Ramsay Hunt Syndrome in Asymptomatic COVID-19 Infection: A Case Report and a Literature Review
Abstract
:1. Introduction
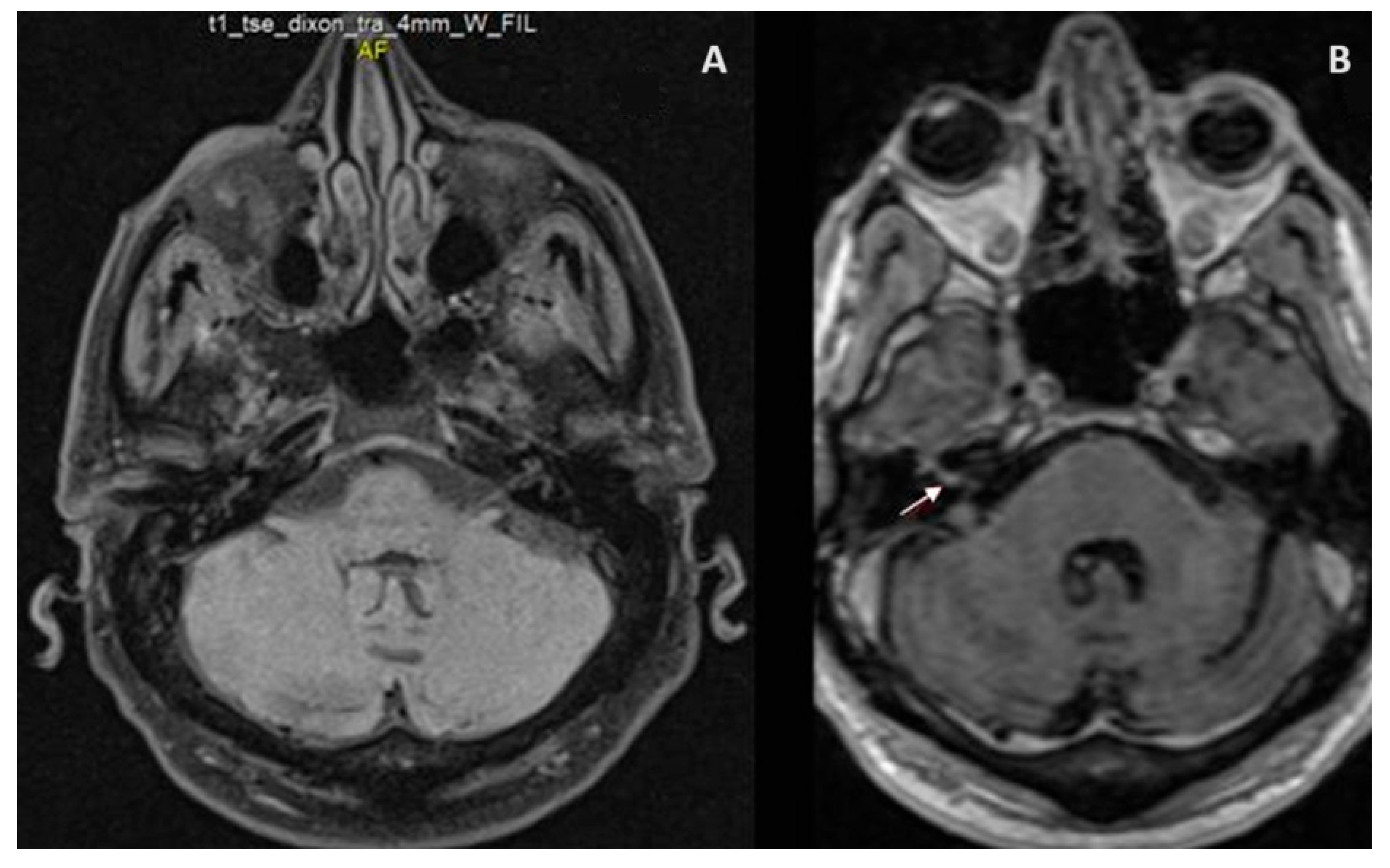
2. Case Presentation
3. Discussion
3.1. Available Case Reports of Ramsay Hunt Syndrome in the Context of COVID-19
3.2. Clinical Presentation of Ramsay Hunt Syndrome in the Context of COVID-19 Infection
3.3. Pathophysiology of Ramsay Hunt Syndrome in the Context of COVID-19
3.4. Evaluation of Ramsay Hunt Syndrome in the Context of COVID-19
3.5. Management of Ramsay Hunt Syndrome in the Context of COVID-19
3.6. Clinical Outcomes and Prognostic Factors of Ramsay Hunt Syndrome in the Context of COVID-19
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, M.; Jain, A.; Mehta, N. Ramsay Hunt Syndrome: Viral Infection Coexisting with COVID-19. Ann. Otol. Neurotol. 2021, 4, 79–82. [Google Scholar] [CrossRef]
- Codeluppi, L.; Venturelli, F.; Rossi, J.; Fasano, A.; Toschi, G.; Pacillo, F.; Cavallieri, F.; Giorgi Rossi, P.; Valzania, F. Facial palsy during the COVID-19 pandemic. Brain Behav. 2021, 11, e01939. [Google Scholar] [CrossRef]
- Costello, F.; Dalakas, M.C. Cranial neuropathies and COVID-19: Neurotropism and autoimmunity. Neurology 2020, 95, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Silva, M.T.T.; Soares, C.N.; Coutinho, R.; Oliveira, H.S.; Afonso, L.; Espíndola, O.; Leite, A.C.; Araujo, A. Peripheral facial nerve palsy associated with COVID-19. J. Neurovirol. 2020, 26, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Algaadi, S.A. Herpes zoster and COVID-19 infection: A coincidence or a causal relationship? Infection 2022, 50, 289–293. [Google Scholar] [CrossRef]
- Maia, C.M.F.; Marques, N.P.; de Lucena, E.H.G.; de Rezende, L.F.; Martelli, D.R.B.; Martelli-Júnior, H. Increased number of Herpes Zoster cases in Brazil related to the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 104, 732–733. [Google Scholar] [CrossRef]
- Shafiee, A.; Amini, M.J.; Arabzadeh Bahri, R.; Jafarabady, K.; Salehi, S.A.; Hajishah, H.; Mozhgani, S.H. Herpesviruses reactivation following COVID-19 vaccination: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 278. [Google Scholar] [CrossRef]
- Katsikas Triantafyllidis, K.; Giannos, P.; Mian, I.T.; Kyrtsonis, G.; Kechagias, K.S. Varicella Zoster Virus Reactivation Following COVID-19 Vaccination: A Systematic Review of Case Reports. Vaccines 2021, 9, 1013. [Google Scholar] [CrossRef]
- Hunt, J.R. On herpetic inflammmation of the geniculate ganglion. A new syndrome and its complications. Nerv. Ment. Dis. 1907, 34, 73–96. [Google Scholar] [CrossRef]
- Crouch, A.E.; Hohman, M.H.; Moody, M.P.; Andaloro, C. Ramsay Hunt Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Alonzo-Correa, C.; Camacho-Martínez, E.; Bustamante-Arias, A.; Rodriguez-Garcia, A. Ramsay Hunt syndrome in a young COVID-19 patient. Pan Am. J. Ophthalmol. 2021, 3, 33. [Google Scholar]
- Chu, W.K.; Lin, K.Y.; Sun, H.Y.; Chen, Y.C.; Cheng, A. Herpes zoster aseptic meningitis and Ramsay Hunt syndrome in an immunocompetent young adult post mild COVID-19-A coincidence? J. Microbiol. Immunol. Infect. 2023, 56, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, F.; Butnariu, I.; Cojocaru, F.M.; Anghel, D.N.; Mihai, E.D.; Tuță, S. Zoster Cranial Polyneuropathy in a COVID-19 Patient. Am. J. Case Rep. 2021, 22, e934658. [Google Scholar] [CrossRef] [PubMed]
- López-Blanco, R.; Cazorla-Garcia, R.; Barbero-Bordallo, N.; Fernández-Ferro, J. Neurological infections during the COVID-19 pandemic. Neurologia 2020, 35, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, M.; Corriols-Noval, P.; López-Simón, E.; Morales-Angulo, C. Ramsay Hunt syndrome following mRNA SARS-COV-2 vaccine. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2022, 40, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.J.; Chou, O.H.I.; Cheung, B.M.Y. Ramsay Hunt syndrome following COVID-19 vaccination. Postgrad. Med. J. 2022, 98, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Lakhoua, G.; Charfi, O.; Dabbeche, S.; Zaiem, A.; Kastalli, S.; Daghfous, R.; Aidli, S.E. An atypical Ramsey Hunt syndrome after COVID-19 immunization. Therapie 2023, 78, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, R.M. Ramsay Hunt Syndrome With Cranial Polyneuropathy and Delayed Facial Nerve Palsy: A Case Report. Cureus 2022, 14, e27434. [Google Scholar] [CrossRef]
- Pavlidis, P.; Cámara, R.J.A.; Kekes, G.; Gouveris, H. Bilateral taste disorders in patients with Ramsay Hunt syndrome and Bell palsy. Ann. Neurol. 2018, 83, 807–815. [Google Scholar] [CrossRef]
- Lozada-Nur, F.; Chainani-Wu, N.; Fortuna, G.; Sroussi, H. Dysgeusia in COVID-19: Possible Mechanisms and Implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 344–346. [Google Scholar] [CrossRef]
- Elsaie, M.L.; Youssef, E.A.; Nada, H.A. Herpes zoster might be an indicator for latent COVID-19 infection. Dermatol. Ther. 2020, 33, e13666. [Google Scholar] [CrossRef]
- Adam, I. Is herpes zoster being an indicator for COVID-19 infection? Dermatol. Ther. 2020, 33, e13846. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Gershon, M.D.; Breuer, J.; Levin, M.J.; Oaklander, A.L.; Griffiths, P.D. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010, 48, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Ramsay Hunt syndrome and mRNA SARS-COV-2 vaccination. Enferm. Infecc. Microbiol. Clin. 2022, 40, 48. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Gerada, C.; Campbell, T.M.; Kennedy, J.J. Manipulation of the innate immune response by varicella zoster virus. Front. Immunol. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, S.; Yao, Y.; Li, Y.; Zhang, G. Dermatologists may need to pay more attention to herpes zoster during the pandemic of COVID-19. Infect. Dis. 2020, 52, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Catucci, O.; Amatulli, F.; Cefalo, A.; Lazzaro, R.; Palazzo, D.; Aityan, S.K.; et al. Immunity Profiling of COVID-19 Infection, Dynamic Variations of Lymphocyte Subsets, a Comparative Analysis on Four Different Groups. Microorganisms 2021, 9, 2036. [Google Scholar] [CrossRef]
- Bhavsar, A.; Lonnet, G.; Wang, C.; Chatzikonstantinidou, K.; Parikh, R.; Brabant, Y.; Servotte, N.; Shi, M.; Widenmaier, R.; Aris, E. Increased Risk of Herpes Zoster in Adults ≥50 Years Old Diagnosed With COVID-19 in the United States. Open Forum Infect. Dis. 2022, 9, ofac118. [Google Scholar] [CrossRef]
- Grammatikos, A.; Bright, P.; Bhatnagar, R.; Johnston, S. How to investigate a suspected immune deficiency in adults. Respir. Med. 2020, 171, 106100. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Gilden, D.H. Ramsay Hunt syndrome. J. Neurol. Neurosurg. Psychiatry 2001, 71, 149–154. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Herpes Zoster Diagnosis, Testing, Lab Methods; Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2023. Available online: https://www.cdc.gov/shingles/hcp/diagnosis-testing.html (accessed on 10 May 2023).
- Lindström, J.; Grahn, A.; Zetterberg, H.; Studahl, M. Cerebrospinal fluid viral load and biomarkers of neuronal and glial cells in Ramsay Hunt syndrome. Eur. J. Neurosci. 2016, 44, 2944–2949. [Google Scholar] [CrossRef]
- Murakami, S.; Hato, N.; Horiuchi, J.; Honda, N.; Gyo, K.; Yanagihara, N. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: Significance of early diagnosis and treatment. Ann. Neurol. 1997, 41, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Hutchins, T.; Palacios, E. Ramsay Hunt syndrome, type I. Ear Nose Throat J. 2007, 86, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Monsanto, R.D.; Bittencourt, A.G.; Bobato Neto, N.J.; Beilke, S.C.; Lorenzetti, F.T.; Salomone, R. Treatment and Prognosis of Facial Palsy on Ramsay Hunt Syndrome: Results Based on a Review of the Literature. Int. Arch. Otorhinolaryngol. 2016, 20, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Uri, N.; Greenberg, E.; Kitzes-Cohen, R.; Doweck, I. Acyclovir in the treatment of Ramsay Hunt syndrome. Otolaryngol. Head Neck Surg. 2003, 129, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.W.; Lee, D.H.; Jun, B.C.; Chang, K.H.; Park, Y.S. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx 2007, 34, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kinishi, M.; Amatsu, M.; Mohri, M.; Saito, M.; Hasegawa, T.; Hasegawa, S. Acyclovir improves recovery rate of facial nerve palsy in Ramsay Hunt syndrome. Auris Nasus Larynx 2001, 28, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Aizawa, H.; Ohtani, F.; Sawa, H.; Fukuda, S. Varicella-zoster virus DNA level and facial paralysis in Ramsay Hunt syndrome. Ann. Otol. Rhinol. Laryngol. 2004, 113, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Coulson, S.; Croxson, G.R.; Adams, R.; Oey, V. Prognostic factors in herpes zoster oticus (ramsay hunt syndrome). Otol. Neurotol. 2011, 32, 1025–1030. [Google Scholar] [CrossRef]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef]
| Authors | López-Blanco et al., 2020 [14] | Mehta et al., 2021 [1] | Alonzo-Correa et al., 2021 [11] | Antonescu et al., 2021 [13] | Chu et al., 2023 [12] |
|---|---|---|---|---|---|
| Demographic and clinical data | 67-year-old male (information NP) | 32-year-old previously healthy male | 25-year-old healthy female | 54-year-old female receiving steroids | 25-year-old immunocompetent male |
| RHS clinical presentation | Right facial paralysis, otalgia, headache, fever, right ear and soft palate skin rash, and gait ataxia | Left facial weakness, left pinna vesicular eruption, hearing loss, tinnitus, and difficulty in communication following COVID-19 symptoms; afebrile at presentation 20 days following symptoms onset | Left earlobe vesicular eruption and jawline/neck pain, followed by left hyperacusis (two days later) then left facial palsy/paresthesia, ageusia, and eye symptoms (one day later) | Acute vestibular syndrome followed by left abducens nerve palsy, right peripheral facial palsy, right ear vesicular rash (a few days later), and severe right hearing loss (one month later) | RHS and aseptic meningitis three weeks following COVID-19 symptoms: right facial palsy/hypoesthesia, right facial and mastoid tenderness, hearing impairment, headache, dizziness, vomiting, nystagmus, afebrile; followed by right ear vesicles |
| COVID-19 presentation | No clinical, laboratory, or neuroimaging evidence | Fever, sore throat | Fatigue; no fever, cough, dyspnea, or anosmia | No fever, cough, dyspnea, myalgia, or arthralgia | Fever, sore throat |
| Investigations |
|
|
|
|
|
| Management |
|
|
|
|
|
| Outcomes | Improvement in ataxia but persistence of facial paralysis | Mild improvement in tinnitus, complete resolution of facial weakness, speech discrimination score of 70% with bilateral hearing aid; no further changes at one month | Significant improvement following one month of treatment, recovery at two months | Significant improvement at six months (complete remission of vestibular and oculomotor symptoms, improvement but persistence of facial asymmetry, severe right hearing loss) | Alleviation of headache, vomiting, and dizziness; persistence of abnormal MRI signals at one month (right cochlea, semicircular canals, and facial nerve) suggesting unresolved facial palsy |
| Authors | Rodríguez-Martín et al., 2022 [15] | Woo et al., 2022 [16] | Lakhoua et al., 2022 [17] |
|---|---|---|---|
| Demographic and clinical data | 78-year-old female with a history of childhood poliomyelitis and untreated arterial hypertension | 37-year-old previously healthy male | 65-year-old male with treated arterial hypertension; no history of chickenpox infection or varicella vaccine |
| RHS clinical presentation | Malaise, gait instability, nausea, right otalgia and vesicles/crusted lesions, right-side predominant hearing loss, right facial nerve palsy, and left nystagmus three days following COVID-19 vaccine (BNT162b2 mRNA) | Fever, right otalgia, right ear and canal vesicles, right hearing loss, vertigo, tinnitus, hearing loss, facial palsy, tongue numbness, and dysgeusia two days following the COVID-19 vaccine (BNT162b2 mRNA) | Vesicular eruption three days after the first shot of the COVID-19 vaccine (BNT162b2 mRNA), left hemifacial paralysis and pain, and cutaneous exacerbation one week after the second shot |
| Investigations |
|
| Normal CBCD four days following the first shot; VZV antibodies not performed |
| Management | NP | NP |
|
| Outcomes | Persistence of instability and hearing loss and slight improvement in facial paralysis at two weeks | NP | Improvement in facial paralysis within one-month, rash resolution at four months, persistence of pain/tingling at six months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, W.A.R.; Lizzeik, D.; Berro, J.; Faddoul, S.; El Dassouki, M.; Shatila, A.R.; Chalah, M.A.; Ayache, S.S. Ramsay Hunt Syndrome in Asymptomatic COVID-19 Infection: A Case Report and a Literature Review. J. Clin. Med. 2023, 12, 7407. https://doi.org/10.3390/jcm12237407
Ayoub WAR, Lizzeik D, Berro J, Faddoul S, El Dassouki M, Shatila AR, Chalah MA, Ayache SS. Ramsay Hunt Syndrome in Asymptomatic COVID-19 Infection: A Case Report and a Literature Review. Journal of Clinical Medicine. 2023; 12(23):7407. https://doi.org/10.3390/jcm12237407
Chicago/Turabian StyleAyoub, Wissam Al Rida, Dina Lizzeik, Jana Berro, Sami Faddoul, Mohamad El Dassouki, Abdul Rahman Shatila, Moussa A. Chalah, and Samar S. Ayache. 2023. "Ramsay Hunt Syndrome in Asymptomatic COVID-19 Infection: A Case Report and a Literature Review" Journal of Clinical Medicine 12, no. 23: 7407. https://doi.org/10.3390/jcm12237407
APA StyleAyoub, W. A. R., Lizzeik, D., Berro, J., Faddoul, S., El Dassouki, M., Shatila, A. R., Chalah, M. A., & Ayache, S. S. (2023). Ramsay Hunt Syndrome in Asymptomatic COVID-19 Infection: A Case Report and a Literature Review. Journal of Clinical Medicine, 12(23), 7407. https://doi.org/10.3390/jcm12237407










