Path to Precision Medicine: Incorporating Blood-Based Biomarkers in Stroke Rehabilitation. Ann. Rehabil. Med. 2021, 45, 341–344. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, C.; Gong, L.; Zhang, Y. Health related quality of life in stroke patients and risk factors associated with patients for return to work. Medicine 2019, 98, e15130. [Google Scholar] [CrossRef]
- Fatema, Z.; Sigamani, A.; Vikneswaran, G.; Manuel, D. ‘Quality of life at 90 days after stroke and its correlation to activities of daily living’: A prospective cohort study. J. Stroke Cerebrovasc. Dis. 2022, 31, 106806. [Google Scholar] [CrossRef]
- Baker, K.; Cano, S.J.; Playford, E.D. Outcome measurement in stroke: A scale selection strategy. Stroke 2011, 42, 1787–1794. [Google Scholar] [CrossRef]
- Cieza, A.; Fayed, N.; Bickenbach, J.; Prodinger, B. Refinements of the ICF Linking Rules to strengthen their potential for establishing comparability of health information. Disabil. Rehabil. 2019, 41, 574–583. [Google Scholar] [CrossRef]
- Stucki, G.; Ewert, T.; Cieza, A. Value and application of the ICF in rehabilitation medicine. Disabil. Rehabil. 2002, 24, 932–938. [Google Scholar] [CrossRef]
- Lemmens, R.J.; Timmermans, A.A.; Janssen-Potten, Y.J.; Smeets, R.J.; Seelen, H.A. Valid and reliable instruments for arm-hand assessment at ICF activity level in persons with hemiplegia: A systematic review. BMC Neurol. 2012, 12, 21. [Google Scholar] [CrossRef]
- Cantero-Tellez, R.; Naughton, N.; Algar, L.; Valdes, K. Outcome measurement of hand function following mirror therapy for stroke rehabilitation: A systematic review. J. Hand Ther. 2019, 32, 277–291.e1. [Google Scholar] [CrossRef]
- Van Gils, A.; Meyer, S.; Van Dijk, M.; Thijs, L.; Michielsen, M.; Lafosse, C.; Truyens, V.; Oostra, K.; Peeters, A.; Thijs, V.; et al. The Adult Assisting Hand Assessment Stroke: Psychometric Properties of an Observation-Based Bimanual Upper Limb Performance Measurement. Arch. Phys. Med. Rehabil. 2018, 99, 2513–2522. [Google Scholar] [CrossRef]
- Santisteban, L.; Teremetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Poole, J.L.; Whitney, S.L. Assessments of motor function post stroke: A review. Phys. Occup. Ther. Geriatr. 2001, 19, 1–22. [Google Scholar] [CrossRef]
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201–211. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair. 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Hope, T.M.; Friston, K.; Price, C.J.; Leff, A.P.; Rotshtein, P.; Bowman, H. Recovery after Stroke: Not So Proportional after All? Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Quinn, T.; Dawson, J.; Walters, M.; Lees, K. Functional outcome measures in contemporary stroke trials. Int. J. Stroke 2009, 4, 200–205. [Google Scholar] [CrossRef]
- Burke, J.W.; McNeill, M.; Charles, D.; Morrow, P.; Crosbie, J.; McDonough, S. Serious games for upper limb rehabilitation following stroke. In Proceedings of the 2009 Conference in Games and Virtual Worlds for Serious Applications, Coventry, UK, 23–24 March 2009; pp. 103–110. [Google Scholar]
- Meng, L.; Jiang, X.; Qin, H.; Fan, J.; Zeng, Z.; Chen, C.; Zhang, A.; Dai, C.; Wu, X.; Akay, Y.M.; et al. Automatic Upper-Limb Brunnstrom Recovery Stage Evaluation via Daily Activity Monitoring. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2589–2599. [Google Scholar] [CrossRef]
- Nam, K.E.; Lim, S.H.; Kim, J.S.; Hong, B.Y.; Jung, H.Y.; Lee, J.K.; Yoo, S.D.; Pyun, S.-B.; Lee, K.M.; Lee, K.J. When does spasticity in the upper limb develop after a first stroke? A nationwide observational study on 861 stroke patients. J. Clin. Neurosci. 2019, 66, 144–148. [Google Scholar] [CrossRef]
- Daly, J.J.; Ruff, R.L.; Osman, S.; Hull, J.J. Response of prolonged flaccid paralysis to FNS rehabilitation techniques. Disabil. Rehabil. 2000, 22, 565–573. [Google Scholar] [CrossRef]
- Lin, Y.F.; Liu, X.H.; Cui, Z.Y.; Song, Z.T.; Zou, F.; Chen, S.G.; Kang, X.Y.; Ye, B.; Wang, Q.; Tian, J.; et al. Weakened Effective Connectivity Related to Electroacupuncture in Stroke Patients with Prolonged Flaccid Paralysis: An EEG Pilot Study. Neural Plast. 2021, 2021, 6641506. [Google Scholar] [CrossRef]
- Correa-Forero, V.; Pinilla-Monsalve, G.D.; Valderrama-Chaparro, J.A.; Amaya-Gonzalez, P. Cryptococcal meningitis presenting as acute flaccid paralysis: A case report. J. Infect. Public Health 2020, 13, 143–148. [Google Scholar] [CrossRef]
- Ramroop, H.; Cruz, R. Electrodiagnostic Evaluation of Motor Neuron Disease; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ivanhoe, C.B.; Reistetter, T.A. Spasticity: The misunderstood part of the upper motor neuron syndrome. Am. J. Phys. Med. Rehabil. 2004, 83 (Suppl. S10), S3–S9. [Google Scholar] [CrossRef]
- Lackritz, H.; Parmet, Y.; Frenkel-Toledo, S.; Banina, M.C.; Soroker, N.; Solomon, J.M.; Liebermann, D.G.; Levin, M.F.; Berman, S. Effect of post-stroke spasticity on voluntary movement of the upper limb. J. Neuroeng. Rehabil. 2021, 18, 81. [Google Scholar] [CrossRef]
- Stewart, J.C.; Cramer, S.C. Patient-reported measures provide unique insights into motor function after stroke. Stroke 2013, 44, 1111–1116. [Google Scholar] [CrossRef]
- Baker, K.; Barrett, L.; Playford, E.D.; Aspden, T.; Riazi, A.; Hobart, J. Measuring arm function early after stroke: Is the DASH good enough? J. Neurol. Neurosurg. Psychiatry 2016, 87, 604–610. [Google Scholar] [CrossRef]
- Hoang-Kim, A.; Pegreffi, F.; Moroni, A.; Ladd, A. Measuring wrist and hand function: Common scales and checklists. Injury 2011, 42, 253–258. [Google Scholar] [CrossRef]
- Badalamente, M.; Coffelt, L.; Elfar, J.; Gaston, G.; Hammert, W.; Huang, J.; Lattanza, L.; Macdermid, J.; Merrell, G.; Netscher, D.; et al. Measurement scales in clinical research of the upper extremity, part 2: Outcome measures in studies of the hand/wrist and shoulder/elbow. J. Hand Surg. Am. 2013, 38, 407–412. [Google Scholar] [CrossRef]
- Kocyigit, B.F.; Akaltun, M.S. Assessment of responsiveness of four hand-related scales in stroke patients. Acta Neurol. Belg. 2021, 121, 1633–1639. [Google Scholar] [CrossRef]
- Morris, J.H.; Van Wijck, F. Responses of the less affected arm to bilateral upper limb task training in early rehabilitation after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2012, 93, 1129–1137. [Google Scholar] [CrossRef]
- Koszewicz, M.; Szydlo, M.; Gosk, J.; Wieczorek, M.; Slotwinski, K.; Budrewicz, S. The Relevance of Collision Tests and Quantitative Sensory Testing in Diagnostics and Postoperative Outcome Prediction in Carpal Tunnel Syndrome. Front. Neurol. 2022, 13, 900562. [Google Scholar] [CrossRef]
- Saini, A.; Zucker-Levin, A.; McMillan, B.; Kumar, P.; Donkers, S.; Levin, M.C. A Descriptive Correlational Study to Evaluate Three Measures of Assessing Upper Extremity Function in Individuals with Multiple Sclerosis. Mult. Scler. Int. 2021, 2021, 5588335. [Google Scholar] [CrossRef]
- Roorda, L.D.; Houwink, A.; Smits, W.; Molenaar, I.W.; Geurts, A.C. Measuring upper limb capacity in poststroke patients: Development, fit of the monotone homogeneity model, unidimensionality, fit of the double monotonicity model, differential item functioning, internal consistency, and feasibility of the stroke upper limb capacity scale, SULCS. Arch. Phys. Med. Rehabil. 2011, 92, 214–227. [Google Scholar] [CrossRef]
- Sunderland, A.; Tinson, D.; Bradley, L.; Hewer, R.L. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1267–1272. [Google Scholar] [CrossRef]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; Waller, S.M. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Yancosek, K.E.; Howell, D. A narrative review of dexterity assessments. J. Hand Ther. 2009, 22, 258–270. [Google Scholar] [CrossRef]
- Gustafsson, L.A.; Turpin, M.J.; Dorman, C.M. Clinical utility of the Chedoke Arm and Hand Activity Inventory for stroke rehabilitation. Can. J. Occup. Ther. 2010, 77, 167–173. [Google Scholar] [CrossRef]
- Chen, P.; Liu, T.-W.; Tse, M.M.; Lai, C.K.; Tsoh, J.; Ng, S.S. The Predictive Role of Hand Section of Fugl–Meyer Assessment and Motor Activity Log in Action Research Arm Test in People With Stroke. Front. Neurol. 2022, 13, 926130. [Google Scholar] [CrossRef]
- Yozbatiran, N.; Der-Yeghiaian, L.; Cramer, S.C. A standardized approach to performing the action research arm test. Neurorehabilit. Neural Repair 2008, 22, 78–90. [Google Scholar] [CrossRef]
- Ikbali Afsar, S.; Mirzayev, I.; Umit Yemisci, O.; Cosar Saracgil, S.N. Virtual Reality in Upper Extremity Rehabilitation of Stroke Patients: A Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 3473–3478. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Almeida, C.S.; Freias, L.C.; Santana, R.; Fernandes, G.; Fonseca Junior, P.R.; Moura, R.C.F. Use of the Box and Block Test for the evaluation of manual dexterity in individuals with central nervous system disorders: A systematic review. Man. Ther. Posturology Rehabil. J. 2016, 14, 1–17. [Google Scholar] [CrossRef]
- Solaro, C.; Di Giovanni, R.; Grange, E.; Mueller, M.; Messmer Uccelli, M.; Bertoni, R.; Brichetto, G.; Tacchino, A.; Patti, F.; Pappalardo, A.; et al. Box and block test, hand grip strength and nine-hole peg test: Correlations between three upper limb objective measures in multiple sclerosis. Eur. J. Neurol. 2020, 27, 2523–2530. [Google Scholar] [CrossRef]
- Beebe, J.A.; Lang, C.E. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. J. Neurol. Phys. Ther. 2009, 33, 96–103. [Google Scholar] [CrossRef]
- Moreno-Morente, G.; Hurtado-Pomares, M.; Terol Cantero, M.C. Bibliometric Analysis of Research on the Use of the Nine Hole Peg Test. Int. J. Environ. Res. Public Health 2022, 19, 10080. [Google Scholar] [CrossRef]
- Krumlinde-Sundholm, L.; Lindkvist, B.; Plantin, J.; Hoare, B. Development of the assisting hand assessment for adults following stroke: A Rasch-built bimanual performance measure. Disabil. Rehabil. 2019, 41, 472–480. [Google Scholar] [CrossRef]
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Taub, E.; Uswatte, G.; Morris, D.; Giuliani, C.; Light, K.E.; Nichols-Larsen, D.; Investigators, E. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA 2006, 296, 2095–2104. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pollock, A.; Pohl, M.; Kugler, J.; Elsner, B. Systematic review with network meta-analysis of randomized controlled trials of robotic-assisted arm training for improving activities of daily living and upper limb function after stroke. J. Neuroeng. Rehabil. 2020, 17, 83. [Google Scholar] [CrossRef]
- Arwert, H.; Schut, S.; Boiten, J.; Vliet Vlieland, T.; Meesters, J. Patient reported outcomes of hand function three years after stroke. Top. Stroke Rehabil. 2018, 25, 13–19. [Google Scholar] [CrossRef]
- Arcidiacone, S.; Panuccio, F.; Tusoni, F.; Galeoto, G. A systematic review of the measurement properties of the Michigan Hand Outcomes Questionnaire (MHQ). Hand Surg. Rehabil. 2022, 41, 542–551. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, C.L.; Huang, Y.Z.; Chen, H.C.; Chen, C.Y.; Wu, C.Y.; Lin, K.C. Augmented efficacy of intermittent theta burst stimulation on the virtual reality-based cycling training for upper limb function in patients with stroke: A double-blinded, randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 91. [Google Scholar] [CrossRef]
- RH, J. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar]
- Resnik, L.; Adams, L.; Borgia, M.; Delikat, J.; Disla, R.; Ebner, C.; Walters, L.S. Development and evaluation of the activities measure for upper limb amputees. Arch. Phys. Med. Rehabil. 2013, 94, 488–494.e4. [Google Scholar] [CrossRef]
- Sezer, N.; Yavuzer, G.; Sivrioglu, K.; Basaran, P.; Koseoglu, B.F. Clinimetric properties of the Duruoz hand index in patients with stroke. Arch. Phys. Med. Rehabil. 2007, 88, 309–314. [Google Scholar] [CrossRef]
- Duruöz, M.T.; Poiraudeau, S.; Fermanian, J.; Menkes, C.-J. Functional Disability Scale That Assesses Functional. Population 1996, 23, 1167–1172. [Google Scholar]
- Lefevre-Colau, M.M.; Poiraudeau, S.; Fermanian, J.; Etchepare, F.; Alnot, J.Y.; Le Viet, D.; Leclercq, C.; Oberlin, C.; Bargy, F.; Revel, M. Responsiveness of the Cochin rheumatoid hand disability scale after surgery. Rheumatology 2001, 40, 843–850. [Google Scholar] [CrossRef][Green Version]
- Brower, L.M.; Poole, J.L. Reliability and validity of the Duruöz Hand Index in persons with systemic sclerosis (scleroderma). Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2004, 51, 805–809. [Google Scholar] [CrossRef]
- Duruoz, M.T.; Cerrahoglu, L.; Dincer-Turhan, Y.; Kursat, S. Hand function assessment in patients receiving haemodialysis. Swiss Med. Wkly. 2003, 133, 433–438. [Google Scholar] [CrossRef]
- Subazwari, S.A.B.; Abrar, A.; Shahid, Z.; Manzoor, S.; Hadiqa, H.; Shafique, H.I. Comparison of Effects of Neurodevelopmental Treatment versus Motor Relearning Program on Upper Limb Spasticity in Chronic Stroke Patients. A Randomized Control Trial. Int. Med. J. 2021, 29, 7723–7729. [Google Scholar]
- Blackburn, M.; van Vliet, P.; Mockett, S.P. Reliability of measurements obtained with the modified Ashworth scale in the lower extremities of people with stroke. Phys. Ther. 2002, 82, 25–34. [Google Scholar] [CrossRef]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Paediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef]
- Gracies, J.M.; Marosszeky, J.E.; Renton, R.; Sandanam, J.; Gandevia, S.C.; Burke, D. Short-term effects of dynamic lycra splints on upper limb in hemiplegic patients. Arch. Phys. Med. Rehabil. 2000, 81, 1547–1555. [Google Scholar] [CrossRef]
- Baricich, A.; Carda, S.; Bertoni, M.; Maderna, L.; Cisari, C. A single-blinded, randomized pilot study of botulinum toxin type A combined with non-pharmacological treatment for spastic foot. J. Rehabil. Med. 2008, 40, 870–872. [Google Scholar] [CrossRef]
- SooHoo, N.F.; McDonald, A.P.; Seiler, J.G., 3rd; McGillivary, G.R. Evaluation of the construct validity of the DASH questionnaire by correlation to the SF-36. J. Hand Surg. Am. 2002, 27, 537–541. [Google Scholar] [CrossRef]
- Dalton, E.; Lannin, N.A.; Laver, K.; Ross, L.; Ashford, S.; McCluskey, A.; Cusick, A. Validity, reliability and ease of use of the disabilities of arm, shoulder and hand questionnaire in adults following stroke. Disabil. Rehabil. 2017, 39, 2504–2511. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Turgeon, T.; Richards, R.S.; Beadle, M.; Roth, J.H. Patient rating of wrist pain and disability: A reliable and valid measurement tool. J. Orthop. Trauma. 1998, 12, 577–586. [Google Scholar] [CrossRef]
- Wehrli, M.; Hensler, S.; Schindele, S.; Herren, D.B.; Marks, M. Measurement Properties of the Brief Michigan Hand Outcomes Questionnaire in Patients With Dupuytren Contracture. J. Hand Surg. Am. 2016, 41, 896–902. [Google Scholar] [CrossRef]
- Wilcke, M.T.; Abbaszadegan, H.; Adolphson, P.Y. Evaluation of a Swedish version of the patient-rated wrist evaluation outcome questionnaire: Good responsiveness, validity, and reliability, in 99 patients recovering from a fracture of the distal radius. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2009, 43, 94–101. [Google Scholar] [CrossRef]
- Tuna, Z.; Mete, O.; Tore, G.; Baglan Yentur, S.; Varan, O.; Goker, B.; Oskay, D. Validity of the Patient-Rated Wrist Evaluation questionnaire in rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 1714–1718. [Google Scholar] [CrossRef]
- Leite, J.C.; Jerosch-Herold, C.; Song, F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet. Disord. 2006, 7, 78. [Google Scholar] [CrossRef]
- Weinstock-Zlotnick, G.; Mehta, S.P. A structured literature synthesis of wrist outcome measures: An evidence-based approach to determine use among common wrist diagnoses. J. Hand Ther. 2016, 29, 98–110. [Google Scholar] [CrossRef]
- Mehta, S.P.; Weinstock-Zlotnick, G.; Akland, K.L.; Hanna, M.M.; Workman, K.J. Using Carpal Tunnel Questionnaire in clinical practice: A systematic review of its measurement properties. J. Hand Ther. 2020, 33, 493–506. [Google Scholar] [CrossRef]
- Lin, C.; Loochtan, A.I.; Dresser, B.; Chang, J.; Farjat, A.E.; Choudhury, K.; Hobson-Webb, L.D. Is carpal tunnel syndrome present in acute stroke patients? An investigative study using clinical and imaging screening tools. J. Clin. Neurosci. 2017, 39, 111–113. [Google Scholar] [CrossRef]
- Houwink, A.; Nijland, R.H.; Geurts, A.C.; Kwakkel, G. Functional recovery of the paretic upper limb after stroke: Who regains hand capacity? Arch. Phys. Med. Rehabil. 2013, 94, 839–844. [Google Scholar] [CrossRef]
- Branco, J.P.; Oliveira, S.; Pinheiro, J.P.; Ferreira, P.L. Assessing upper limb function: Transcultural adaptation and validation of the Portuguese version of the Stroke Upper Limb Capacity Scale. BMC Sports Sci. Med. Rehabil. 2017, 9, 15. [Google Scholar] [CrossRef]
- Smedes, F.; van der Salm, A.; Koel, G.; Oosterveld, F. Manual mobilization of the wrist: A pilot study in rehabilitation of patients with a chronic hemiplegic hand post-stroke. J. Hand Ther. 2014, 27, 209–215; quiz 216. [Google Scholar] [CrossRef]
- Salvietti, G.; Hussain, I.; Cioncoloni, D.; Taddei, S.; Rossi, S.; Prattichizzo, D. Compensating Hand Function in Chronic Stroke Patients Through the Robotic Sixth Finger. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 142–150. [Google Scholar] [CrossRef]
- Sampaio, C.; Ferreira, J.J.; Pinto, A.A.; Crespo, M.; Ferro, J.M.; Castro-Caldas, A. Botulinum toxin type A for the treatment of arm and hand spasticity in stroke patients. Clin. Rehabil. 1997, 11, 3–7. [Google Scholar] [CrossRef]
- Penta, M.; Tesio, L.; Arnould, C.; Zancan, A.; Thonnard, J.L. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke 2001, 32, 1627–1634. [Google Scholar] [CrossRef]
- Ekstrand, E.; Alt Murphy, M.; Sunnerhagen, K.S. Clinical interpretation and cutoff scores for manual ability measured by the ABILHAND questionnaire in people with stroke. Top. Stroke Rehabil. 2023, 30, 21–31. [Google Scholar] [CrossRef]
- Lai, S.M.; Studenski, S.; Duncan, P.W.; Perera, S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002, 33, 1840–1844. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, G.; Cho, D.Y.; Kim, H.Y.; Lee, J.-Y.; Kim, S.; Park, S.-B.; Shin, J.-H. Comparisons between end-effector and exoskeleton rehabilitation robots regarding upper extremity function among chronic stroke patients with moderate-to-severe upper limb impairment. Sci. Rep. 2020, 10, 1806. [Google Scholar] [CrossRef]
- Reddon, J.R.; Gill, D.M.; Gauk, S.E.; Maerz, M.D. Purdue Pegboard: Test-retest estimates. Percept. Mot. Ski. 1988, 66, 503–506. [Google Scholar] [CrossRef]
- Lawson, I. Purdue pegboard test. Occup. Med. 2019, 69, 376–377. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Yang, K.I.; Kim, D.-e.; Kim, S.A. The relationship between sleep disturbance and functional status in mild stroke patients. Ann. Rehabil. Med. 2015, 39, 545–552. [Google Scholar] [CrossRef]
- Brogårdh, C.; Persson, A.L.; Sjölund, B.H. Intra-and inter-rater reliability of the Sollerman hand function test in patients with chronic stroke. Disabil. Rehabil. 2007, 29, 145–154. [Google Scholar] [CrossRef]
- Blomgren, I.; Blomqvist, G.; Ejeskar, A.; Fogdestam, I.; Volkman, R.; Edshage, S. Hand function after replantation or revascularization of upper extremity injuries: A follow-up study of 21 cases operated on 1979–1985 in Göteborg. Scand. J. Plast. Reconstr. Surg. 1988, 22, 93–101. [Google Scholar]
- Dellhag, B.; Burckhardt, C.S. Predictors of hand function in patients with rheumatoid arthritis. Arthritis Care Res. 1995, 8, 16–20. [Google Scholar] [CrossRef]
- Levin, M.F.; Desrosiers, J.; Beauchemin, D.; Bergeron, N.; Rochette, A. Development and validation of a scale for rating motor compensations used for reaching in patients with hemiparesis: The reaching performance scale. Phys. Ther. 2004, 84, 8–22. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Margolese, G.; Turolla, A.; Saposnik, G.; Levin, M.F. Responsiveness of the Reaching Performance Scale for Stroke. Arch. Phys. Med. Rehabil. 2023, 104, 1588–1595. [Google Scholar] [CrossRef]
- Hidayat, A.A.; Arief, Z.; Yuniarti, H. LOVETT scalling with MYO armband for monitoring finger muscles therapy of post-stroke people. In Proceedings of the 2016 International Electronics Symposium (IES), Denpasar, Indonesia, 29–30 September 2016; pp. 66–70. [Google Scholar]
- Niklas, K.; Niklas, A.; Puszczewicz, M.; Wolska-Bulach, A.; Tykarski, A. Polymyositis induced by atorvastatin. Kardiol. Pol. 2015, 73, 1336. [Google Scholar] [CrossRef]
- Giannini, F.; Cioni, R.; Mondelli, M.; Padua, R.; Gregori, B.; D’Amico, P.; Padua, L. A new clinical scale of carpal tunnel syndrome: Validation of the measurement and clinical-neurophysiological assessment. Clin. Neurophysiol. 2002, 113, 71–77. [Google Scholar] [CrossRef]
- Luo, Z.; Lo, W.L.A.; Bian, R.; Wong, S.; Li, L. Advanced quantitative estimation methods for spasticity: A literature review. J. Int. Med. Res. 2020, 48, 300060519888425. [Google Scholar] [CrossRef]
- Garcia-Bernal, M.I.; Gonzalez-Garcia, P.; Casuso-Holgado, M.J.; Cortes-Vega, M.D.; Heredia-Rizo, A.M. Measuring Mechanical Properties of Spastic Muscles After Stroke. Does Muscle Position During Assessment Really Matter? Arch. Phys. Med. Rehabil. 2022, 103, 2368–2374. [Google Scholar] [CrossRef]
- Patrick, E.; Ada, L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin. Rehabil. 2006, 20, 173–182. [Google Scholar] [CrossRef]
- Haugh, A.B.; Pandyan, A.D.; Johnson, G.R. A systematic review of the Tardieu Scale for the measurement of spasticity. Disabil. Rehabil. 2006, 28, 899–907. [Google Scholar] [CrossRef]
- Yam, W.K.; Leung, M.S. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J. Child. Neurol. 2006, 21, 1031–1035. [Google Scholar] [CrossRef]
- Love, S.; Gibson, N.; Smith, N.; Bear, N.; Blair, E.; Australian Cerebral Palsy Register, G. Interobserver reliability of the Australian Spasticity Assessment Scale (ASAS). Dev. Med. Child. Neurol. 2016, 58 (Suppl. S2), 18–24. [Google Scholar] [CrossRef]
- Gomez-Cuaresma, L.; Lucena-Anton, D.; Gonzalez-Medina, G.; Martin-Vega, F.J.; Galan-Mercant, A.; Luque-Moreno, C. Effectiveness of Stretching in Post-Stroke Spasticity and Range of Motion: Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1074. [Google Scholar] [CrossRef]
- Marques, I.A.; Silva, M.B.; Silva, A.N.; Luiz, L.M.D.; Soares, A.B.; Naves, E.L.M. Measurement of post-stroke spasticity based on tonic stretch reflex threshold: Implications of stretch velocity for clinical practice. Disabil. Rehabil. 2019, 41, 219–225. [Google Scholar] [CrossRef]
- Sorinola, I.O.; White, C.M.; Rushton, D.N.; Newham, D.J. Electromyographic response to manual passive stretch of the hemiplegic wrist: Accuracy, reliability, and correlation with clinical spasticity assessment and function. Neurorehabil. Neural Repair. 2009, 23, 287–294. [Google Scholar] [CrossRef]
- Jarque-Bou, N.J.; Sancho-Bru, J.L.; Vergara, M. A Systematic Review of EMG Applications for the Characterization of Forearm and Hand Muscle Activity during Activities of Daily Living: Results, Challenges, and Open Issues. Sensors 2021, 21, 3035. [Google Scholar] [CrossRef]
- Grimm, F.; Naros, G.; Gharabaghi, A. Compensation or Restoration: Closed-Loop Feedback of Movement Quality for Assisted Reach-to-Grasp Exercises with a Multi-Joint Arm Exoskeleton. Front. Neurosci. 2016, 10, 280. [Google Scholar] [CrossRef]
- Cheung, V.C.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef]
- Mogk, J.P.; Keir, P.J. Crosstalk in surface electromyography of the proximal forearm during gripping tasks. J. Electromyogr. Kinesiol. 2003, 13, 63–71. [Google Scholar] [CrossRef]
- Anastasiev, A.; Kadone, H.; Marushima, A.; Watanabe, H.; Zaboronok, A.; Watanabe, S.; Matsumura, A.; Suzuki, K.; Matsumaru, Y.; Ishikawa, E. Supervised Myoelectrical Hand Gesture Recognition in Post-Acute Stroke Patients with Upper Limb Paresis on Affected and Non-Affected Sides. Sensors 2022, 22, 8733. [Google Scholar] [CrossRef]
- Kamen, G. Essentials of Electromyography; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- Kim, J.H. The effects of training using EMG biofeedback on stroke patients upper extremity functions. J. Phys. Ther. Sci. 2017, 29, 1085–1088. [Google Scholar] [CrossRef]
- Xia, W.; Dai, R.; Xu, X.; Huai, B.; Bai, Z.; Zhang, J.; Jin, M.; Niu, W. Cortical mapping of active and passive upper limb training in stroke patients and healthy people: A functional near-infrared spectroscopy study. Brain Res. 2022, 1788, 147935. [Google Scholar] [CrossRef]
- Collinger, J.L.; Wodlinger, B.; Downey, J.E.; Wang, W.; Tyler-Kabara, E.C.; Weber, D.J.; McMorland, A.J.; Velliste, M.; Boninger, M.L.; Schwartz, A.B. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 2013, 381, 557–564. [Google Scholar] [CrossRef]
- Soekadar, S.R.; Birbaumer, N.; Slutzky, M.W.; Cohen, L.G. Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 2015, 83, 172–179. [Google Scholar] [CrossRef]
- Cherry-Allen, K.M.; French, M.A.; Stenum, J.; Xu, J.; Roemmich, R.T. Opportunities for Improving Motor Assessment and Rehabilitation After Stroke by Leveraging Video-Based Pose Estimation. Am. J. Phys. Med. Rehabil. 2023, 102, S68–S74. [Google Scholar] [CrossRef]
- Kwakkel, G.; van Wegen, E.E.H.; Burridge, J.H.; Winstein, C.J.; van Dokkum, L.E.H.; Alt Murphy, M.; Levin, M.F.; Krakauer, J.W. Standardized Measurement of Quality of Upper Limb Movement After Stroke: Consensus-Based Core Recommendations From the Second Stroke Recovery and Rehabilitation Roundtable. Neurorehabil. Neural Repair. 2019, 33, 951–958. [Google Scholar] [CrossRef]
- Rahimi, F.; Eyvazpour, R.; Salahshour, N.; Azghani, M.R. Objective assessment of spasticity by pendulum test: A systematic review on methods of implementation and outcome measures. Biomed. Eng. Online 2020, 19, 82. [Google Scholar] [CrossRef]
- De-la-Torre, R.; Ona, E.D.; Balaguer, C.; Jardon, A. Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review. Sensors 2020, 20, 5251. [Google Scholar] [CrossRef]
- Simbaña, E.D.O.; Baeza, P.S.-H.; Huete, A.J.; Balaguer, C. Review of automated systems for upper limbs functional assessment in neurorehabilitation. IEEE Access 2019, 7, 32352–32367. [Google Scholar] [CrossRef]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of Using Virtual Reality-Supported Exercise Therapy for Upper Extremity Motor Rehabilitation in Patients With Stroke: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef]
- Park, M.; Ko, M.H.; Oh, S.W.; Lee, J.Y.; Ham, Y.; Yi, H.; Choi, Y.; Ha, D.; Shin, J.H. Effects of virtual reality-based planar motion exercises on upper extremity function, range of motion, and health-related quality of life: A multicenter, single-blinded, randomized, controlled pilot study. J. Neuroeng. Rehabil. 2019, 16, 122. [Google Scholar] [CrossRef]
- Lee, P.Y.; Huang, J.C.; Tseng, H.Y.; Yang, Y.C.; Lin, S.I. Effects of Trunk Exercise on Unstable Surfaces in Persons with Stroke: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 9135. [Google Scholar] [CrossRef]
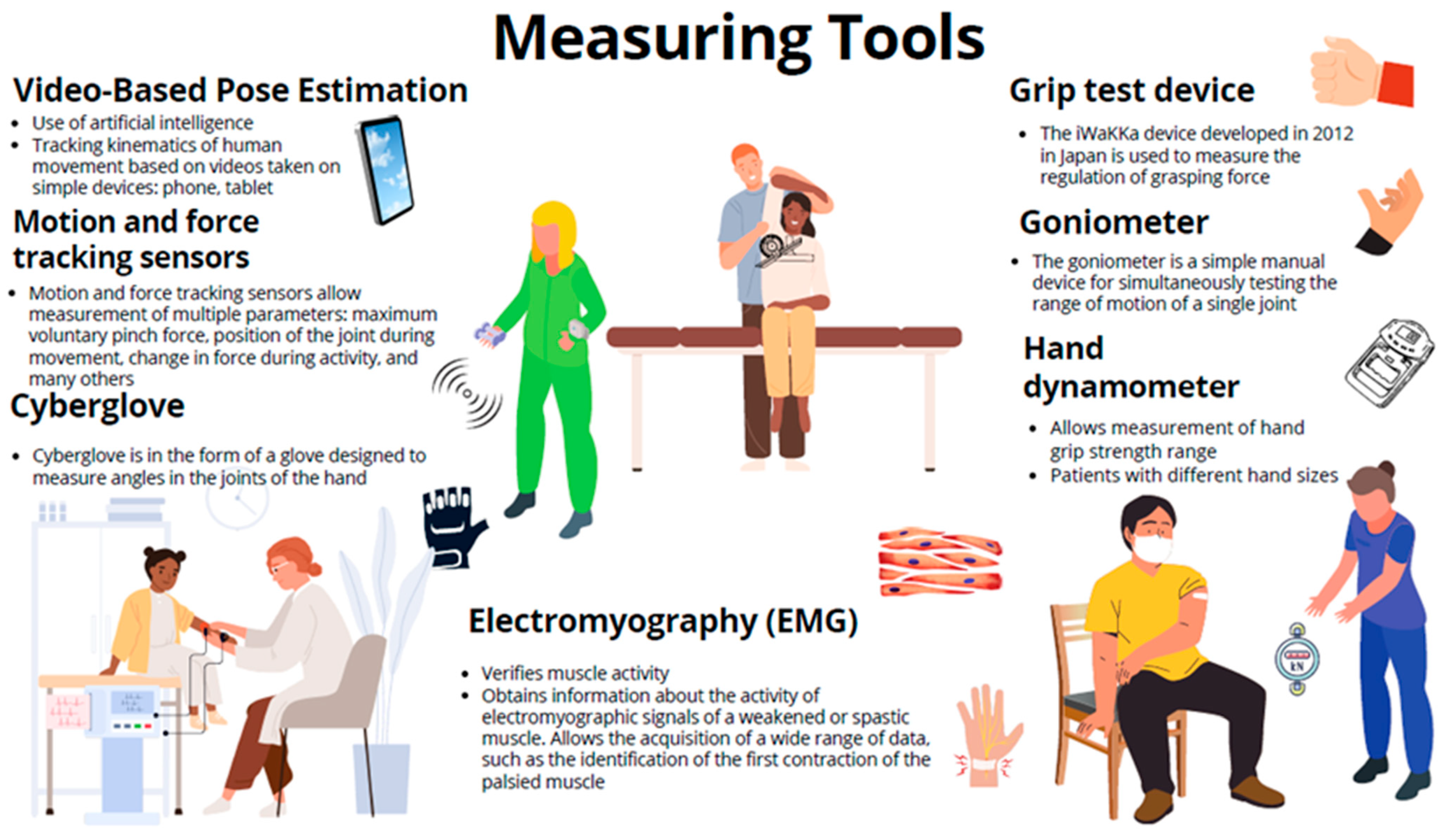
- Kaneno, T.; Sato, A.; Akizuki, K.; Yamaguchi, A.; Yasaki, K.; Morita, Y. Assessing the adjustability of grasping force using the iWakka in elderly individuals. J. Phys. Ther. Sci. 2017, 29, 2215–2219. [Google Scholar] [CrossRef]
- Phan, G.H.; Solanki, V.K.; Quang, N.H. Combining 3D Motion Tracker with IMU Sensor Signals of Muscles to Discover Macro-and Microvibration for Stroke Rehabilitation. In Bio-Inspired Motor Control Strategies for Redundant and Flexible Manipulator with Application to Tooling Tasks; Springer: Berlin/Heidelberg, Germany, 2022; pp. 57–68. [Google Scholar]
- Bakhtina, V.A.; Marinushkin, P.S.; Levitskiy, A.A.; Ilminskaya, A.A.; Abroskina, M.V.; Prokopenko, S.V. Portable Accelerometer-Based System for Aiding Elbow Extension in Post-Stroke Individuals. In Proceedings of the 2019 20th International Conference of Young Specialists on Micro/Nanotechnologies and Electron Devices (EDM), Erlagol, Russia, 29 June–3 July 2019; pp. 626–630. [Google Scholar]
- Borboni, A.; Villafane, J.H.; Mulle, C.; Valdes, K.; Faglia, R.; Taveggia, G.; Negrini, S. Robot-Assisted Rehabilitation of Hand Paralysis After Stroke Reduces Wrist Edema and Pain: A Prospective Clinical Trial. J. Manip. Physiol. Ther. 2017, 40, 21–30. [Google Scholar] [CrossRef]
- Boomkamp-Koppen, H.G.; Visser-Meily, J.M.; Post, M.W.; Prevo, A.J. Poststroke hand swelling and oedema: Prevalence and relationship with impairment and disability. Clin. Rehabil. 2005, 19, 552–559. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Borges, L.R.; Fernandes, A.B.; Oliveira Dos Passos, J.; Rego, I.A.O.; Campos, T.F. Action observation for upper limb rehabilitation after stroke. Cochrane Database Syst. Rev. 2022, 8, CD011887. [Google Scholar] [CrossRef]
- Gregor, S.; Saumur, T.M.; Crosby, L.D.; Powers, J.; Patterson, K.K. Study Paradigms and Principles Investigated in Motor Learning Research After Stroke: A Scoping Review. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100111. [Google Scholar] [CrossRef]
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A.; American Heart Association Council on Cardiovascular Nursing and the Stroke Council. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the American Heart Association. Stroke 2010, 41, 2402–2448. [Google Scholar] [CrossRef]
- Prange-Lasonder, G.B.; Alt Murphy, M.; Lamers, I.; Hughes, A.M.; Buurke, J.H.; Feys, P.; Keller, T.; Klamroth-Marganska, V.; Tarkka, I.M.; Timmermans, A.; et al. European evidence-based recommendations for clinical assessment of upper limb in neurorehabilitation (CAULIN): Data synthesis from systematic reviews, clinical practice guidelines and expert consensus. J. Neuroeng. Rehabil. 2021, 18, 162. [Google Scholar] [CrossRef]
- Chalos, V.; van der Ende, N.A.M.; Lingsma, H.F.; Mulder, M.; Venema, E.; Dijkland, S.A.; Berkhemer, O.A.; Yoo, A.J.; Broderick, J.P.; Palesch, Y.Y.; et al. National Institutes of Health Stroke Scale: An Alternative Primary Outcome Measure for Trials of Acute Treatment for Ischemic Stroke. Stroke 2020, 51, 282–290. [Google Scholar] [CrossRef]
- Vidmar, T.; Goljar Kregar, N.; Puh, U. Reliability of the Modified Ashworth Scale After Stroke for 13 Muscle Groups. Arch. Phys. Med. Rehabil. 2023, 104, 1606–1611. [Google Scholar] [CrossRef]
- Wright, V. Prosthetic outcome measures for use with upper limb amputees: A systematic review of the peer-reviewed literature, 1970 to 2009. JPO J. Prosthet. Orthot. 2009, 21, P3–P63. [Google Scholar] [CrossRef]
- Lindner, H.Y.; Natterlund, B.S.; Hermansson, L.M. Upper limb prosthetic outcome measures: Review and content comparison based on International Classification of Functioning, Disability and Health. Prosthet. Orthot. Int. 2010, 34, 109–128. [Google Scholar] [CrossRef]
- Swingler, G.H.; Volmink, J.; Ioannidis, J.P. Number of published systematic reviews and global burden of disease: Database analysis. BMJ 2003, 327, 1083–1084. [Google Scholar] [CrossRef]
- Kosorok, M.R.; Laber, E.B. Precision Medicine. Annu. Rev. Stat. Appl. 2019, 6, 263–286. [Google Scholar] [CrossRef]
- Bonnechere, B. Integrating Rehabilomics into the Multi-Omics Approach in the Management of Multiple Sclerosis: The Way for Precision Medicine? Genes 2022, 14, 63. [Google Scholar] [CrossRef]
- Lynning, M.; Svane, C.; Westergaard, K.; Bergien, S.; Gunnersen, S.; Skovgaard, L. Tension and trauma releasing exercises for people with multiple sclerosis–An exploratory pilot study. J. Tradit. Complement. Med. 2021, 11, 383–389. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Petersen, T.H.; Kirk, H.; Forman, C.; Svane, C.; Kofoed-Hansen, M.; Boesen, F.; Lorentzen, J. Spasticity in adults with cerebral palsy and multiple sclerosis measured by objective clinically applicable technique. Clin. Neurophysiol. 2018, 129, 2010–2021. [Google Scholar] [CrossRef]
- Rosenthal, O.; Wing, A.M.; Wyatt, J.L.; Punt, D.; Miall, R.C. Mapping upper-limb motor performance after stroke—A novel method with utility for individualized motor training. J. Neuroeng. Rehabil. 2017, 14, 127. [Google Scholar] [CrossRef]
- Israely, S.; Leisman, G.; Carmeli, E. Improvement in arm and hand function after a stroke with task-oriented training. BMJ Case Rep. 2017, 2017, bcr2017219250. [Google Scholar] [CrossRef]
- Liu, F.; Tsang, R.C.; Zhou, J.; Zhou, M.; Zha, F.; Long, J.; Wang, Y. Relationship of Barthel Index and its Short Form with the Modified Rankin Scale in acute stroke patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 105033. [Google Scholar] [CrossRef]
- Schneidert, M.; Hurst, R.; Miller, J.; Üstün, B. The role of environment in the International Classification of Functioning, Disability and Health (ICF). Disabil. Rehabil. 2003, 25, 588–595. [Google Scholar] [CrossRef]
- Vergara, M.; Sancho-Bru, J.L.; Gracia-Ibanez, V.; Perez-Gonzalez, A. An introductory study of common grasps used by adults during performance of activities of daily living. J. Hand Ther. 2014, 27, 225–233; quiz 234. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Petersen, N.T.; Crone, C.; Sinkjaer, T. Stretch reflex regulation in healthy subjects and patients with spasticity. Neuromodulation 2005, 8, 49–57. [Google Scholar] [CrossRef]
- JW, L. Pathophysiology of spasticity and clinical experience with baclofen. In Spasticity: Disordered Motor Control; Year Book Med. Pub.: Chicago, IL, USA, 1980; pp. 185–204. [Google Scholar]
- Radomski, M.V.; Anheluk, M.; Arulanantham, C.; Finkelstein, M.; Flinn, N. Implementing evidence-based practice: A context analysis to examine use of task-based approaches to upper-limb rehabilitation. Br. J. Occup. Ther. 2018, 81, 285–289. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wu, C.Y.; Lin, K.C.; Chang, Y.F.; Chen, C.L.; Liu, J.S. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke 2009, 40, 1386–1391. [Google Scholar] [CrossRef]
- Kielhofner, G. A Model of Human Occupation: Theory and Application; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Walder, K.; Molineux, M.; Bissett, M.; Whiteford, G. Occupational adaptation–analyzing the maturity and understanding of the concept through concept analysis. Scand. J. Occup. Ther. 2021, 28, 26–40. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Zhang, Y.; Ni, J.; Huang, J.; Wu, Y.; Li, M. Interventions targeting psychosocial adaptation in people with stroke: A scoping review. Patient Educ. Couns. 2023, 113, 107751. [Google Scholar] [CrossRef]
- Proud, E.L.; Miller, K.J.; Bilney, B.; Morris, M.E.; McGinley, J.L. Construct validity of the 9-Hole Peg Test and Purdue Pegboard Test in people with mild to moderately severe Parkinson’s disease. Physiotherapy 2020, 107, 202–208. [Google Scholar] [CrossRef]
- Velstra, I.-M.; Ballert, C.S.; Cieza, A. A systematic literature review of outcome measures for upper extremity function using the international classification of functioning, disability, and health as reference. PM&R 2011, 3, 846–860. [Google Scholar]
- Hsieh, C.-L.; Hsueh, I.-P.; Chiang, F.-M.; Lin, P.-H. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing 1998, 27, 107–113. [Google Scholar] [CrossRef]
- Barreca, S.R.; Stratford, P.W.; Lambert, C.L.; Masters, L.M.; Streiner, D.L. Test-retest reliability, validity, and sensitivity of the Chedoke arm and hand activity inventory: A new measure of upper-limb function for survivors of stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1616–1622. [Google Scholar] [CrossRef]
- Temporiti, F.; Mandaresu, S.; Calcagno, A.; Coelli, S.; Bianchi, A.M.; Gatti, R.; Galli, M. Kinematic evaluation and reliability assessment of the Nine Hole Peg Test for manual dexterity. J. Hand Ther. 2023, 36, 560–567. [Google Scholar] [CrossRef]
- Johansson, G.M.; Hager, C.K. A modified standardized nine hole peg test for valid and reliable kinematic assessment of dexterity post-stroke. J. Neuroeng. Rehabil. 2019, 16, 8. [Google Scholar] [CrossRef]
- Everard, G.; Otmane-Tolba, Y.; Rosselli, Z.; Pellissier, T.; Ajana, K.; Dehem, S.; Auvinet, E.; Edwards, M.G.; Lebleu, J.; Lejeune, T. Concurrent validity of an immersive virtual reality version of the Box and Block Test to assess manual dexterity among patients with stroke. J. Neuroeng. Rehabil. 2022, 19, 7. [Google Scholar] [CrossRef]
- Turtle, B.; Porter-Armstrong, A.; Stinson, M. The reliability of the graded Wolf Motor Function Test for stroke. Br. J. Occup. Ther. 2020, 83, 585–594. [Google Scholar] [CrossRef]
- Wolf, S.; Gerloff, C.; Backhaus, W. Predictive Value of Upper Extremity Outcome Measures After Stroke-A Systematic Review and Metaregression Analysis. Front. Neurol. 2021, 12, 675255. [Google Scholar] [CrossRef]
- Maggio, M.G.; Latella, D.; Maresca, G.; Sciarrone, F.; Manuli, A.; Naro, A.; De Luca, R.; Calabro, R.S. Virtual Reality and Cognitive Rehabilitation in People With Stroke: An Overview. J. Neurosci. Nurs. 2019, 51, 101–105. [Google Scholar] [CrossRef]
- Tan, M.; Li, H.; Wang, X. Analysis of the current status of rehabilitation motivation and its influencing factors in older adults with stroke: A cross-sectional study. Front. Aging Neurosci. 2023, 15, 1186681. [Google Scholar] [CrossRef]
- Perna, R.; Harik, L. The role of rehabilitation psychology in stroke care described through case examples. NeuroRehabilitation 2020, 46, 195–204. [Google Scholar] [CrossRef]
| The Brunnstrom Stages of Stroke Recovery | |
|---|---|
| Stage | Meaning |
| 1 | Flaccid paralysis. No voluntary movement and reflexes. |
| 2 | Some spastic tone. No voluntary movement. A small amount of movement may be elicited through facilitation. |
| 3 | Spasticity is marked. Synergistic movements may be elicited voluntarily. |
| 4 | Spasticity decreases. Muscle control increases. Synergistic movements predominate. |
| 5 | Spasticity wanes. Complex movements begin although synergies are still present. |
| 6 | Coordination reappears. Spasticity disappears completely. Complex coordinated movements are almost fully present. |
| 7 | Normal functions returns. |
| Type of Outcome Measure | Details and Description | Patient Specification |
|---|---|---|
| Fugl-Meyer Assessment of Motor Recovery Upper Extremity (FMA—UE) | The upper extremity motor section of the Fugl-Meyer Assessment (FMA) measures the level of impairment using Brunnstrom’s stages of recovery after stroke. The assessment includes arm movements in and out of synergy, reflexes, the ability to isolate shoulder, elbow, and wrist movements, and grasping objects. The FMA-UE consists of five main domains: motor function, sensory function, balance, joint range of motion (ROM), and joint pain. The subscales can be administered separately. To conduct the assessment, a tennis ball and a round container are required. The subscales can be managed separately [5,58,94,95]. | Neurological [96]. |
| Chedoke Arm and Hand Inventory (CAHAI) | The CAHAI evaluates the ability to perform everyday bimanual activities in stroke patients. It assesses various aspects of hand and arm function, such as coordination, grip, dexterity, and upper limb strength. The inventory is specifically designed for use in the stroke population [97]. | Neurological [97]. |
| Action Research Arm Test (ARAT) | The ARAT is designed to evaluate the upper limb function in neurological patients. The test consists of 19 items divided into four sections: grasping, gripping, pinching, and gross movement. It enables the quantification of these skills [98,99]. | Neurological [98]. |
| Box and Block Test (BBT) | The BBT is used to assess the manual dexterity of the hand. During the test, patients move 2.5 cm blocks as quickly as possible within a short period of time, using only their thumb and index finger. The attempt to move the blocks lasts 60 s [100]. | Neurological [101,102]. |
| Nine Hole Peg Test (9-HPT) | The 9-HPT involves placing nine pegs on a specially designed board. Once placed, the pegs must be removed using only one hand [103]. The test is timed, and patients are instructed to complete the task as quickly as possibe without sacrificing accuracy. | Neurological [104]. |
| Adult Assisting Hand Assessment Stroke (Ad-AHA) | The scale consists of 19 items, which are assessed by observing the patient’s performance during functional activities, “present”, or “sandwich” tasks. It tests ambidextritye, as these tasks require the patient to coordinate and use their affected hand in collaboration with their unaffected hand to accomplish the activity effectively [105]. | Neurological [58]. |
| Wolf Motor Function Test (WMFT) | The WMFT is an assessment tool designed to evaluate upper limb motor function in post-stroke patients. The test comprises a series of reaching and manipulation activities that patients are required to perform within a set time frame [106]. It assesses various aspects of upper limb function, such as grip strength, dexterity, coordination, and the ability to perform functional tasks efficiently. | Neurological [106]. |
| Motricity Index (MI) | Was developed to measure limb motor function and muscle strength in paralyzed stroke patients. For the upper limb assessment, the MI evaluates the shoulder abduction, elbow flexion, and pinch grip [107]. | Neurological [107]. |
| Michigan Hand Outcomes Questionnaire (MHQ) | It is a self-completion questionnaire. It includes 57 items and covers six domains: general hand function, daily activities, pain, work performance, aesthetics, and patient satisfaction with the functional capabilities of the hand [108]. In neurological patients, it is used for hemiparesis and nerve compression [109]. | Orthopedic, rheumatoid arthritis, and neurological [108,109]. |
| Motor Activity Log (MAL) | The MAL is a tool used to assess the impaired arm based on 14 daily activities performed routinely throughout the day. The scale evaluates the quality of movement (Quality of Movement or QOM) and the amount of use (Amount of Use or AOU) in which the patient utilizes the affected arm [110]. | Neurological [110]. |
| Jebsen–Taylor Hand Function Test (JHFT) | The test is a standardized assessment that consists of seven parts and evaluates unilateral hand functions. The test measures the patient’s ability to perform various tasks that mimic everyday activities, such as picking up small objects, writing, and manipulating items. The items needed to perform the test include a paper clip, cans, and coins [111]. | Neurological and amputation status [112]. |
| Duruöz Hand Index (DHI) | The DHI is a self-reported questionnaire consisting of 18 questions related to hand function. These questions focus on various daily activities that involve the use of hands, such as buttoning, writing, cutting food, opening doors, and lifting objects, from five domains (kitchen, dressing, hygiene, office, and other). Each question requires the respondent to rate their ability to perform the activity on a scale from zero (no difficulty) to five (unable to perform the task). The index evaluates ambidextrous dexterity and provides a total score, with higher scores indicating greater impairment in hand function [113]. | Neurological (stroke) [113], rheumatoid arthritis [114], osteoarthritis [115], systemic scleroderma [116], and hemodialysis patients [117]. |
| Ashworth Scale (AS) Modified Ashworth Scale (MAS) | The AS is a 5-point numerical scale used to assess spasticity. Scores range from 0 to 4, with 0 indicating no resistance and 4 indicating a limb that is rigid in flexion or extension [118]. The MAS is a 6-point scale that expands on the original AS, with scores ranging from 0 to 4, and an additional rating of 1+ for more precise assessment. Muscle evaluation is conducted by measuring passive stiffness, joint range of motion, and grip and movement ability [119]. Both scales are designed to assess muscle tone and spasticity in patients. | Neurological patients with spasticity after botulinum toxin injection [118,119,120]. |
| Tardieu Scale (TS) Modified Tardieu Scale (MTS) | The TS is a five-step scale used to assess spasticity. It evaluates two parameters: the degree of spasticity (a scale that assesses the quality of a muscle’s response to stretching) and the angle of spasticity (the angle at which the muscle’s response occurs). Assessments are conducted at three speeds: as slow as possible (V1), falling under gravity (V2), and as fast as possible (V3) [121,122]. The MTS takes into account muscle responses to passive movement at two different speeds (low and high). In the high-speed measurement, the joint moves as fast as possible through its full range of motion. The angle at which the muscles first activate the stretch reflex is measured as R1. The angle of full passive range of motion (ROM) is R2. The difference between these angles (R2-R1) represents the potential ROM [120]. Both scales are designed to assess muscle tension and spasticity in patients. | Neurological patients with spasticity after botulinum toxin injection [120,122]. |
| Disability of Arm-Shoulder-Hand questionnaire (DASH) | The DASH is a self-reported questionnaire consisting of 30 items that assess various concerns and functions related to the arm, shoulder, and hand. Each item offers five response options, allowing patients to rate their level of difficulty or discomfort. While the DASH is predominantly used in orthopedic patients [86,123], it can also be employed, with some modifications, in neurological patients such as those with stroke or multiple sclerosis [85]. | Orthopedic, musculoskeletal diseases, neurological, and stroke [124]. |
| Patient-Rated Wrist Evaluation questionnaire (PRWE) | The PRWE is a self-administered questionnaire specifically designed for assessing wrist-related conditions. It consists of 15 self-completion items that focus on evaluating two subscales: wrist pain and function [125]. The pain subscale contains five items about pain experienced in various situations (resting, specific movements, lifting, and daily activities). The function subscale includes items that evaluate the patient’s wrist function in specific (such as turning the doorknob) and usual activities (daily living). | Orthopedic patients, surgical patients after fracture of the distal root of the radius and scaphoid bone, dysfunction of the distal root of the prominence-ulnar bone, carpal tunnel syndrome [114,115,116,125,126,127], and rheumatoid arthritis [128]. Rarely, neurological patients [124]. |
| Carpal Tunnel Questionnaire scales (CTQ) | The CTQ is a patient-reported outcome measure used to assess symptom severity and functional status of individuals with carpal tunnel syndrome (CTS) or other wrist-related issues. The CTQ comprises two subscales: the Symptom Severity Scale (SSS) and the Functional Status Scale (FSS). Symptom Severity Scale (SSS): The SSS is designed to evaluate the severity of symptoms associated with carpal tunnel syndrome or wrist problems. It includes questions about the frequency and intensity of symptoms, such as numbness, tingling, pain, and weakness, as well as their impact on sleep and daily activities. Patients rate their symptoms on a scale, typically ranging from one (mildest) to five (most severe). Functional Status Scale (FSS): The FSS assesses the patient’s functional status and their ability to perform daily activities involving the affected hand and wrist. It consists of questions related to activities, such as writing, buttoning clothes, gripping objects, and carrying out household tasks. Patients rate their ability to perform these activities on a scale, typically ranging from one (no difficulty) to five (unable to do) [129,130]. | Patients with carpal tunnel syndrome (CTS) and after wrist surgery [122], neurological patients, and stroke [129,131,132]. |
| Upper Extremity Function Scale (UEFS) | The UEFS is a patient-reported outcome measure used to evaluate the impact of upper limb impairment on the ability of patients to perform daily activities. The scale is applicable to both orthopedic and neurological conditions affecting the upper extremity function [88]. The UEFS comprises eight activities that involve the use of the upper extremity. These activities include writing, sleeping, washing dishes, lifting small objects with fingers, driving a car for more than 30 min, opening doors, taking a milk jug out of the refrigerator, and opening jars [91]. | Neurological and orthopedic [91]. |
| Stroke Upper Limb Capacity Scale (SULCS) | The SULCS is a clinical assessment tool designed to evaluate the functional capacity of the upper limb in stroke patients. It consists of 10 items that reflect a range of daily living activities, from simple to more complex tasks, involving the upper extremity. The SULCS is divided into three categories, assessing different aspects of upper limb function: Proximal Functioning (three items): These items evaluate the ability to perform activities that primarily involve the shoulder and elbow joints. Basic Hand and Finger Control (four items): These items assess the ability to perform tasks requiring basic grasp and manipulation skills with the hand and fingers. Advanced Distal Functioning (three items): These items evaluate the ability to perform more complex tasks involving precise finger movements and dexterity [133,134]. | Neurological [133,134]. |
| Frenchay Arm Test (FAT) | The FAT is a clinical assessment tool used to evaluate activity limitations in the upper extremity, particularly among stroke patients. It is designed to assess the patient’s ability to perform functional tasks that involve of manipulation of objects. The test consists of five tasks: Holding a ruler with the affected hand while drawing lines with the unaffected hand; Grasping and lifting a cylindrical object (e.g., a glass or cup) and performing a drinking motion; Picking up a small object, such as a paper clip, and placing it onto a surface; Grasping a comb and performing a combing motion. Picking up and placing a paper clip onto the edge of a sheet of paper [135]. | Post-stroke neurological patients with spasticity after botulinum toxin injection [136,137]. |
| ABILHAND questionnaire | The ABILHAND is designed to assess manual dexterity and hand function. This assessment is conducted through a structured interview process. It consists of questions related to 23 bimanual activities, which the patient evaluates as impossible, difficult, or easy [138,139]. | Neurological, stroke, and rheumatoid arthritis [1,138]. |
| Stroke Impact Scale Hand (SIS Hand) | Part of the Stroke Impact Scale which assesses eight domains: mobility, communication, emotions, strength, hand function, memory, thinking, participation, and ability to perform independent activities of daily living, the SIS Hand focuses on hand function and dexterity. It consists of five items that evaluate the ability to perform tasks, such as carrying heavy objects or opening jar [140]. | Neurological and stroke [140,141]. |
| Purdue Pegboard Test | The Purdue Pegboard Test is a time-based assessment designed to evaluate an individual’s manual dexterity and hand-eye coordination. The test involves placing as many pegs as possible into the holes on a specialized board within a 30 s timeframe. This is followed by folding pegs, pads, and collars as quickly as possible within a 1 min interval. The tasks are performed individually with each hand and then simultaneously with both hands [142,143]. | Originally developed for occupational physicians to assess the manual dexterity of candidates for industrial assembly line work, the Purdue Pegboard Test has since been adapted for broader applications. It is now used to evaluate the progress of orthopedic patients recovering from hand injuries and surgeries, as well as neurological patients undergoing rehabilitation [142,143,144]. |
| Sollerman Hand Function Test (SHFT) | The SHFT is a comprehensive assessment designed to evaluate the quality of hand movements, with a particular emphasis on grasping skills, within a specified time frame. The test consists of 20 subtests, each targeting various hand-related tasks that simulate daily activities [145]. The subtests mimic real-life tasks (cutting with scisors, buttoning and unbuttoning, etc.) and are administered by a trained professional. | Surgical, post-injury, orthopedic rheumatoid arthritis, and neurological after stroke [134,135,136,145,146,147]. |
| Reaching Performance Scale for Stroke (RPSS) | The RPSS is used to assess the quality of movement during two tasks of reaching and grasping with the upper limb and compensatory movements. During the tasks, the patient is trying to reach objects that are far away and close by [148]. A scale used to characterize improvements in upper limb motor skills [149]. | Neurological patients with hemiparesis and patients after stroke [148,149]. |
| Lovett scale | A five-grade scale for measuring muscle strength [150]. | Neurological patients, after stroke, and patients with reduced muscle strength [150,151]. |
| Outcome Measures | Muscle Strength | Range of Motion | Muscle Tension | Execution of the Movement | Grasping | Dexterity | Coordination | Manual Skills | Manipulation of Objects | Self-Report Questionnaire | Pain |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BBT | + | + | + | + | + | + | |||||
| ARAT | + | + | + | + | + | ||||||
| CAHAI | + | + | + | + | + | + | |||||
| FM-UE | + | + | + | + | + | + | + | ||||
| 9-HPT | + | + | + | + | + | ||||||
| WMFT | + | + | + | + | + | + | + | ||||
| MI | + | + | + | + | |||||||
| MAL | + | + | + | + | + | + | |||||
| AS | + | ||||||||||
| MAS | + | ||||||||||
| TS | + | ||||||||||
| MTS | + | ||||||||||
| Ad-AHA | + | + | + | + | + | + | |||||
| JHFT | + | + | + | + | + | + | |||||
| MHQ | + | + | + | + | + | + | + | + | |||
| DHI | + | + | + | + | + | + | + | ||||
| DASH | + | + | + | + | + | + | + | + | |||
| PRWE | + | + | + | + | + | + | + | + | |||
| CTQ | + | + | + | + | + | + | + | + | |||
| UEFS | + | + | + | + | + | + | |||||
| SULCS | + | + | + | + | + | + | |||||
| FAT | + | + | + | + | + | ||||||
| ABILHAND | + | + | + | + | + | + | + | ||||
| SISHAND | + | + | + | + | + | + | + | + | |||
| PEGBOARD | + | + | + | + | + | + | |||||
| SHFT | + | + | + | + | + | + | |||||
| RPSS | + | + | + | + | + | ||||||
| LOVETT | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marek, K.; Redlicka, J.; Miller, E.; Zubrycki, I. Objectivizing Measures of Post-Stroke Hand Rehabilitation through Multi-Disciplinary Scales. J. Clin. Med. 2023, 12, 7497. https://doi.org/10.3390/jcm12237497
Marek K, Redlicka J, Miller E, Zubrycki I. Objectivizing Measures of Post-Stroke Hand Rehabilitation through Multi-Disciplinary Scales. Journal of Clinical Medicine. 2023; 12(23):7497. https://doi.org/10.3390/jcm12237497
Chicago/Turabian StyleMarek, Klaudia, Justyna Redlicka, Elżbieta Miller, and Igor Zubrycki. 2023. "Objectivizing Measures of Post-Stroke Hand Rehabilitation through Multi-Disciplinary Scales" Journal of Clinical Medicine 12, no. 23: 7497. https://doi.org/10.3390/jcm12237497
APA StyleMarek, K., Redlicka, J., Miller, E., & Zubrycki, I. (2023). Objectivizing Measures of Post-Stroke Hand Rehabilitation through Multi-Disciplinary Scales. Journal of Clinical Medicine, 12(23), 7497. https://doi.org/10.3390/jcm12237497








