Abstract
Aim: The present study aimed to investigate the impact of mild tricuspid regurgitation (TR) on the exercise capacity or clinical outcomes in patients with chronic heart failure (CHF). Methods and Results: The study enrolled 511 patients with CHF who underwent cardiopulmonary exercise testing (CPET) between 2013 and 2018. The primary outcome was a composite of heart failure hospitalization and death. Patients with mild TR (n = 324) or significant TR (moderate or greater; n = 60) displayed worse NHYA class and reduced exercise capacity on CPET than those with non-TR (n = 127), but these were more severely impaired in patients with significant TR. A total of 90 patients experienced events over a median follow-up period of 3.3 (interquartile range 0.8–5.5) years. Patients with significant TR displayed a higher risk of events, while patients with mild TR had a 3.0-fold higher risk of events than patients with non-TR (hazard ratio (HR) 3.01; 95% confidence interval (CI), 1.50–6.07). Multivariate Cox regression analysis showed that, compared with non-TR, mild TR was associated with increased adverse events, even after adjustment for co-variates (HR 2.97; 95% CI, 1.35–6.55). Conclusions: TR severity was associated with worse symptoms, reduced exercise capacity, and poor clinical outcomes. Even patients with mild TR had worse clinical characteristics than those with non-TR.
1. Introduction
Tricuspid regurgitation (TR) is commonly observed in patients with cardiovascular disease (CVD) [,,]. In patients with chronic heart failure (CHF), TR typically develops secondarily to right heart remodeling and dilation caused by elevated left ventricular (LV) filling pressure due to LV systolic or diastolic dysfunction, subsequent post-capillary pulmonary hypertension (PH), and pulmonary vascular remodeling (i.e., combined pre- and post-capillary PH (CpcPH)), which, in turn, leads to more severe right ventricular (RV) afterload [,].
Previous studies have reported that among patients with CVD, those with moderate and significant TR displayed worse symptoms and poorer clinical outcomes than those without significant TR [,,,,,,]. Moreover, a recent study, using the National Echocardiography Database of Australia, demonstrated that even mild TR was independently associated with a significant increase in mortality in individuals who underwent echocardiography with or without CHF []. The majority of patients with CHF have mild TR rather than significant TR; nevertheless, the adverse effects of mild TR in patients with CHF are not well delineated.
We hypothesized that patients with mild TR have worse clinical characteristics, such as impaired functional capacity, and clinical outcomes than patients without TR. Therefore, investigating these clinical parameters may be of use in the risk stratification of patients with CHF. To test this hypothesis, we investigated the impact of mild TR on exercise intolerance and clinical outcomes in patients with CHF who underwent cardiopulmonary exercise testing (CPET).
2. Methods
2.1. Study Design
This was a single-center, observational, retrospective study that included consecutive patients whose exercise capacity was assessed by upright CPET using a ramp protocol between January 2013 and December 2018. CHF was defined as the evidence of heart failure (HF) based on the current European Society of Cardiology (ESC) diagnostic guideline [] or previous HF-related hospitalization. All patients underwent CPET for assessing the exercise capacity were screened eligibility. Among the eligible patients, we excluded (1) patients without data of outcomes; (2) patients without CHF (defined above); and (3) patients with no echocardiography or B-type natriuretic peptide within 12 months from the day of CPET.
The study protocol was approved by the Ethics Committee of Hokkaido University Hospital (IRB No. 022-0048). The investigation conformed with the principles outlined in the Declaration of Helsinki. All the authors had full access to the data and take responsibility for its integrity.
2.2. Study Population
From the 707 consecutively enrolled patients who underwent CPET in this study, patients without outcome data (n = 10), echocardiography, or B-type natriuretic peptide (BNP) measurements within 12 months from the day of CPET (n = 69)or those who did not meet the HF criteria (n = 117) were excluded. Ultimately, 511 patients were examined. Patients were then divided into three groups according to the severity of TR: non-TR, mild TR, and significant TR (defined as moderate or severe TR) []. A study diagram of patient enrollment and grouping is shown in Figure 1.
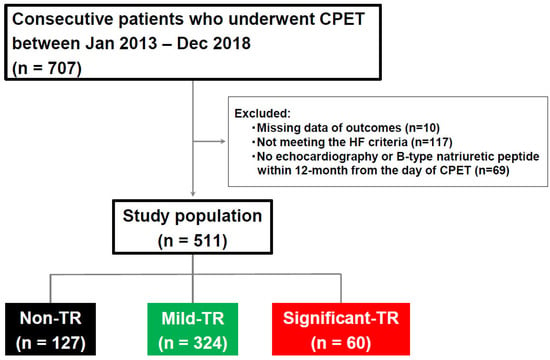
Figure 1.
Flow diagram of the present study. Study population was divided into three groups by the severity of TR. CPET, cardiopulmonary exercise testing; HF, heart failure; TR, tricuspid regurgitation.
2.3. Cardiopulmonary Exercise Testing
A symptom-limited standard upright cycle ergometry exercise using a cycle ergometer was performed, as described previously [].
2.4. Cardiac Structure, Function, and Hemodynamics
Two-dimensional, M-mode, doppler, and tissue doppler echocardiography were performed according to the American Society of Echocardiography guidelines []. TR severity was classified as non (including trivial), mild, and significant (moderate or greater) according to the current recommendations for noninvasive evaluation of native valvular regurgitation []. The etiology of TR was defined as either primary or secondary. Primary TR was classified as documented congenital or organic causes of TR, and the others were classified as secondary TR [].
The method of invasive hemodynamics assessment has been described previously [].
2.5. Outcome Assessment
The primary outcome of interest was the composite of hospitalization due to worsening HF and death. The definition of death was all-cause death and that of hospitalization was hospitalization due to worsening heart failure. HF hospitalizations and mortality data were ascertained from medical records adjudicated by a single cardiologist (K.N.). Patient follow-up was initiated on the day of CPET.
2.6. Statistical Analyses
Continuous variables are presented as means ± standard deviations when normally distributed and as medians and interquartile ranges (IQRs) when non-normally distributed. Group differences were first compared using one-way ANOVA, the Kruskal–Wallis H test, or χ2 test, as appropriate. The Tukey honestl significant difference test or Steel–Dwass test was then used to compare between the individual groups. Event rates were compared using Kaplan–Meier curve analysis. Risk of the composite outcome was compared among the three patient groups according to the severity of TR (non-TR, mild TR, and significant TR). A Cox proportional hazards model was used to calculate the hazard ratio (HR) with a corresponding 95% confidence interval (95%CI), with adjustments for age, sex, moderate or greater mitral regurgitation (MR), and E/e’ ratio, which were the same co-variates used in a previous study [].
A two-sided p value of <0.05 was considered statistically significant. All data were analyzed using JMP 16.0 (SAS Institute Inc., Cary, NC, USA) and Stata MP64 version 15 (StataCorp, College Station, TX, USA).
3. Results
3.1. Baseline Characteristics
Overall, 25% of patients with CHF had non-TR, 63% had mild TR, and 12% had significant TR (Figure 2). Patients with TR were older with lower body mass index and had a higher prevalence of atrial fibrillation, moderate or greater MR, and poorer kidney function as compared with those with non-TR (Table 1). Patients with significant TR displayed lower prevalence of ischemic heart disease and significant aortic regurgitation as compared with other groups. There was no significant difference in terms of heart failure types classified by LVEF among the three groups. Patients with TR displayed higher plasma BNP levels, but patients with significant TR displayed higher BNP as compared to those with mild TR. Patients with significant TR displayed smaller LV diastolic dimension with larger LV mass index as compared to patients with non-TR or mild TR. Left atrial (LA) volume, RV dimension, and TR velocity were greater in patients with TR, but these abnormalities were the greatest in significant TR patients. Symptoms of HF assessed by NYHA functional class were worse in patients with mild TR than in patients with non-TR but were the worst in patients with significant TR (Figure 2).
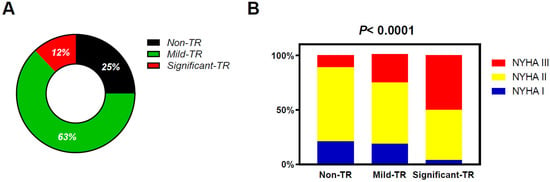
Figure 2.
Distribution patterns of the TR severity in this study population (A) and the relation between the TR severity and NHYA functional class (B). TR, tricuspid regurgitation.

Table 1.
Baseline vharacteristics.
3.2. Invasive Hemodynamics
Patients with significant TR displayed higher right atrial pressure (RAP) with higher pulmonary artery (PA) pressure as compared to those with non- or mild TR, while pulmonary capillary wedge pressure (PCWP) and pulmonary vascular resistance (PVR) were similar across the three groups. Patients with TR displayed more severe RV afterload, such as reduced pulmonary artery compliance (PAC) and elevated PA Ea as compared with those with non-TR. These were more severely impaired in patients with significant TR as compared to those with mild TR (Table 1).
3.3. Cardiopulmonary Exercise Testing
Baseline heart rate was similar among the three groups, while peak heart rate and peak systolic blood pressure were lower in patients with significant TR as compared to those with the other two groups (Table 2). Patients with significant TR displayed lower peak oxygen consumption (VO2), anaerobic threshold, and O2 pulse compared to those with non- or mild TR. Patients with TR displayed higher minute ventilation (VE)/carbon dioxide volume (VCO2) slope and lower peak workload achievements as compared with those without TR, but these values were the most severely impaired in patients with significant TR (Figure 3, Table 2). Peak respiratory exchange ratio (RER) was similar across all groups and were all more than 1.10.

Table 2.
Cardiopulmonary exercise testing.
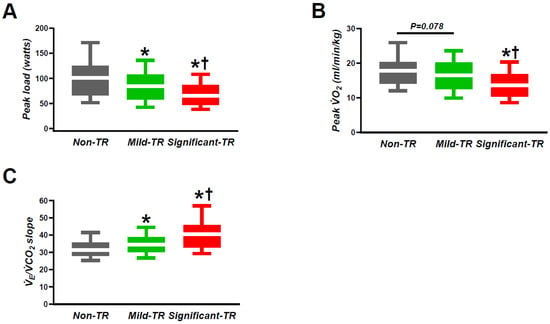
Figure 3.
The relation between TR severity and the representative parameters in CPET: (A) peak load; (B) peak VO2; (C) VE/VCO2 slope. CPET, cardiopulmonary exercise testing; TR, tricuspid regurgitation; VCO2, carbon dioxide volume; VO2, oxygen consumption; VE, minute ventilation. * p < 0.05 vs. non-TR. † p < 0.05 vs. mild TR.
3.4. Tricuspid Regurgitation Severity and Clinical Outcomes
Over a median follow-up period of 3.3 (IQR 0.8–5.5) years, 90 patients experienced the composite endpoint, including 35 deaths (7%) and 67 HF hospitalizations (13%). Patients with significant TR displayed a higher risk of events compared to patients with non-TR (HR 5.22; 95% CI, 2.35–11.6) and to patients with mild TR (HR 1.73; 95% CI, 1.03–2.93). However, patients with mild TR showed a 3.0–fold higher risk of events as compared with those without TR (HR 3.01; 95% CI, 1.50–6.07) (Figure 4). In multivariable Cox regressions analyses, mild TR was associated with an increased risk of morbidity and mortality as compared with that of non-TR (HR 2.97; 95% CI, 1.35–6.55), while significant TR was associated with higher risks of adverse outcomes as compared to those with mild TR (HR 2.04; 95% CI, 1.13–3.67), even after adjusting for age, sex, moderate or greater MR, and E/e’ ratio, which were the same co-variates used in a previous study (Table 3) [].
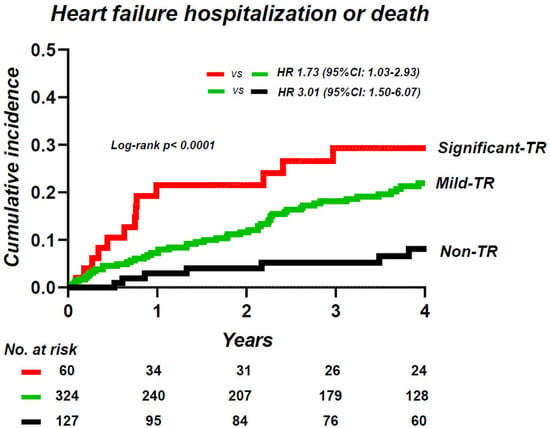
Figure 4.
Kaplan–Meier analysis of composite of heart failure hospitalization and death, categorized by the severity of TR. TR, tricuspid regurgitation.

Table 3.
Univariable and multivariable Cox proportional hazard model for composite events.
4. Discussion
The major finding of the present study was that the TR severity was associated with worse HF symptoms, reduced exercise capacity, and increased morbidity and mortality, and that in patients with CHF who underwent CPET, even patients with mild TR displayed worse clinical characteristics and outcomes than patients with non-TR. These findings suggest that the severity assessment of TR is useful for risk stratification in these patients.
4.1. Tricuspid Regurgitation in Chronic Heart Failure
In the early stages of HF, the LA compensates for LV dysfunction through its reservoir and booster functions as an important barrier between the LV and the lung []. However, as the disease progresses, dilation and dysfunction of LA advance over time. This progression leads to LA myopathy and is associated with post-capillary PH that increases RV afterload and leads to right-side heart dysfunction and the development of atrial and/or ventricle functional TR due to the dilatation of the annulus [,,]. In the present study, patients with mild TR displayed greater LA volume and more severe pulsatile pulmonary vascular load (lower PAC and higher PA Ea) and greater RV dilation than those of non-TR patients. These parameters were more severely impaired in patients with significant TR than in the other groups.
4.2. Tricuspid Regurgitation and Functional Capacity
Many patients with TR are often asymptomatic [], but in the advanced stages of the disease, patients display edema, anorexia, chronic liver or kidney congestion, or exertional fatigue [,]. Although the cause of impaired exercise capacity in TR patients is complex, a previous study that investigated the hemodynamics of exercise on right heart catheterization with a simultaneous expired gas analysis demonstrated that impaired exercise capacity (e.g., lower peak VO2) was related to an inability to adequately increase cardiac output (CO) response to exercise, coupled with elevated right and left heart filling pressures in patients with symptomatic grade 3 or 4 TR without significant left heart disease [], wherein patients with mild TR were not emphasized. This impairment in perfusion was coupled with abnormal increases in left heart filling pressure that was related to elevated right heart pressure and enhanced diastolic ventricular interdependence (DVI) rather than a primary left heart lesion because LV transmural pressure (calculated as PCWP minus RAP) dropped with exercise, consistent with inadequate LV preload, despite elevated left-sided filling pressure [,,]. When RV and pericardial pressures are low, LV end-diastolic pressure (LVEDP) reflects LV preload (LV end-diastolic volume (LVEDV)). However, in the presence of right heart enlargement and pericardial restraint, LVEDP and LVEDV become uncoupled [,,]. This phenomenon is commonly observed in patients with significant TR and in those with persistent AF, obesity, RV dysfunction, or CpcPH [,,,]. Patients with these conditions demonstrate findings of exaggerated pericardial restraint and failure to augment CO, which may contribute to poor functional capacity. Similarly, in the present study, the LV ejection fraction was similar between all groups, but the RV dimension and LA volume were greater in patients with mild TR than in patients without TR, and these abnormalities were most prominent in significant TR patients. The enlargement of RV dimensions and LA volume leads to total heart enlargement that enhances the pericardial restraint and subsequent DVI. Therefore, these cardiac structural abnormalities may be related to a reduction in the augmented CO response to exercise, which further leads to reduced exercise capacity or worsening symptoms of HF.
4.3. Tricuspid Regurgitation and Clinical Outcomes in Chronic Heart Failure
Numerous studies have demonstrated that significant TR is associated with adverse outcomes in patients with CVD, such as HF with reduced EF, HF with preserved EF, left side valvular disease, or primary PAH [,,,,]. However, the available data are scarce on the prognostic impact of mild TR in patients with CVD including CHF, despite a study from the National Echocardiography Database of Australia that first reported that even mild TR was independently associated with a significant increase in mortality in individuals with or without HF []. Herein, we showed that TR severity is independently associated with HF hospitalization or death, and that, compared with non-TR, even mild TR can increase the risk of adverse events, suggesting that TR severity may be useful for risk stratification in patients with CHF.
There are several limitations in this study. This is a single-center study. The study sample size was small, thereby hindering our ability to generalize the findings. Since the study population was enrolled between 2013 and 2018, the use of angiotensin receptor-neprilysin inhibitors or sodium-glucose cotransporter 2 inhibitors was limited.
5. Conclusions
TR severity was associated with exercise intolerance and adverse outcomes, where even mild TR was related to worse HF symptoms, reduced exercise capacity, and increased risk of morbidity and mortality in patients with CHF, suggesting that TR severity may be useful for risk stratification in these patients. Further study is required to identify therapeutic methods targeting TR to improve outcomes in patients with CHF.
Author Contributions
Conceptualization, K.O. and Y.T. (Yoji Tamaki); Methodology, K.N., S.I., K.O., Y.Y., S.T., Y.T. (Yuki Takahashi), K.S., S.N., A.T., Y.K., T.S., K.K. and T.N.; Software, H.A.; Investigation, K.O.; Data curation, K.N., S.I., Y.Y., Y.M., S.T., Y.S., K.M., H.A., Y.T. (Yoji Tamaki), S.K., Y.T. (Yuki Takahashi), K.S., S.N., A.T., Y.K., T.S. and K.K.; Writing—original draft, K.N., S.I., K.O., Y.M., S.T., Y.S., K.M., H.A., Y.T. (Yoji Tamaki) and A.T.; Writing—review & editing, T.N. and T.A.; Visualization, K.O. and S.K.; Supervision, T.N. and T.A.; Funding acquisition, K.O. All authors have read and agreed to the published version of the manuscript.
Funding
Omote was supported by the JSPS KAKENHI Grant-in-Aid for Young Scientists Start-up (Grant no. 19K23955) and Young Scientists (Grant no. 20K17139 and 23K15089), the Fukuda Foundation for Medical Technology, the Daiwa Securities Health Foundation, and the Hokkaido Heart Association Grant for Research.
Institutional Review Board Statement
The study protocol was approved by the Ethics Committee of Hokkaido University Hospital (IRB No. 022-0048). The investigation conformed with the principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors wish to thank the staff of Hokkaido University Hospital Cardiopulmonary Exercise Test Laboratory for their invaluable support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akintoye, E.; Wang, T.K.M.; Nakhla, M.; Ali, A.H.; Fava, A.M.; Akyuz, K.; Popovic, Z.B.; Pettersson, G.B.; Gillinov, A.M.; Xu, B.; et al. Quantitative Echocardiographic Assessment and Optimal Criteria for Early Intervention in Asymptomatic Tricuspid Regurgitation. JACC. Cardiovasc. Imaging 2023, 16, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bartko, P.E.; Arfsten, H.; Frey, M.K.; Heitzinger, G.; Pavo, N.; Cho, A.; Neuhold, S.; Tan, T.C.; Strunk, G.; Hengstenberg, C.; et al. Natural History of Functional Tricuspid Regurgitation: Implications of Quantitative Doppler Assessment. JACC. Cardiovasc. Imaging 2019, 12, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Vanermen, H.; Maisano, F.; Guidotti, A.; La Canna, G.; Alfieri, O. The growing clinical importance of secondary tricuspid regurgitation. J. Am. Coll. Cardiol. 2012, 59, 703–710. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2019, 40, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Obokata, M.; Omote, K.; Iwano, H.; Ikoma, T.; Okada, K.; Yoshida, K.; Kato, T.; Kurosawa, K.; Nagai, T.; et al. Functional Tricuspid Regurgitation and Right Atrial Remodeling in Heart Failure With Preserved Ejection Fraction. Am. J. Cardiol. 2022, 162, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.J.; Nishimura, R.A.; Borlaug, B.A. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ. Heart Fail. 2014, 7, 911–917. [Google Scholar] [CrossRef]
- Amano, M.; Izumi, C.; Taniguchi, T.; Morimoto, T.; Miyake, M.; Nishimura, S.; Kitai, T.; Kato, T.; Kadota, K.; Ando, K.; et al. Impact of concomitant tricuspid regurgitation on long-term outcomes in severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 353–360. [Google Scholar] [CrossRef]
- Chen, L.; Larsen, C.M.; Le, R.J.; Connolly, H.M.; Pislaru, S.V.; Murphy, J.G.; McGoon, M.D.; Frantz, R.P.; Kane, G.C. The prognostic significance of tricuspid valve regurgitation in pulmonary arterial hypertension. Clin. Respir. J. 2018, 12, 1572–1580. [Google Scholar] [CrossRef]
- Maeder, M.T.; Holst, D.P.; Kaye, D.M. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 2008, 14, 824–830. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef]
- Nishi, H.; Takahashi, T.; Matsumiya, G.; Takano, H.; Ichikawa, H.; Miyagawa, S.; Sawa, Y. Preoperative assessment of congestive liver dysfunction using technetium-99 m galactosyl human serum albumin liver scintigraphy in patients with severe valvular heart disease. Surg. Today 2007, 37, 564–569. [Google Scholar] [CrossRef]
- Offen, S.; Playford, D.; Strange, G.; Stewart, S.; Celermajer, D.S. Adverse Prognostic Impact of Even Mild or Moderate Tricuspid Regurgitation: Insights from the National Echocardiography Database of Australia. J. Am. Soc. Echocardiogr. 2022, 35, 810–817. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Shimono, Y.; Ishizaka, S.; Omote, K.; Nakamura, K.; Yasui, Y.; Mizuguchi, Y.; Takenaka, S.; Aoyagi, H.; Tamaki, Y.; Sato, T.; et al. Impact of Cardiac Power Output on Exercise Capacity and Clinical Outcome in Patients With Chronic Heart Failure. Am. J. Cardiol. 2023, 206, 4–11. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Borlaug, B.A. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur. Heart J. 2018, 39, 2825–2835. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Reddy, Y.N.V. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Carter, R.E.; Melenovsky, V.; DeSimone, C.V.; Gaba, P.; Killu, A.; Naksuk, N.; Lerman, L.; Asirvatham, S.J. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2017, 10, e003612. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Schaff, H.V.; Pochettino, A.; Pedrotty, D.M.; Asirvatham, S.J.; Abel, M.D.; Carter, R.E.; Mauermann, W.J. Pericardiotomy Enhances Left Ventricular Diastolic Reserve with Volume Loading in Humans. Circulation 2018, 138, 2295–2297. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).