Heartburn’s Hidden Impact: A Narrative Review Exploring Gastroesophageal Reflux Disease (GERD) as a Cardiovascular Disease Risk Factor
Abstract
:1. Introduction
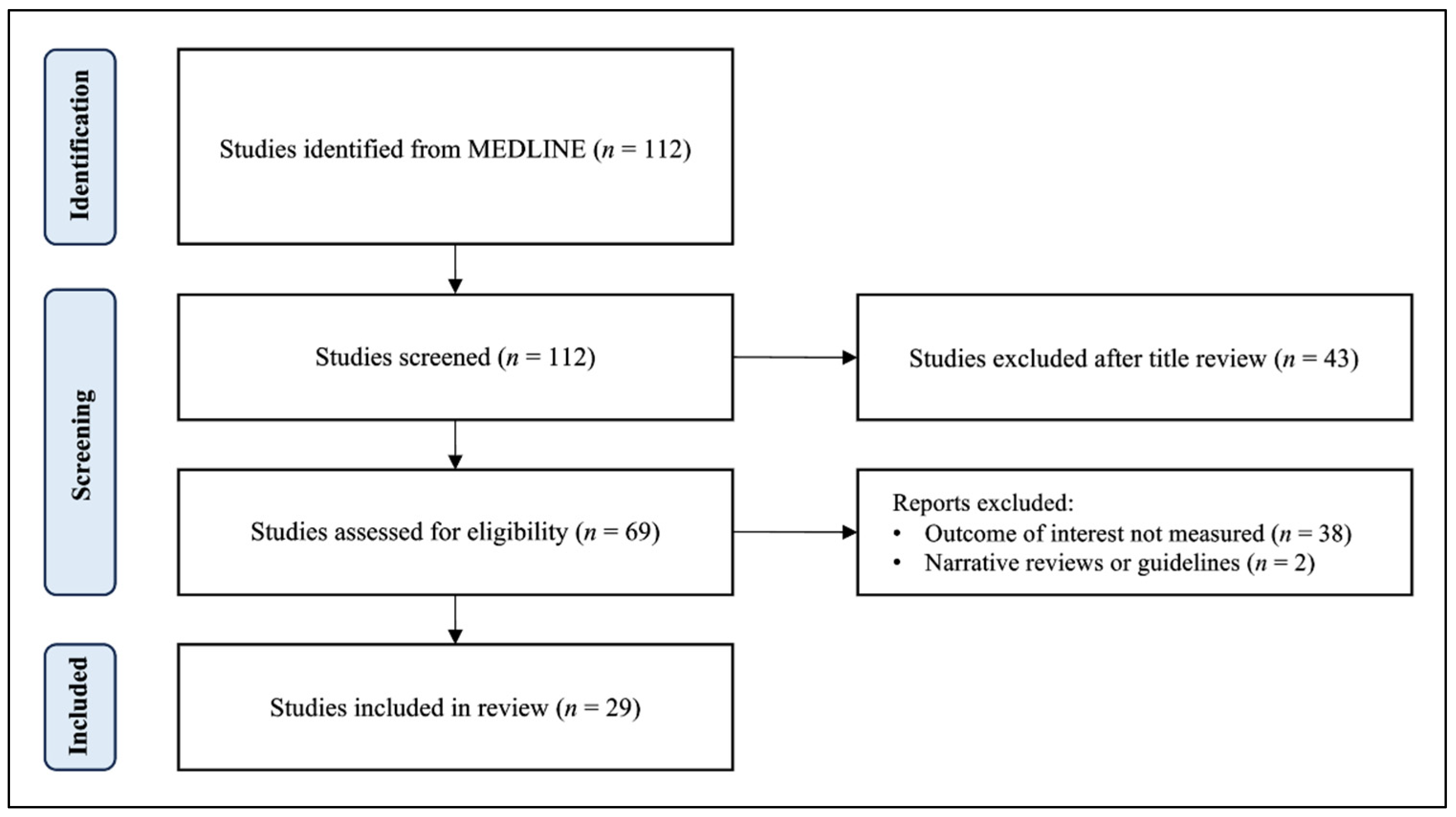
2. Materials and Methods
3. Results and Discussion
3.1. Association between GERD and Coronary Atherosclerosis
3.2. Association between GERD and Myocardial Ischemia
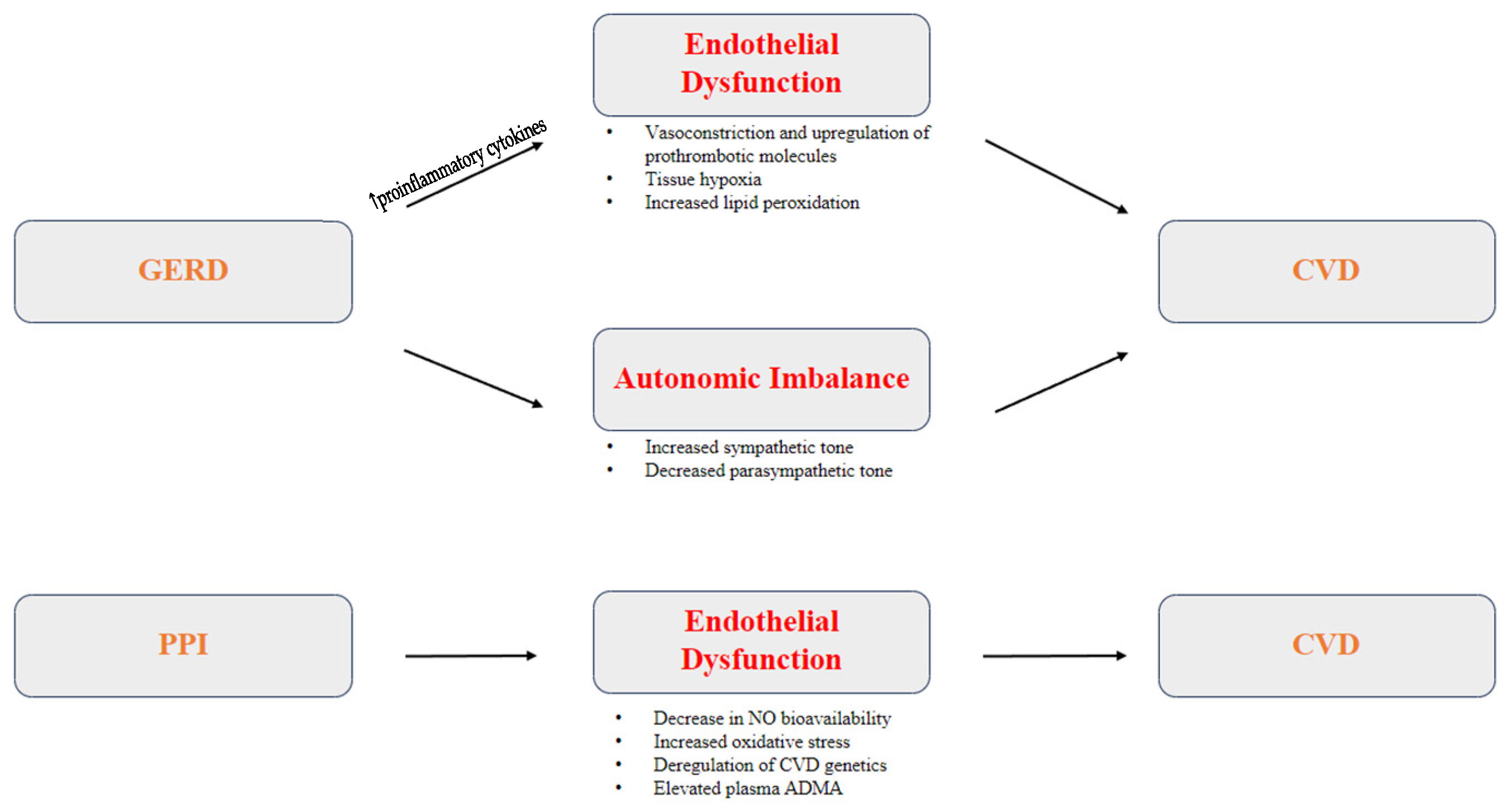
3.3. Pathophysiology of GERD as a Risk Factor for CVD
3.3.1. Inflammation and Endothelial Dysfunction
3.3.2. Autonomic Imbalance
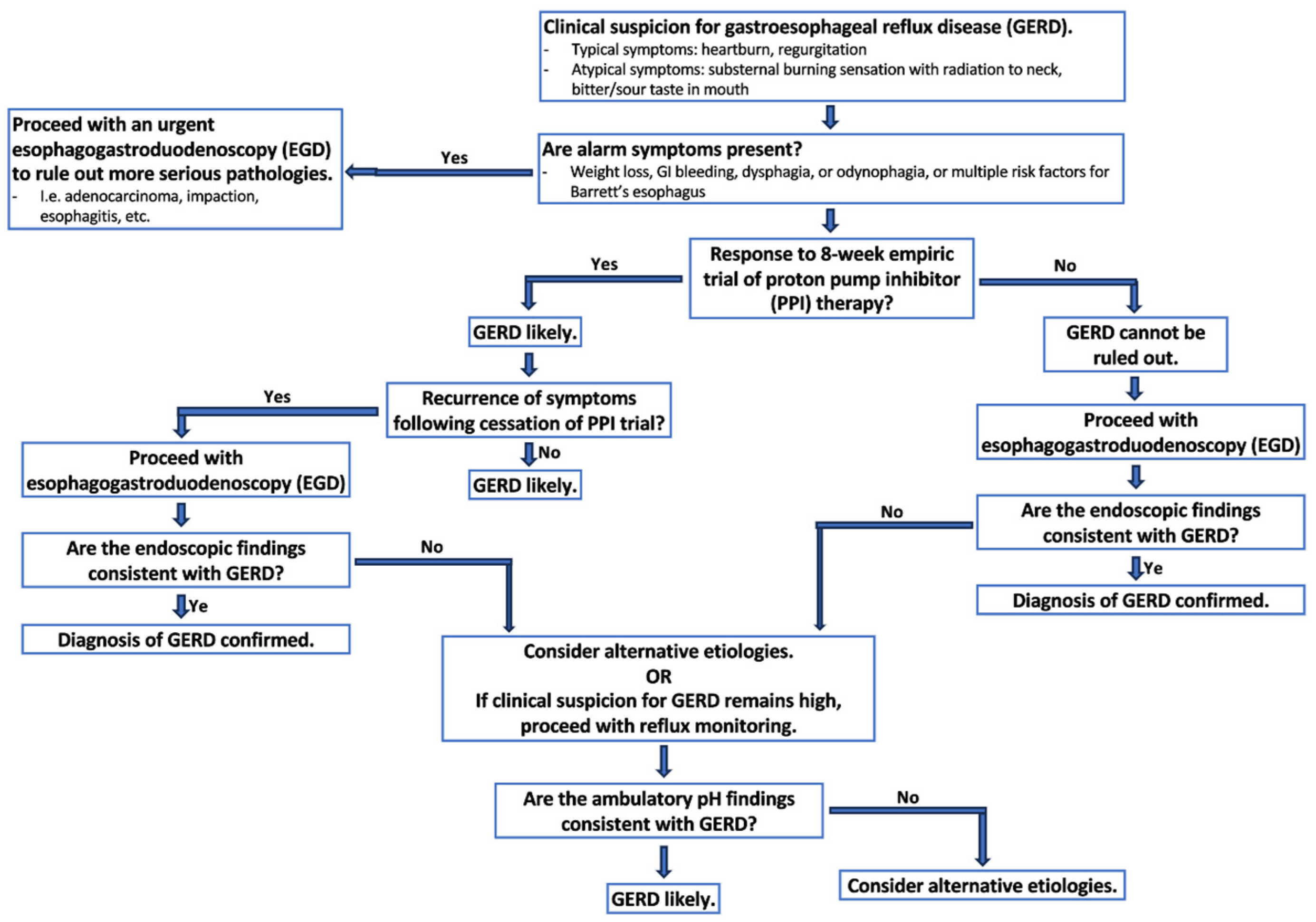
3.4. Diagnosing GERD
3.5. Management of GERD and Its Relation to CVD
3.5.1. Lifestyle Modifications
3.5.2. Medical Therapy
3.5.3. Mechanical Interventions
4. Future Direction of Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Liu, S.; Li, Z.; Wang, R. Global, regional and national burden of gastroesophageal reflux disease, 1990–2019: Update from the GBD 2019 study. Ann. Med. 2022, 54, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147. [Google Scholar] [CrossRef] [PubMed]
- Maret-Ouda, J.; Santoni, G.; Xie, S.; Rosengren, A.; Lagergren, J. Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events. Cardiovasc. Drugs Ther. 2022, 36, 1121–1128. [Google Scholar] [CrossRef]
- Khomenko, L.; Vnukova, A.; Dvoiashkina, Y. Features of endothelial dysfunction in elderly persons with coronary heart disease and concomitant gastroesophageal reflux disease. Georgian Med. News 2019, 78–82. [Google Scholar]
- Landi, S.N.; Sandler, R.S.; Pate, V.; Lund, J.L. No increase in risk of acute myocardial infarction in privately insured adults prescribed proton pump inhibitors vs histamine-2 receptor antagonists (2002–2014). Gastroenterology 2018, 154, 861–873.e6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Lochhead, P.; Joshi, A.D.; Cao, Y.; Ma, W.; Khalili, H.; Rimm, E.B.; Rexrode, K.M.; Chan, A.T. No significant association between proton pump inhibitor use and risk of stroke after adjustment for lifestyle factors and indication. Gastroenterology 2018, 154, 1290–1297.e1. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; LePendu, P.; Bauer-Mehren, A.; Ghebremariam, Y.T.; Iyer, S.V.; Marcus, J.; Nead, K.T.; Cooke, J.P.; Leeper, N.J. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS ONE 2015, 10, e0124653. [Google Scholar] [CrossRef]
- Liu, Y.; He, S.; Chen, Y.; Xu, J.; Tang, C.; Tang, Y.; Luo, G. Acid reflux in patients with coronary artery disease and refractory chest pain. Intern. Med. 2013, 52, 1165–1171. [Google Scholar] [CrossRef]
- Schmidt, M.; Johansen, M.B.; Robertson, D.J.; Maeng, M.; Kaltoft, A.; Jensen, L.O.; Tilsted, H.; Bøtker, H.E.; Sørensen, H.T.; Baron, J.A. Concomitant use of clopidogrel and proton pump inhibitors is not associated with major adverse cardiovascular events following coronary stent implantation. Aliment. Pharmacol. Ther. 2012, 35, 165–174. [Google Scholar] [CrossRef]
- Gupta, E.; Bansal, D.; Sotos, J.; Olden, K. Risk of adverse clinical outcomes with concomitant use of clopidogrel and proton pump inhibitors following percutaneous coronary intervention. Dig. Dis. Sci. 2010, 55, 1964–1968. [Google Scholar] [CrossRef]
- Jansson, C.; Nordenstedt, H.; Wallander, M.-A.; Johansson, S.; Johnsen, R.; Hveem, K.; Lagergren, J. Severe symptoms of gastro-oesophageal reflux disease are associated with cardiovascular disease and other gastrointestinal symptoms, but not diabetes: A population-based study. Aliment. Pharmacol. Ther. 2008, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, Y.; Fu, T.; Lu, S.; Shi, W.; Zhao, J.; Li, S.; Li, X.; Yuan, S.; Larsson, S.C. Risk of incident cardiovascular disease among patients with gastrointestinal disorder: Prospective cohort study of 340,862 individuals. medRxiv 2023. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.S.; Choi, S.Y.; Yang, S.Y. Association between gastroesophageal reflux disease and coronary atherosclerosis. PLoS ONE 2022, 17, e0267053. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, C.; Kao, C. Association between gastroesophageal reflux disease and coronary heart disease. Medicine 2016, 95, e4089. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, L.; Zheng, L. A Mendelian randomization study to assess the genetic liability of gastroesophageal reflux disease for cardiovascular diseases and risk factors. Hum. Mol. Genet. 2022, 31, 4275–4285. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, E.; Bassotti, G.; Buonafede, G.; Serra, A.M.; Dughera, L.; Orzan, F.; Casoni, R.; Chistolini, F.; Morelli, A.; Emanuelli, G. Noncardiac chest pain of esophageal origin in patients with and without coronary artery disease. Hepatogastroenterology 2005, 52, 792–795. [Google Scholar]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Eisa, M.; Sandhu, A.; Prakash, R.; Ganocy, S.J.; Fass, R. The Risk of Acute Myocardial Infarction in Patients With Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2020, 26, 471–476. [Google Scholar] [CrossRef]
- Lei, W.Y.; Wang, J.H.; Wen, S.H.; Yi, C.H.; Hung, J.S.; Liu, T.T.; Orr, W.C.; Chen, C.L. Risk of acute myocardial infarction in patients with gastroesophageal reflux disease: A nationwide population-based study. PLoS ONE 2017, 12, e0173899. [Google Scholar] [CrossRef]
- Teragawa, H.; Oshita, C.; Ueda, T. History of gastroesophageal reflux disease in patients with suspected coronary artery disease. Heart Vessel. 2019, 34, 1631–1638. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.; L’Allier, P.L.; Elgharib, N.; Tardif, J.C. Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc. Health Risk Manag. 2006, 2, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, J.; McCrum, E.; Rumley, A.; Patterson, C.; Salomaa, V.; Lowe, G.; Evans, A. Association of European population levels of thrombotic and inflammatory factors with risk of coronary heart disease: The MONICA Optional Haemostasis Study. Eur. Heart J. 2005, 26, 332–342, discussion 317–318. [Google Scholar] [CrossRef] [PubMed]
- Shridas, P.; Tannock, L.R. Role of serum amyloid A in atherosclerosis. Curr. Opin. Lipidol. 2019, 30, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Oparin, A.; Vnukova, A. The Role of Endothelial Dysfunction in the Mechanism of Gastroesophageal Reflux Disease Development in Patients with Ischemic Heart Disease. Acta Clin. Croat. 2017, 56, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chan, W.-L.; Luo, J.-C.; Chen, Y.-C.; Chen, T.-J.; Chung, C.-M.; Huang, P.-H.; Lin, S.-J.; Chen, J.-W.; Leu, H.-B. Gastroesophageal reflux disease and atrial fibrillation: A nationwide population-based study. PLoS ONE 2012, 7, e47575. [Google Scholar] [CrossRef]
- Dobrek, L.; Nowakowski, M.; Syguła, A.; Lipczyński, A.; Barylak, H.; Herman, R.M.; Thor, P.J. 24-hour heart rate variability in patients with gastroesophageal reflux disease. Folia Medica Cracoviensia 2005, 46, 53–64. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=17037287 (accessed on 17 October 2023).
- Cuomo, R.; De Giorgi, F.; Adinolfi, L.; Sarnelli, G.; Loffredo, F.; Efficie, E.; Verde, C.; Savarese, M.F.; Usai, P.; Budillon, G. Oesophageal acid exposure and altered neurocardiac function in patients with GERD and idiopathic cardiac dysrhythmias. Aliment. Pharmacol. Ther. 2006, 24, 361–370. [Google Scholar] [CrossRef]
- Kaya, H.; Barutcu, S. Gastroesophageal reflux disease is associated with abnormal ventricular repolarization indices. Turk. J. Gastroenterol. 2019, 30, 1021–1024. [Google Scholar] [CrossRef]
- Tougas, G.; Spaziani, R.; Hollerbach, S.; Djuric, V.; Pang, C.; Upton, A.R.M.; Fallen, E.L.; Kamath, M.V. Cardiac autonomic function and oesophageal acid sensitivity in patients with non-cardiac chest pain. Gut 2001, 49, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2022, 117, 2756. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, R.; Gyawali, C.P.; Pandolfino, J.E.; CGIT GERD Consensus Conference Participants. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 984–994.e1, Erratum in Clin. Gastroenterol. Hepatol. 2022, 20, 2156. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Lee, J.; Gupta, N.; Gaddam, S.; Smith, B.K.; Wani, S.B.; Sullivan, D.K.; Rastogi, A.; Bansal, A.; Donnelly, J.E.; et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: A prospective intervention trial. Obesity 2013, 21, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.C.; Somers, S.C.; Fuchs, C.S.; Kelly, C.P.; Camargo, C.A., Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N. Engl. J. Med. 2006, 354, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Ness-Jensen, E.; Lindam, A.; Lagergren, J.; Hveem, K. Weight loss and reduction in gastroesophageal reflux. A prospective population-based cohort study: The HUNT study. Am. J. Gastroenterol. 2013, 108, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Ness-Jensen, E.; Lindam, A.; Lagergren, J.; Hveem, K. Tobacco Smoking Cessation and Improved Gastroesophageal Reflux: A Prospective Population-Based Cohort Study: The HUNT Study. Am. J. Gastroenterol. 2014, 109, 171–177. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Liakoni, E. Tobacco use disorder and cardiovascular health. Addiction 2022, 117, 1128–1138. [Google Scholar] [CrossRef]
- Kohata, Y.; Fujiwara, Y.; Watanabe, T.; Kobayashi, M.; Takemoto, Y.; Kamata, N.; Yamagami, H.; Tanigawa, T.; Shiba, M.; Watanabe, T.; et al. Long-Term Benefits of Smoking Cessation on Gastroesophageal Reflux Disease and Health-Related Quality of Life. PLoS ONE 2016, 11, e0147860, Erratum in PLoS ONE 2016, 11, e0150554. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Mone, I.; Kraja, B.; Bregu, A.; Duraj, V.; Sadiku, E.; Hyska, J.; Burazeri, G. Adherence to a predominantly Mediterranean diet decreases the risk of gastroesophageal reflux disease: A cross-sectional study in a South Eastern European population. Dis. Esophagus 2016, 29, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.P.; Kaur, M.; Bhavanam, S.; Mulaka, G.S.R.; Ishfaq, L.; Vempati, R.; C, M.F.; Kandepi, H.V.; Er, R.; Sahu, S.; et al. A Systematic Review of the Effects of Smoking on the Cardiovascular System and General Health. Cureus 2023, 15, e38073. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, M.; Thakker, J.; Kherallah, R.; Kitakaze, M.; Jneid, H.; Angiolillo, D.J.; Birnbaum, Y. Antacid Therapy in Coronary Artery Disease and Heart Failure: Proton Pump Inhibitors vs. H2 Receptor Blockers. Cardiovasc. Drugs Ther. 2022. [Google Scholar] [CrossRef]
- Ahmed, A.; Clarke, J.O. Proton Pump Inhibitors (PPI) [Updated 1 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557385/ (accessed on 18 September 2023).
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef] [PubMed]
- Nugent, C.C.; Falkson, S.R.; Terrell, J.M. H2 Blockers. [Updated 11 December 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525994/ (accessed on 18 September 2023).
- Abdul-Hussein, M.; Freeman, J.; Castell, D. Concomitant Administration of a Histamine2 Receptor Antagonist and Proton Pump Inhibitor Enhances Gastric Acid Suppression. Pharmacotherapy 2015, 35, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Luo, Y.; Yang, X. Inappropriate Use of Proton Pump Inhibitors Increases Cardiovascular Events in Patients with Coronary Heart Disease. Int. J. Gen. Med. 2022, 15, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Hamzeloo-Moghadam, M.; Rezaei Tavirani, M.; Jahani-Sherafat, S.; Rezaei Tavirani, S.; Esmaeili, S.; Ansari, M.; Ahmadzadeh, A. Side effects of omeprazole: A system biology study. Gastroenterol. Hepatol. Bed Bench 2021, 14, 334–341. [Google Scholar] [PubMed]
- Teperikidis, E.; Boulmpou, A.; Potoupni, V.; Kundu, S.; Singh, B.; Papadopoulos, C. Does the long-term administration of proton pump inhibitors increase the risk of adverse cardiovascular outcomes? A ChatGPT powered umbrella review. Acta Cardiol. 2023, 78, 980–988. [Google Scholar] [CrossRef]
- Taneja, G.; Thanikachalam, P.V.; Rajput, S.K. Dose and time-dependent toxicological impact of pantoprazole on vascular endothelium and renal tissue. Toxicol. Lett. 2020, 333, 97–104. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; LePendu, P.; Lee, J.C.; Erlanson, D.A.; Slaviero, A.; Shah, N.H.; Leiper, J.; Cooke, J.P. Unexpected effect of proton pump inhibitors: Elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 2013, 128, 845–853. [Google Scholar] [CrossRef]
- Wedemeyer, R.S.; Blume, H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: An update. Drug Saf. 2014, 37, 201–211. [Google Scholar] [CrossRef]
- Kwok, C.S.; Jeevanantham, V.; Dawn, B.; Loke, Y.K. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: Meta-analysis. Int. J. Cardiol. 2013, 167, 965–974. [Google Scholar] [CrossRef]
- Shih, C.; Chen, Y.; Ou, S.; Li, S.; Chen, T.; Wang, S. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int. J. Cardiol. 2014, 177, 292–297. [Google Scholar] [CrossRef]
- Valkhoff, V.E.; ‘t Jong, G.W.; Van Soest, E.M.; Kuipers, E.J.; Sturkenboom, M.C. Risk of recurrent myocardial infarction with the concomitant use of clopidogrel and proton pump inhibitors. Aliment. Pharmacol. Ther. 2011, 33, 77–88. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Yang, H.; Zhang, Y.; Li, H.; Zhou, L.; Lin, J.; Chen, Y.; Hou, Y.; Zhang, X.; et al. Acid suppressants use and risk of atherosclerotic cardiovascular disease in middle-aged and older adults. Atherosclerosis 2022, 358, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Cryer, B.L.; Contant, C.F.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; Lapuerta, P.; Goldsmith, M.A.; Laine, L.; et al. Clopidogrel with or without omeprazole in coronary artery disease. N. Engl. J. Med. 2010, 363, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Unal, Y.; Sargin, Z.; Urun, Y.; Kalayc, B.; Ustundag, Y. The effects of proton pump inhibitors on the development of post-stenting major adverse cardiovascular events in patients with acute coronary syndrome. Med. Sci. 2023, 12, 522. [Google Scholar] [CrossRef]
- Luo, T.; Chen, B.; Zhao, Z.; He, N.; Zeng, Z.; Wu, B.; Fukushima, Y.; Dai, M.; Huang, Q.; Xu, D.; et al. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res. Cardiol. 2013, 108, 342. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Hunter, J.G.; Jones, K.M.; Lee, R.; Smith, B.R.; Mashimo, H.; Sanchez, V.M.; Dunbar, K.B.; Pham, T.H.; Murthy, U.K.; et al. Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N. Engl. J. Med. 2019, 381, 1513–1523. [Google Scholar] [CrossRef]
- Bell, R.; Lipham, J.; Louie, B.E.; Williams, V.; Luketich, J.; Hill, M.; Richards, W.; Dunst, C.; Lister, D.; McDowell-Jacobs, L.; et al. Magnetic Sphincter Augmentation Superior to Proton Pump Inhibitors for Regurgitation in a 1-Year Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 1736–1743.e2. [Google Scholar] [CrossRef]
- Richardson, W.S.; Gorham, J.K.; Neal, N.; Fanelli, R.D. Endoscopic Treatment of Gastroesophageal Reflux Disease. Adv. Surg. 2022, 56, 205–227. [Google Scholar] [CrossRef]
- Kalapala, R.; Karyampudi, A.; Nabi, Z.; Darisetty, S.; Jagtap, N.; Ramchandani, M.; Gupta, R.; Lakhtakia, S.; Goud, R.; Venkat Rao, G.; et al. Endoscopic full-thickness plication for the treatment of PPI-dependent GERD: Results from a randomised, sham controlled trial. Gut 2022, 71, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Rettura, F.; Bronzini, F.; Campigotto, M.; Lambiase, C.; Pancetti, A.; Berti, G.; Marchi, S.; de Bortoli, N.; Zerbib, F.; Savarino, E.; et al. Refractory Gastroesophageal Reflux Disease: A Management Update. Front. Med. 2021, 8, 765061. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Lee, E.; Ahnen, D.; Goyal, R.K.; Hirano, I.; Ramirez, F.; Raufman, J.P.; Sampliner, R.; Schnell, T.; Sontag, S.; et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: Follow-up of a randomized controlled trial. JAMA 2001, 285, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Rudolph-Stringer, V.; Bright, T.; Irvine, T.; Thompson, S.K.; Devitt, P.G.; Game, P.A.; Jamieson, G.G.; Watson, D.I. Randomized Trial of Laparoscopic Nissen Versus Anterior 180 Degree Partial Fundoplication—Late Clinical Outcomes at 15 to 20 years. Ann. Surg. 2022, 275, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zornig, C.; Strate, U.; Fibbe, C.; Emmermann, A.; Layer, P. Nissen vs. Toupet laparoscopic fundoplication. Surg. Endosc. 2002, 16, 758–766. [Google Scholar] [CrossRef] [PubMed]
- LINX® Reflux Management System|J&J medtech. Available online: https://www.jnjmedtech.com/en-US/product/linx-reflux-management-system (accessed on 1 November 2023).
- Ferrari, D.; Asti, E.; Lazzari, V.; Siboni, S.; Bernardi, D.; Bonavina, L. Six to 12-year outcomes of magnetic sphincter augmentation for gastroesophageal reflux disease. Sci. Rep. 2020, 10, 13753. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Siboni, S.; Riva, C.G.; Guerrazzi, G.; Lovece, A.; Bonavina, L. Magnetic Sphincter Augmentation Outcomes in Severe Gastroesophageal Reflux Disease. Front. Med. 2021, 8, 645592. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.P.; Chang, K.J. Endoscopic Management of GERD. Dig. Dis. Sci. 2022, 67, 1455–1468. [Google Scholar] [CrossRef]
- Kalapala, R.; Shah, H.; Nabi, Z.; Darisetty, S.; Talukdar, R.; Nageshwar Reddy, D. Treatment of gastroesophageal reflux disease using radiofrequency ablation (Stretta procedure): An interim analysis of a randomized trial. Indian J. Gastroenterol. 2017, 36, 337–342. [Google Scholar] [CrossRef]
- Trad, K.S.; Barnes, W.E.; Prevou, E.R.; Simoni, G.; Steffen, J.A.; Shughoury, A.B.; Raza, M.; Heise, J.A.; Fox, M.A.; Mavrelis, P.G. The TEMPO Trial at 5 Years: Transoral Fundoplication (TIF 2.0) Is Safe, Durable, and Cost-effective. Surg. Innov. 2018, 25, 149–157. [Google Scholar] [CrossRef]
- Kaindlstorfer, A.; Koch, O.O.; Antoniou, S.A.; Asche, K.U.; Granderath, F.A.; Pointner, R. A randomized trial on endoscopic full-thickness gastroplication versus laparoscopic antireflux surgery in GERD patients without hiatal hernias. Surg. Laparosc. Endosc. Percutaneous Tech. 2013, 23, 212–222. [Google Scholar] [CrossRef]
| Year | Authors | Title | Study Type | Conclusions |
|---|---|---|---|---|
| 2023 | Chen J et al. | Risk of incident cardiovascular disease among patients with gastrointestinal disorder: prospective cohort study of 340,862 individuals | Prospective cohort study |
|
| 2023 | Geng T et al. | Proton pump inhibitor use and risks of cardiovascular disease and mortality in patients with type 2 diabetes | Population-based cohort | PPI use is associated with a higher risk of CVD events and mortality among patients with type 2 diabetes mellitus. |
| 2023 | Sun L et al. | Helicobacter pylori infection and risk of cardiovascular disease | Meta-analysis | Helicobacter pylori infection is associated with a mildly increased risk of CVD. |
| 2023 | Teperikidis E et al. | Does the long-term administration of proton pump inhibitors increase the risk of adverse cardiovascular outcomes? A ChatGPT powered umbrella review | Umbrella review | A causal relationship between PPI use and an increased risk of MACE cannot be ruled out. |
| 2022 | Song J et al. | Association between gastroesophageal reflux disease and coronary atherosclerosis | Retrospective cohort study | GERD was associated with higher degrees of coronary atherosclerosis by CACS (p = 0.008) but did not increase the risk of a higher CACS (OR = 1.018, 95% CI 0.865–1.198). |
| 2022 | Sun X et al. | A Mendelian randomization study to assess the genetic liability of gastroesophageal reflux disease for cardiovascular diseases and risk factors | Mendelian randomization study | GERD was associated with 7 CVD outcomes and 9 cardiovascular risk factors. |
| 2022 | Ma Y et al. | Acid suppressants use and risk of atherosclerotic cardiovascular disease in middle-aged and older adults | Prospective cohort study | PPI use is associated with increased risk of ASCVD, particularly amongst participants without indications for medications. |
| 2022 | Maret-Ouda et al. | Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events [3] | Retrospective cohort study | In patients who receive clopidogrel after PCI, concomitant use of PPI may increase the risk of major cardiovascular events. |
| 2021 | Bell E et al. | Association of proton pump inhibitors with higher risk of cardiovascular disease and heart failure | Prospective cohort study | Long-term PPI use was associated with twice the risk of total CVD and HF compared with nonusers. |
| 2021 | Rooney M et al. | Proton pump inhibitor use, hypomagnesemia and risk of cardiovascular diseases: The atherosclerosis risk in communities (ARIC) study | Prospective cohort study | PPI users had a higher prevalence of hypomagnesemia than nonusers. PPI users also had higher CVD risk than nonusers; however, it appears unlikely that hypomagnesemia explains associations of PPIs with CVD risk. |
| 2020 | Eisa M et al. | The risk of acute myocardial infarction in patients with gastroesophageal reflux disease | Observational | GERD is a risk factor for AMI, higher than male gender and obesity. |
| 2020 | Wang B et al. | A meta-analysis of the association between helicobacter pylori infection and risk of atherosclerotic cardiovascular disease. | Meta-analysis | H pylori infection increases the risk of adverse cardiovascular events by 51%, with an even greater effect on AMI (OR = 1.80, 95% CI 1.42–2.26) and cerebrovascular disease (OR = 1.54, 95% CI 1.27–1.89). |
| 2019 | Teragawa H et al. | History of gastroesophageal reflux disease in patients with suspected coronary artery disease | Experimental | The presence of GERD may increase the incidence of vasospastic angina in patients with suspected coronary artery disease (OR 7.8; p < 0.01). |
| 2019 | Khomenko et al. | Features of endothelial dysfunction in elderly persons with coronary heart disease and concomitant gastroesophageal reflux disease [4] | Experimental | Endothelial dysfunction manifests itself as a decrease in stable nitric oxide metabolite levels and an increase in endothelin-1 levels, disturbance of celiac trunk regional blood flow, causing a decrease in esophageal tissue resistance, leading to lower esophageal sphincter dysfunction |
| 2018 | Landi et al. | No increase in risk of acute myocardial infarction in privately insured adults prescribed proton pump inhibitors vs. histamine-2 receptor antagonists (2002–2014) [5] | Retrospective cohort study | No difference in increased risk of AMIs with PPIs versus H2RAs. |
| 2018 | Nguyen et al. | No significant association between proton pump inhibitor use and risk of stroke after adjustment for lifestyle factors and indication [6] | Retrospective Cohort Study | No significant association between PPI use and ischemic stroke, after accounting for indications for PPI use. Prior reports of an increased risk of stroke may be due to residual confounding related to chronic conditions associated with PPI use. |
| 2017 | Oparin et al. | The role of endothelial dysfunction in the mechanism of gastroesophageal reflux disease development in patients with ischemic heart disease | Experimental | In patients with ischemic heart disease and concomitant GERD, endothelial dysfunction manifested by a significant increase in the levels of endothelin-1 and lipid peroxidation products, with decreased levels of nitric oxide metabolites, regional blood flow and quality of life. |
| 2017 | Lei W et al. | Risk of acute myocardial infarction in patients with gastroesophageal reflux disease: A nationwide population-based study | Prospective cohort study | GERD was associated with a higher risk of developing an AMI compared to controls (HR = 1.48; 95% CI: 1.31–1.66, p < 0.001). |
| 2016 | Chen C et al. | Association between gastroesophageal reflux disease and coronary heart disease | Population-based cohort | GERD was associated with a higher risk of developing coronary heart disease compared to controls (aHR = 1.67, 95% CI = 1.34–2.08) and in patients with GERD who were treated with PPI therapy for more than 1 year compared to those treated for less than 1 year (aHR = 1.56, 95% CI = 1.39–1.74). |
| 2015 | Shah et al. | Proton pump inhibitor usage and the risk of myocardial infarction in the general population [7] | Systematic review | GERD patients exposed to PPIs have a 1.16 fold increased risk of AMI, regardless of clopidogrel use. |
| 2014 | Shih C et al. | Proton pump inhibitor use represents an independent risk factor for myocardial infarction | Propensity-score matched study case-crossover study | Use of PPIs may be independently associated with an increased risk of MI. However, the benefits of PPIs may greatly outweigh the risks of adverse cardiovascular effects, with number needed to harm of 4357. |
| 2014 | Unal et al. | The effects of proton pump inhibitors on the development of post-stenting major adverse cardiovascular events in patients with acute coronary syndrome | Prospective cohort study | ADMA and copeptin levels may be significantly increased in patients started on imminent DAPT and PPI therapy after PCI. |
| 2013 | Ghebremariam Y et al. | Unexpected effect of proton pump inhibitors | Observational | Biochemical, cellular, ex vivo, and in vivo data revealing that PPIs directly interact with and significantly inhibit human DDAH activity, thereby increasing endothelial and serum ADMA levels. The increase in ADMA levels would be anticipated to impair vascular NOS activity, to increase oxidative stress, to reduce vasodilator function, and to impair vasoprotective mechanisms. |
| 2013 | Luo T et al. | Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function | Experimental | H2R activation exaggerates myocardial I/R injury by promoting myocardial mitochondrial dysfunction and by increasing cardiac vascular endothelial permeability. |
| 2013 | Liu et al. | Acid reflux in patients with coronary artery disease and refractory chest pain [8] | Prospective cohort study | Refractory chest pain in patients with CAD can be partially noncardiac chest pain (NCCP) secondary to acid reflux. The combined use of common cardiac drugs may predispose or aggravate GERD. Short-term proton pump inhibitor (PPI) therapy not only restores a normal esophageal pH, but also significantly improves the general health-related quality of life (HRQL) of patients. |
| 2012 | Schmidt et al. | Concomitant use of clopidogrel and proton pump inhibitors is not associated with major adverse cardiovascular events following coronary stent implantation [9] | Retrospective cohort study | The use of PPIs as a class did not modify the protective effect of clopidogrel, but its use was associated with major adverse cardiovascular events itself, particularly among patients having used PPIs before percutaneous coronary intervention. |
| 2010 | Gupta et al. | Risk of adverse clinical outcomes with concomitant use of clopidogrel and proton pump inhibitors following percutaneous coronary intervention [10] | Concomitant use of clopidogrel and PPI in post-PCI patients is associated with a higher risk of MACE. | |
| 2008 | Jansson C et al. | Severe symptoms of gastro-oesophageal reflux disease are associated with cardiovascular disease and other gastrointestinal symptoms, but not diabetes: a population-based study | Population-based, cross-sectional, case-control study | Myocardial infarction, angina pectoris, stroke and symptoms of nausea, diarrhea and constipation are associated with GERD. |
| 2001 | Tougas et al. | Cardiac autonomic function and oesophageal acid sensitivity in patients with non-cardiac chest pain | Experimental | Patients with angina-like pain during direct esophageal acidification have decreased resting vagal activity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gries, J.J.; Chen, B.; Virani, S.S.; Virk, H.U.H.; Jneid, H.; Krittanawong, C. Heartburn’s Hidden Impact: A Narrative Review Exploring Gastroesophageal Reflux Disease (GERD) as a Cardiovascular Disease Risk Factor. J. Clin. Med. 2023, 12, 7400. https://doi.org/10.3390/jcm12237400
Gries JJ, Chen B, Virani SS, Virk HUH, Jneid H, Krittanawong C. Heartburn’s Hidden Impact: A Narrative Review Exploring Gastroesophageal Reflux Disease (GERD) as a Cardiovascular Disease Risk Factor. Journal of Clinical Medicine. 2023; 12(23):7400. https://doi.org/10.3390/jcm12237400
Chicago/Turabian StyleGries, Jacob J., Bing Chen, Salim S. Virani, Hafeez Ul Hassan Virk, Hani Jneid, and Chayakrit Krittanawong. 2023. "Heartburn’s Hidden Impact: A Narrative Review Exploring Gastroesophageal Reflux Disease (GERD) as a Cardiovascular Disease Risk Factor" Journal of Clinical Medicine 12, no. 23: 7400. https://doi.org/10.3390/jcm12237400
APA StyleGries, J. J., Chen, B., Virani, S. S., Virk, H. U. H., Jneid, H., & Krittanawong, C. (2023). Heartburn’s Hidden Impact: A Narrative Review Exploring Gastroesophageal Reflux Disease (GERD) as a Cardiovascular Disease Risk Factor. Journal of Clinical Medicine, 12(23), 7400. https://doi.org/10.3390/jcm12237400







