Effect of Repeated Greater Occipital Nerve Block in Patients with Ocular Neuropathic Pain: A Retrospective Observational Study
Abstract
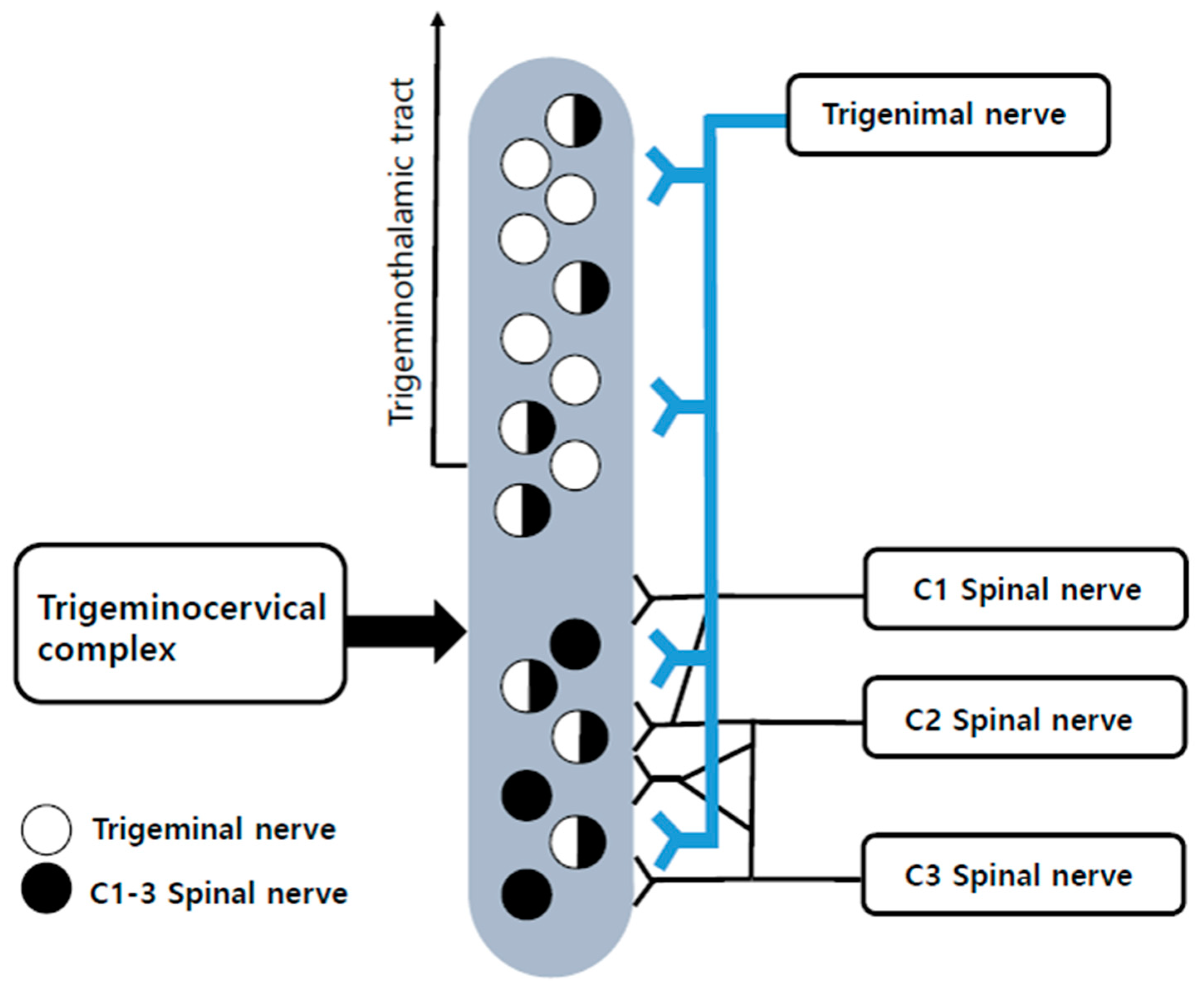
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
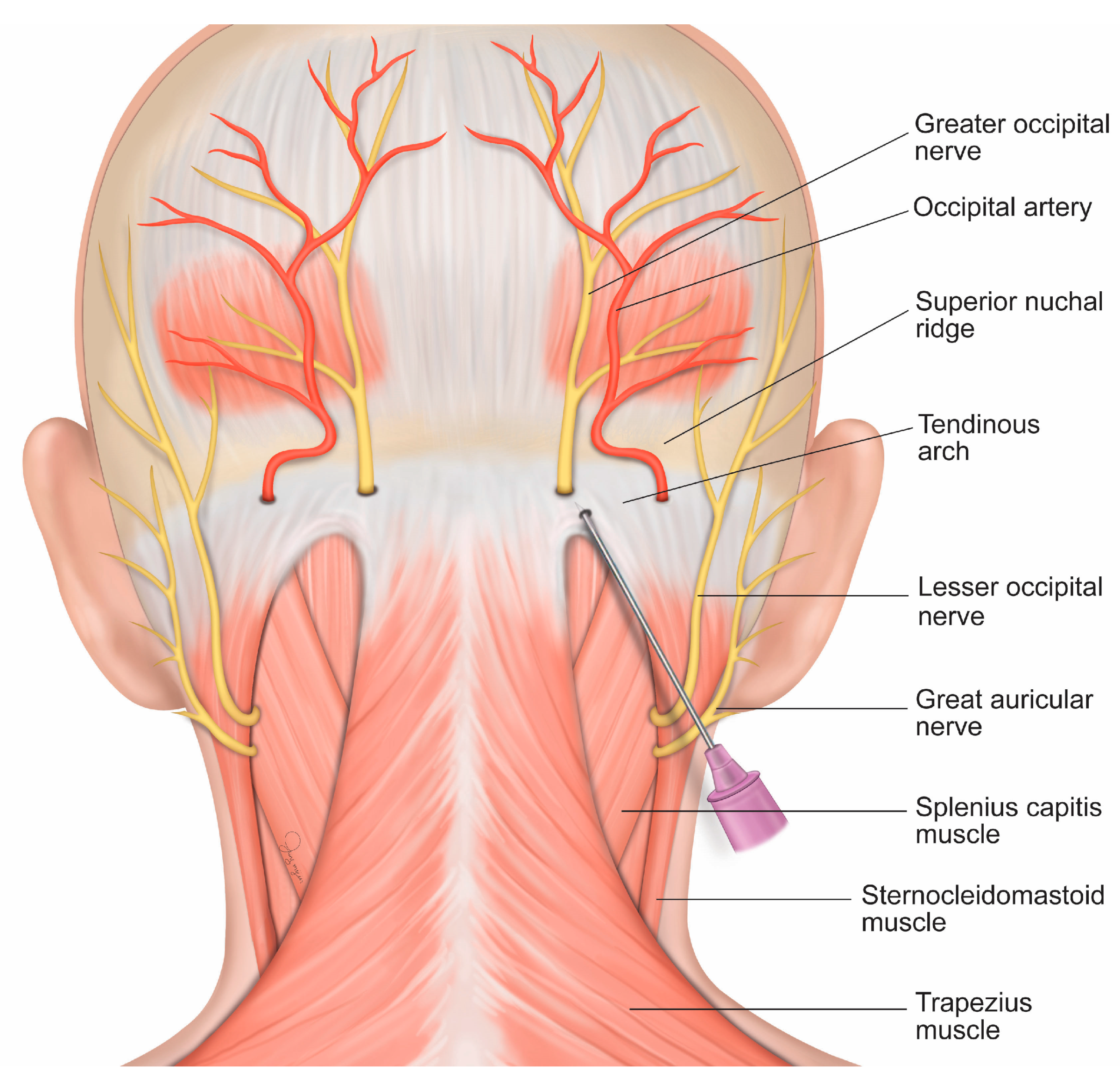
2.3. Intervention
2.4. Outcome Measurement
2.5. Statistical Analysis
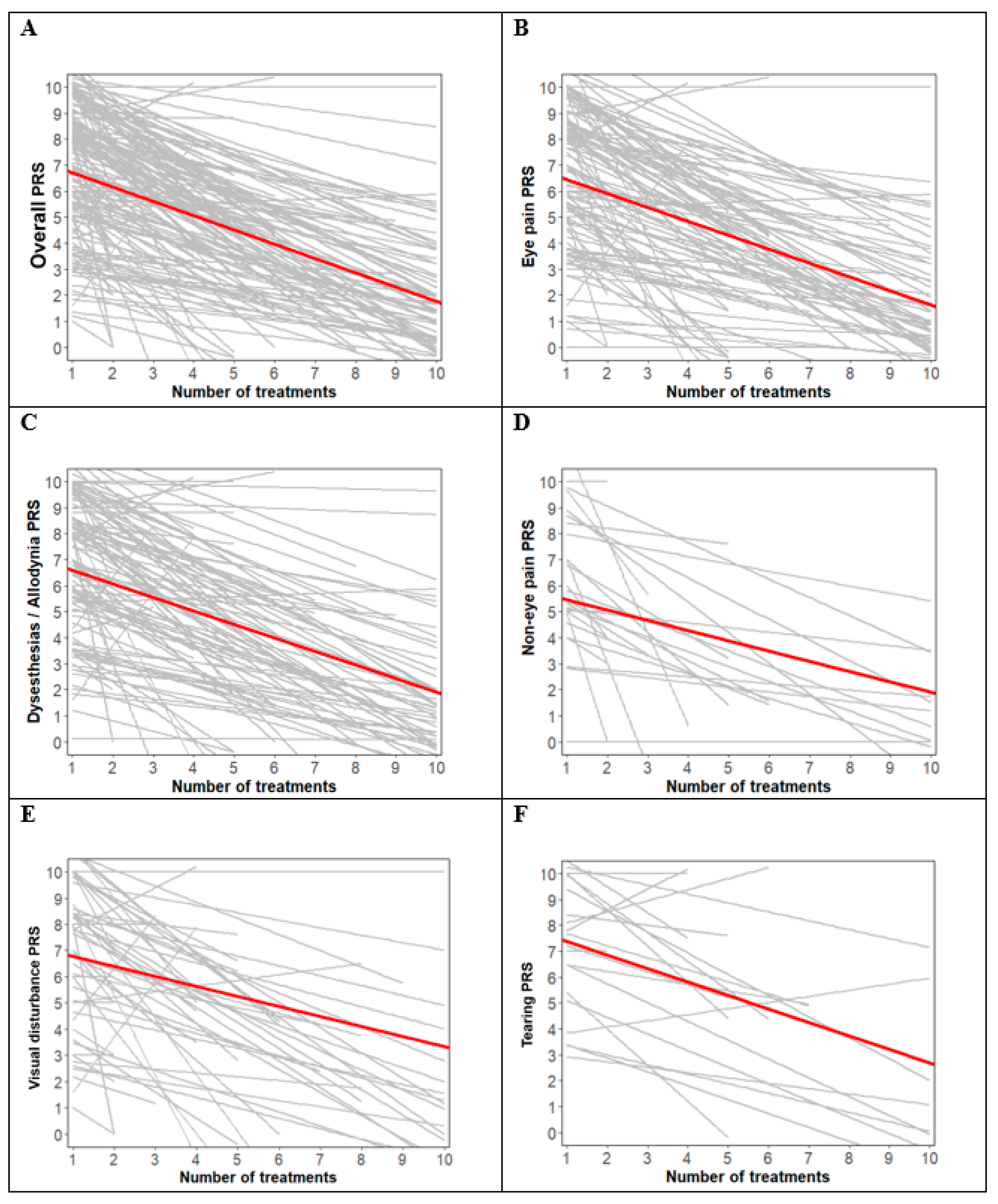
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic corneal pain: Approaches for management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Hamrah, P. Understanding neuropathic corneal pain—Gaps and current therapeutic approaches. Semin. Ophthalmol. 2016, 31, 59–70. [Google Scholar] [CrossRef]
- Friedman, N.J. Impact of dry eye disease and treatment on quality of life. Curr. Opin. Ophthalmol. 2010, 21, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Levitt, R.C.; Felix, E.R.; Sarantopoulos, C.D. Understanding the true burden of dry eye disease. Expert Rev. Ophthalmol. 2015, 10, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Kheirkhah, A.; Cavalcanti, B.M.; Cruzat, A.; Colon, C.; Brown, E.; Borsook, D.; Prüss, H.; Hamrah, P. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: Efficacy and evaluation with in vivo confocal microscopy. Ocul. Surf. 2015, 13, 250–262. [Google Scholar] [CrossRef]
- Ellis, B.D.; Kosmorsky, G.S. Referred ocular pain relieved by suboccipital injection. Headache 1995, 35, 101–103. [Google Scholar] [CrossRef]
- Armbrust, K.R.; Kosmorsky, G.S.; Lee, M.S.; Friedman, D.I. A pain in the eye. Surv. Ophthalmol. 2014, 59, 474–477. [Google Scholar] [CrossRef]
- Piovesan, E.; Kowacs, P.; Tatsui, C.; Lange, M.; Ribas, L.; Werneck, L. Referred pain after painful stimulation of the greater occipital nerve in humans: Evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia 2001, 21, 107–109. [Google Scholar] [CrossRef]
- Naja, Z.M.; El-rajab, M.; Al-tannir, M.A.; Ziade, F.M.; Tawfik, O.M. Repetitive occipital nerve blockade for cervicogenic headache: Expanded case report of 47 adults. Pain Pract. 2006, 6, 278–284. [Google Scholar] [CrossRef]
- Kurose, M.; Meng, I.D. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J. Neurophysiol. 2013, 109, 2517–2522. [Google Scholar] [CrossRef][Green Version]
- Burstein, R.; Yamamura, H.; Malick, A.; Strassman, A.M. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 1998, 79, 964–982. [Google Scholar] [CrossRef]
- Peres, M.F.; Stiles, M.A.; Siow, H.C.; Rozen, T.D.; Young, W.B.; Silberstein, S.D. Greater occipital nerve blockade for cluster headache. Cephalalgia 2002, 22, 520–522. [Google Scholar] [CrossRef]
- Galor, A.; Levitt, R.C.; Felix, E.R.; Martin, E.R.; Sarantopoulos, C.D. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye 2015, 29, 301–312. [Google Scholar] [CrossRef]
- Karadaş, Ö.; Özön, A.Ö.; Özçelik, F.; Özge, A. Greater occipital nerve block in the treatment of triptan-overuse headache: A randomized comparative study. Acta Neurol. Scand. 2017, 135, 426–433. [Google Scholar] [CrossRef]
- Tang, Y.; Kang, J.; Zhang, Y.; Zhang, X. Influence of greater occipital nerve block on pain severity in migraine patients: A systematic review and meta-analysis. Am. J. Emerg. Med. 2017, 35, 1750–1754. [Google Scholar] [CrossRef]
- Cuadrado, M.L.; Aledo-Serrano, Á.; Navarro, P.; López-Ruiz, P.; Fernández-De-Las-Peñas, C.; González-Suárez, I.; Orviz, A.; Fernández-Pérez, C. Short-term effects of greater occipital nerve blocks in chronic migraine: A double-blind, randomised, placebo-controlled clinical trial. Cephalalgia 2017, 37, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Gaul, C.; Roguski, J.; Dresler, T.; Abbas, H.; Totzeck, A.; Görlinger, K.; Diener, H.-C.; Weber, R. Efficacy and safety of a single occipital nerve blockade in episodic and chronic cluster headache: A prospective observational study. Cephalalgia 2017, 37, 873–880. [Google Scholar] [CrossRef]
- Trescot, A.M. Headache management in an interventional pain practice. Pain Physician 2000, 3, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimiadib, N.; Yousefshahi, F.; Abdi, P.; Ghahari, M.; Modjtahedi, B.S. Ocular neuropathic pain: An overview focusing on ocular surface pains. Clin. Ophthalmol. 2020, 14, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lee, M.K.; Kim, J.E.; Kim, H.Z.; Park, S.H.; Tae, J.H.; Choi, S.S. Pain relief scale is more highly correlated with numerical rating scale than with visual analogue scale in chronic pain patients. Pain Physician 2015, 18, E195–E200. [Google Scholar] [PubMed]
- Levitt, A.E.; Galor, A.; Weiss, J.S.; Felix, E.R.; Martin, E.R.; Patin, D.J.; Sarantopoulos, K.D.; Levitt, R.C. Chronic dry eye symptoms after LASIK: Parallels and lessons to be learned from other persistent post-operative pain disorders. Mol. Pain 2015, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Matsuura, M.; Ando, F.; Sahashi, K.; Torii, Y.; Hirose, H. The effect of stellate ganglion block on prolonged post-operative ocular pain. Nihon. Ganka Gakkai Zasshi 2003, 107, 607–612. [Google Scholar] [CrossRef][Green Version]
- Piovesan, E.J.; Kowacs, P.A.; Oshinsky, M.L. Convergence of cervical and trigeminal sensory afferents. Curr. Pain Headache Rep. 2003, 7, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Goadsby, P.J. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 2003, 126, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Busch, V.; Jakob, W.; Juergens, T.; Schulte-Mattler, W.; Kaube, H.; May, A. Occipital nerve blockade in chronic cluster headache patients and functional connectivity between trigeminal and occipital nerves. Cephalalgia 2007, 27, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Winnie, A.P.; Candido, K.D. Chapter 18—Differential neural blockade for the diagnosis of pain. In Pain Management, 2nd ed.; Waldman, S.D., Ed.; Saunders: Philadelphia, PA, USA, 2011; pp. 150–161. [Google Scholar]
- Kanai, A.; Hiruma, H.; Katakura, T.; Sase, S.; Kawakami, T.; Hoka, S. Low-concentration lidocaine rapidly inhibits axonal transport in cultured mouse dorsal root ganglion neurons. Anesthesiology 2001, 95, 675–680. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Matro, R.; Shaw, J.W.; Abbas, M.A.; Silberstein, S.D. Greater occipital nerve block using local anaesthetics alone or with triamcinolone for transformed migraine: A randomised comparative study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 415–417. [Google Scholar] [CrossRef]
- Busch, V.; Jakob, W.; Juergens, T.; Schulte-Mattler, W.; Kaube, H.; May, A. Functional connectivity between trigeminal and occipital nerves revealed by occipital nerve blockade and nociceptive blink reflexes. Cephalalgia 2006, 26, 50–55. [Google Scholar] [CrossRef]
- Haanpää, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; Iannetti, G.D.; et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011, 152, 14–27. [Google Scholar] [CrossRef]
- Spies, D.; Renz, P.F.; Beyer, T.A.; Ciaudo, C. Comparative analysis of differentail gene expression tools for RNA sequencing time course data. Brief. Bioinform 2017, 20, 288–298. [Google Scholar] [CrossRef]
- Kissoon, N.R.; O’Brien, T.G.; Bendel, M.A.; Eldrige, J.S.; Hagedorn, J.M.; Mauck, W.D.; Moeschler, S.M.; Olatoye, O.O.; Pittelkow, T.P.; Watson, J.C.; et al. Comparative effectiveness of landmark-guided greater occipital nerve (GON) block at the superior nuchal line versus ultrasound-guided GON block at the level of C2: A randomized clinical trial (RCT). Clin. J. Pain 2022, 38, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Ko, S.Y.; Bang, M.R.; Jeon, W.J.; Cho, S.Y.; Yeom, J.H.; Shin, W.J.; Kim, K.H. Ultrasound-guided greater occipital nerve block for patients with occipital headache and short term follow up. Korean J. Anesthesiol. 2011, 61, 50–54. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 204) | |
|---|---|
| Sex | |
| Male | 28 (13.7) |
| Female | 176 (86.3) |
| Age, years | 62.50 (55.00–71.00) |
| Height, cm | 158.00 (152.00–162.00) |
| Weight, kg | 58.50 (50.00–62.00) |
| Body Mass Index, kg/m2 | 23.02 (21.06–24.85) |
| History of ONP (months) | 12.00 (3.00–36.00) |
| Surgical history & trauma | |
| Cataract surgery | 76 (37.3) |
| Refractive surgery | 25 (12.3) |
| Eyelid surgery | 26 (12.7) |
| Other eye surgery | 2 (1.0) |
| Trauma | 3 (1.5) |
| Underlying eye diseases | |
| Dry eye | 131 (64.2) |
| Keratitis | 36 (17.6) |
| Corneal ulcer | 7 (3.4) |
| Conjunctivitis | 61 (29.9) |
| Blepharitis | 26 (12.7) |
| Uveitis | 3 (1.5) |
| Tumor | 1 (0.5) |
| Underlying systemic diseases | |
| Diabetes | 14 (6.9) |
| Fibromyalgia | 2 (1.0) |
| Depression | 5 (2.5) |
| Sjögren’s syndrome | 5 (2.5) |
| Symptoms | |
| Eye pain | 147 (72.1) |
| Dysesthesia/Allodynia | 138 (67.6) |
| Noneye pain | 26 (12.7) |
| Visual disturbance | 67 (32.8) |
| Tearing | 18 (8.8) |
| Initial NRS (overall) | 5.00 (4.00–7.00) |
| Initial NRS (eye pain) | 5.00 (4.00–8.00) |
| Initial NRS (dysesthesias/allodynia) | 5.00 (4.00–7.00) |
| Initial NRS (noneye pain) | 6.00 (4.00–8.00) |
| Initial NRS (visual disturbance) | 5.00 (4.00–6.00) |
| Initial NRS (tearing) | 4.00 (3.00–6.50) |
| All Patients (n = 204) | |
|---|---|
| Recurrence | 2 (1.0) |
| Adverse events | |
| Neuritis | 4 (2.0) |
| Infection | 0 (0.0) |
| Transient facial hypoesthesia | 2 (1.0) |
| Dizziness | 0 (0.0) |
| Nausea | 0 (0.0) |
| Skin irritation | 1 (0.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, W.; Choi, J.; Lee, G.; Ma, S.; Lee, S.; Park, S. Effect of Repeated Greater Occipital Nerve Block in Patients with Ocular Neuropathic Pain: A Retrospective Observational Study. J. Clin. Med. 2023, 12, 7454. https://doi.org/10.3390/jcm12237454
Lee J, Park W, Choi J, Lee G, Ma S, Lee S, Park S. Effect of Repeated Greater Occipital Nerve Block in Patients with Ocular Neuropathic Pain: A Retrospective Observational Study. Journal of Clinical Medicine. 2023; 12(23):7454. https://doi.org/10.3390/jcm12237454
Chicago/Turabian StyleLee, Jonghwan, Woochan Park, Jinyoung Choi, Geonho Lee, Seokhyun Ma, Seungcheol Lee, and Sangyoong Park. 2023. "Effect of Repeated Greater Occipital Nerve Block in Patients with Ocular Neuropathic Pain: A Retrospective Observational Study" Journal of Clinical Medicine 12, no. 23: 7454. https://doi.org/10.3390/jcm12237454
APA StyleLee, J., Park, W., Choi, J., Lee, G., Ma, S., Lee, S., & Park, S. (2023). Effect of Repeated Greater Occipital Nerve Block in Patients with Ocular Neuropathic Pain: A Retrospective Observational Study. Journal of Clinical Medicine, 12(23), 7454. https://doi.org/10.3390/jcm12237454







