Colocalization of IgG and IgA Heavy Chains with Kappa and Lambda Light Chains in Glomerular Deposits of IgA Nephropathy Patients Using High-Resolution Confocal Microscopy and Correlation with Oxford MEST-C Scores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Biopsies
2.2. Confocal Microscopy and Routine Immunofluorescence Microscopy
2.3. Statistical Analysis
3. Results
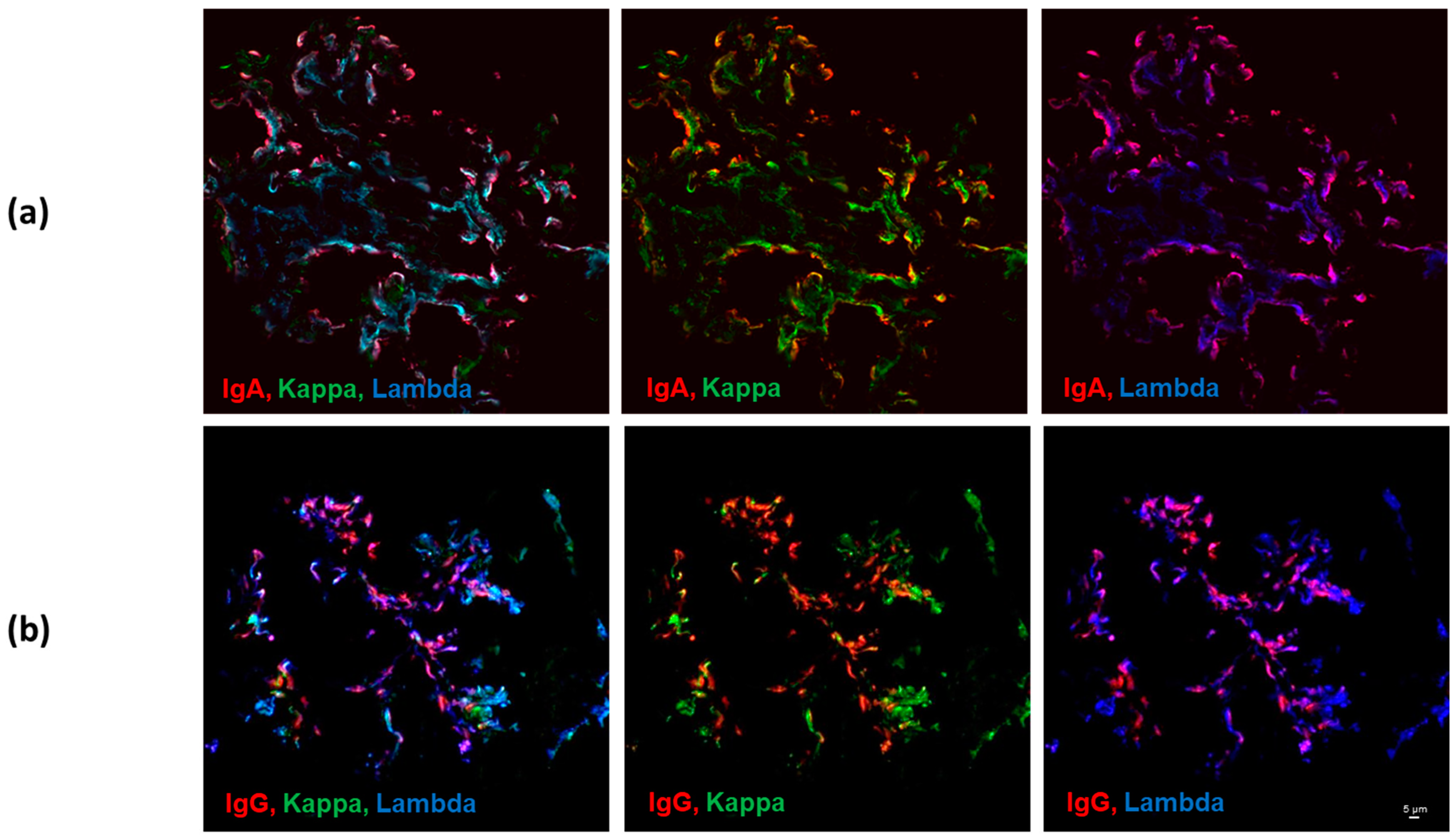
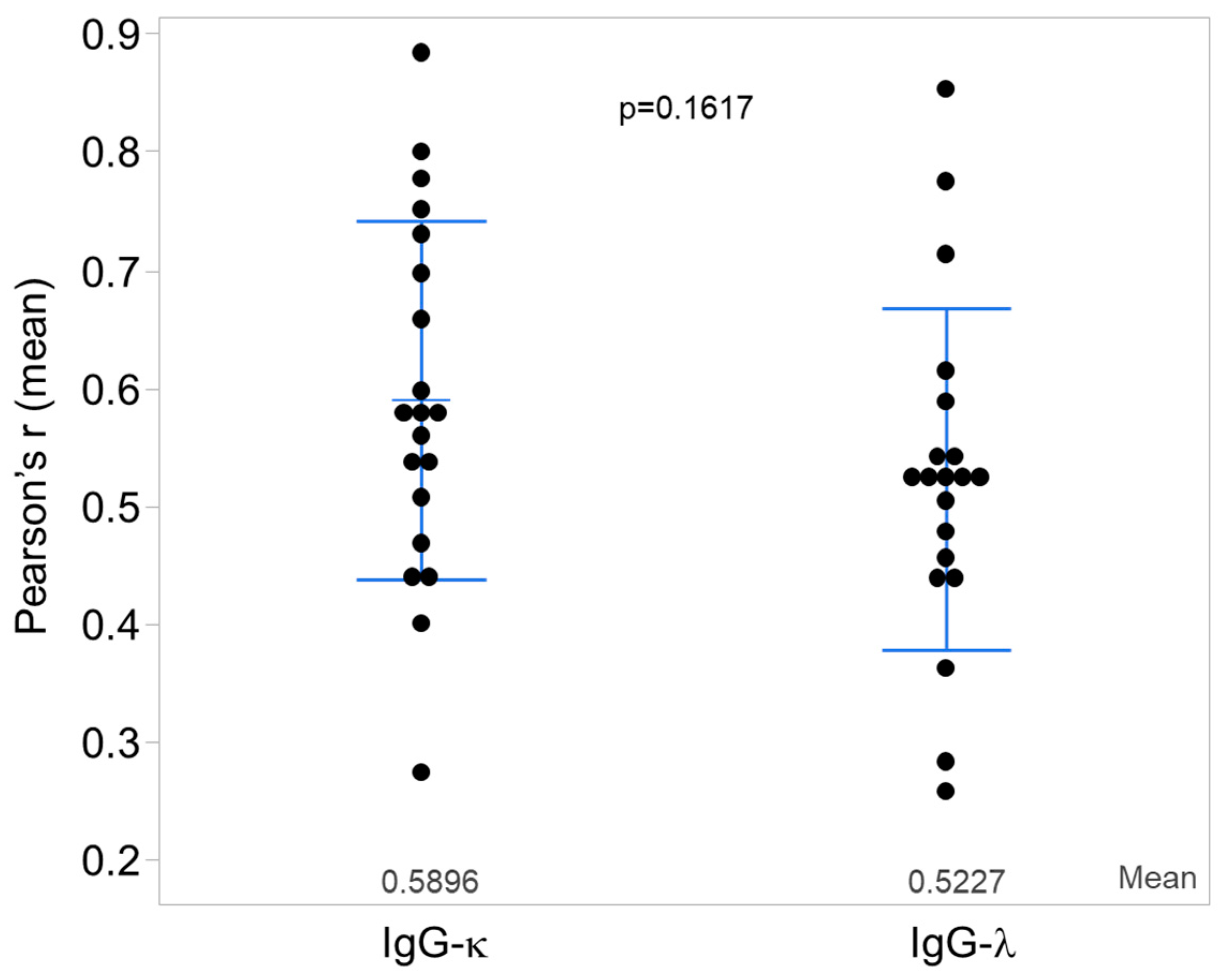
3.1. Kappa and Lambda Light-Chain Colocalization with IgG vs. IgA Heavy Chains
3.2. Correlation of Light Chain—Heavy Chain Colocalization with Oxford MEST-C Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Floege, J.; Moura, I.C.; Daha, M.R. New insights into the pathogenesis of IgA nephropathy. Semin. Immunopathol. 2014, 36, 431–442. [Google Scholar] [CrossRef]
- Haas, M. Heptinstall’s Pathology of the Kidney, 7th ed.; Jennette, J., D’Agati, V., Olson, J., Silva, F., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2015. [Google Scholar]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Placzek, W.J.; Yanagawa, H.; Makita, Y.; Renfrow, M.B.; Julian, B.A.; Rizk, D.V.; Suzuki, Y.; Novak, J.; Suzuki, H. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS ONE 2018, 13, e0190967. [Google Scholar] [CrossRef] [PubMed]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Investig. 1999, 104, 73–81. [Google Scholar] [CrossRef]
- Allen, A.C.; Bailey, E.M.; Brenchley, P.E.; Buck, K.S.; Barratt, J.; Feehally, J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001, 60, 969–973. [Google Scholar] [CrossRef]
- Hiki, Y.; Odani, H.; Takahashi, M.; Yasuda, Y.; Nishimoto, A.; Iwase, H.; Shinzato, T.; Kobayashi, Y.; Maeda, K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001, 59, 1077–1085. [Google Scholar] [CrossRef]
- Knoppova, B.; Reily, C.; Maillard, N.; Rizk, D.V.; Moldoveanu, Z.; Mestecky, J.; Raska, M.; Renfrow, M.B.; Julian, B.A.; Novak, J. The Origin and Activities of IgA1-Containing Immune Complexes in IgA Nephropathy. Front. Immunol. 2016, 7, 117. [Google Scholar] [CrossRef]
- Maillard, N.; Wyatt, R.J.; Julian, B.A.; Kiryluk, K.; Gharavi, A.; Fremeaux-Bacchi, V.; Novak, J. Current Understanding of the Role of Complement in IgA Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1503–1512. [Google Scholar] [CrossRef]
- Bellur, S.S.; Troyanov, S.; Cook, H.T.; Roberts, I.S.; Working Group of International IgA Nephropathy Network and Renal Pathology Society. Immunostaining findings in IgA nephropathy: Correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol. Dial. Transpl. 2011, 26, 2533–2536. [Google Scholar] [CrossRef]
- Suzuki, H.; Fan, R.; Zhang, Z.; Brown, R.; Hall, S.; Julian, B.A.; Chatham, W.W.; Suzuki, Y.; Wyatt, R.J.; Moldoveanu, Z.; et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Investig. 2009, 119, 1668–1677. [Google Scholar] [CrossRef]
- Rizk, D.V.; Saha, M.K.; Hall, S.; Novak, L.; Brown, R.; Huang, Z.Q.; Fatima, H.; Julian, B.A.; Novak, J. Glomerular Immunodeposits of Patients with IgA Nephropathy Are Enriched for IgG Autoantibodies Specific for Galactose-Deficient IgA1. J. Am. Soc. Nephrol. 2019, 30, 2017–2026. [Google Scholar] [CrossRef]
- Moldoveanu, Z.; Suzuki, H.; Reily, C.; Satake, K.; Novak, L.; Xu, N.; Huang, Z.Q.; Knoppova, B.; Khan, A.; Hall, S.; et al. Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J. Autoimmun. 2021, 118, 102593. [Google Scholar] [CrossRef]
- Chen, N.; Nusbaum, P.; Halbwachs-Mecarelli, L.; Lesavre, P. Light-chain composition of serum IgA1 and in vitro IgA1 production in IgA nephropathy. Nephrol. Dial. Transpl. 1991, 6, 846–850. [Google Scholar] [CrossRef]
- Suen, K.K.; Lewis, W.H.; Lai, K.N. Analysis of charge distribution of lambda- and kappa-IgA in IgA nephropathy by focused antigen capture immunoassay. Scand. J. Urol. Nephrol. 1997, 31, 289–293. [Google Scholar] [CrossRef]
- Zachova, K.; Jemelkova, J.; Kosztyu, P.; Ohyama, Y.; Takahashi, K.; Zadrazil, J.; Orsag, J.; Matousovic, K.; Galuszkova, D.; Petejova, N.; et al. Galactose-Deficient IgA1 B cells in the Circulation of IgA Nephropathy Patients Carry Preferentially Lambda Light Chains and Mucosal Homing Receptors. J. Am. Soc. Nephrol. 2022, 33, 908–917. [Google Scholar] [CrossRef]
- Leung, J.C.; Tang, S.C.; Chan, D.T.; Lui, S.L.; Lai, K.N. Increased sialylation of polymeric lambda-IgA1 in patients with IgA nephropathy. J. Clin. Lab. Anal. 2002, 16, 11–19. [Google Scholar] [CrossRef]
- Lai, K.N.; Chan, K.W.; Mac-Moune, F.; Ho, C.P.; Yan, K.W.; Lam, C.W.; Vallance-Owen, J. The immunochemical characterization of the light chains in the mesangial IgA deposits in IgA nephropathy. Am. J. Clin. Pathol. 1986, 85, 548–551. [Google Scholar] [CrossRef]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef]
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Lai, K.N.; Chui, S.H.; Lewis, W.H.; Poon, A.S.; Lam, C.W. Charge distribution of IgA-lambda in IgA nephropathy. Nephron 1994, 66, 38–44. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, F.M.; Lo, S.T.; Lam, C.W. Light chain composition of IgA in IgA nephropathy. Am. J. Kidney Dis. 1988, 11, 425–429. [Google Scholar] [CrossRef]
- Dingess, K.A.; Hoek, M.; van Rijswijk, D.M.H.; Tamara, S.; den Boer, M.A.; Veth, T.; Damen, M.J.A.; Barendregt, A.; Romijn, M.; Juncker, H.G.; et al. Identification of common and distinct origins of human serum and breastmilk IgA1 by mass spectrometry-based clonal profiling. Cell Mol. Immunol. 2023, 20, 26–37. [Google Scholar] [CrossRef]
- Kiryluk, K.; Li, Y.; Moldoveanu, Z.; Suzuki, H.; Reily, C.; Hou, P.; Xie, J.; Mladkova, N.; Prakash, S.; Fischman, C.; et al. GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLoS Genet. 2017, 13, e1006609. [Google Scholar] [CrossRef]
- Kiryluk, K.; Li, Y.; Sanna-Cherchi, S.; Rohanizadegan, M.; Suzuki, H.; Eitner, F.; Snyder, H.J.; Choi, M.; Hou, P.; Scolari, F.; et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012, 8, e1002765. [Google Scholar] [CrossRef]
- Kiryluk, K.; Li, Y.; Scolari, F.; Sanna-Cherchi, S.; Choi, M.; Verbitsky, M.; Fasel, D.; Lata, S.; Prakash, S.; Shapiro, S.; et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat. Genet. 2014, 46, 1187–1196. [Google Scholar] [CrossRef]
- Kiryluk, K.; Sanchez-Rodriguez, E.; Zhou, X.J.; Zanoni, F.; Liu, L.; Mladkova, N.; Khan, A.; Marasa, M.; Zhang, J.Y.; Balderes, O.; et al. Genome-wide association analyses define pathogenic signaling pathways and prioritize drug targets for IgA nephropathy. Nat. Genet. 2023, 55, 1091–1105. [Google Scholar] [CrossRef]
- Liu, L.; Khan, A.; Sanchez-Rodriguez, E.; Zanoni, F.; Li, Y.; Steers, N.; Balderes, O.; Zhang, J.; Krithivasan, P.; LeDesma, R.A.; et al. Genetic regulation of serum IgA levels and susceptibility to common immune, infectious, kidney, and cardio-metabolic traits. Nat. Commun. 2022, 13, 6859. [Google Scholar] [CrossRef]
- Novak, J.; Renfrow, M.B.; King, R.G.; Reily, C.; Green, T.J. Protein-based profiling of the human IgA1 clonal repertoire revealed shared clones of serum polymeric IgA1 and milk secretory IgA1. Cell Mol. Immunol. 2023, 20, 305–307. [Google Scholar] [CrossRef]
- Suzuki, H.; Raska, M.; Yamada, K.; Moldoveanu, Z.; Julian, B.A.; Wyatt, R.J.; Tomino, Y.; Gharavi, A.G.; Novak, J. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J. Biol. Chem. 2014, 289, 5330–5339. [Google Scholar] [CrossRef] [PubMed]
- Muda, A.O.; Feriozzi, S.; Rahimi, S.; Faraggiana, T. Spatial arrangement of IgA and C3 as a prognostic indicator of IgA nephropathy. J. Pathol. 1995, 177, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Giannakakis, K.; Feriozzi, S.; Perez, M.; Faraggiana, T.; Muda, A.O. Aberrantly glycosylated IgA1 in glomerular immune deposits of IgA nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Z.; Lin, S.; Hu, Y.; Liang, Y.; Qiu, W.; Chen, B.; Chen, C. Clinicopathologic and Prognostic Study of Primary IgA Nephropathy With Light Chain lambda Restriction in the Mesangial Deposits. Kidney Int. Rep. 2022, 7, 776–785. [Google Scholar] [CrossRef]
- Ravipati, P.; Freese, R.L.; Royal, V.; Bu, L.; Canetta, P.; Gipson, D.; Kallash, M.; Kiryluk, K.; Nast, C.; Reich, H.N.; et al. Clinicopathologic Significance of Predominant Lambda Light Chain Deposition in IgA Nephropathy. Kidney Int. Rep. 2022, 7, 2462–2473. [Google Scholar] [CrossRef]
- D’Amico, G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin. Nephrol. 2004, 24, 179–196. [Google Scholar] [CrossRef]

| Subject | Age (Years) | Gender | SCr (mg/dL) | eGFR (mL/min/1.73 m2) | UPCR or 24-h Urinary Protein | Oxford Classification | Routine Immunofluorescence Staining | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | E | S | T | C | IgA | IgG | IgM | C3 | κ | λ | ||||||
| 1 | 37 | M | 1.2 | 80 | 1.1 g/g | 0 | 1 | 0 | 0 | 1 | 4 | 0 | 0 | 4 | 1.5 | 2 |
| 2 | 59 | M | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 3.5 | 0 | 0.5 | 2.5 | 1.5 | 2.5 |
| 3 | 24 | M | 1.0 | 108 | 1.2 g/g | 1 | 1 | 1 | 0 | 1 | 3 | 0 | 0.5 | 1.5 | 0 | 2.5 |
| 4 | 54 | F | 11.8 | 3 | NA | 1 | 1 | 0 | 1 | 1 | 2.5 | 0 | 0 | 2 | 0.5 | 1.5 |
| 5 | 32 | M | 0.9 | 116 | 0.8 g/g | 1 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 3 | 2.5 | 3 |
| 6 | 32 | F | 1.7 | 41 | 4.8 g/d | 0 | 0 | 1 | 2 | 0 | 2.5 | 0 | 0.5 | 0.5 | 0.5 | 1.5 |
| 7 | 33 | M | 1.3 | 74 | 0.7 g/g | 0 | 1 | 1 | 0 | 1 | 4 | 0 | 0 | 1.5 | 2 | 2.5 |
| 8 | 25 | M | 1.2 | 86 | 0.8 g/g | 1 | 1 | 0 | 0 | 1 | 4 | 0 | 0 | 4 | 0.5 | 3.5 |
| 9 | 42 | F | 2.2 | 28 | NA | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1.5 | 0 | 1.5 |
| 10 | 48 | M | 1.5 | 57 | 2.0 g/d | 1 | 1 | 1 | 1 | 1 | 4 | 3 | 2 | 3 | 3 | 3 |
| 11 | 29 | F | 1.0 | 78 | NA | 0 | 0 | 1 | 0 | 1 | 3 | 1 | 0 | 2.5 | 0.5 | 2.5 |
| 12 | 40 | F | 0.6 | 116 | 4.5 g/g | 1 | 0 | 1 | 0 | 1 | 4 | 0 | 0 | 4 | 2.5 | 2.5 |
| 13 | 29 | M | 1.1 | 93 | 1.0 g/d | 1 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 2 | 1 | 2.5 |
| 14 | 38 | F | 1.6 | 42 | 1.3 g/g | 0 | 0 | 1 | 1 | 0 | 4 | 0 | 1 | 3 | 3 | 4 |
| 15 | 67 | M | 1.2 | 66 | NA | 0 | 0 | 0 | 0 | 0 | 1.5 | 0 | 0 | 1 | 0.5 | 0.5 |
| 16 | 40 | M | 1.2 | 78 | 1.0 g/g | 1 | 1 | 1 | 0 | 1 | 4 | 0 | 0 | 3.5 | 2 | 3 |
| 17 | 54 | M | 1.7 | 47 | 1.3 g/g | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 3 | 0.5 | 1.5 |
| 18 | 61 | F | 1.4 | 43 | 0.4 g/d | 1 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 2.5 | 2 | 3 |
| 19 | 48 | F | 2.5 | 23 | 1.8 g/g | 1 | 0 | 1 | 1 | 0 | 2.5 | 0 | 0 | 3 | 0 | 1.5 |
| 20 | 21 | M | 1.2 | 88 | 0.3 g/g | 1 | 1 | 1 | 0 | 0 | 4 | 0 | 1 | 4 | 2 | 2 |
| Biopsies | M0/M1 | E0/E1 | S0/S1 | T0/T1/T2 | C0/C1/C2 |
|---|---|---|---|---|---|
| All (20) | 7/13 | 11/9 | 7/13 | 14/5/1 | 10/10/0 |
| Kappa ≥ 1+ * | 4/7 | 6/5 | 3/8 | 8/3/0 | 7/4/0 |
| Lambda ≥ 1+ * | 6/13 | 10/9 | 6/13 | 13/5/1 | 9/10/0 |
| Pearson’s r IgG-κ | Pearson’s r IgG-λ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | x | SD | n | x | SD | p-Value | N | X | SD | n | x | SD | p-Value | |
| Oxford Score | Score 0 | Score 1 (or T1/T2) (C1/C2) | Score 0 | Score 1 (or T1/T2) (C1/C2) | ||||||||||
| M | 7 | 0.559 | 0.132 | 13 | 0.606 | 0.164 | 0.525 | 7 | 0.470 | 0.137 | 13 | 0.551 | 0.146 | 0.242 |
| E | 11 | 0.539 | 0.142 | 9 | 0.652 | 0.147 | 0.050 | 11 | 0.505 | 0.107 | 9 | 0.544 | 0.186 | 0.562 |
| S | 7 | 0.604 | 0.130 | 13 | 0.582 | 0.167 | 0.762 | 7 | 0.518 | 0.111 | 13 | 0.525 | 0.164 | 0.916 |
| T | 14 | 0.590 | 0.159 | 5 | 0.612 | 0.150 | 0.964 | 14 | 0.504 | 0.145 | 5 | 0.593 | 0.146 | 0.594 |
| C | 10 | 0.528 | 0.127 | 10 | 0.651 | 0.154 | 0.033 | 10 | 0.529 | 0.082 | 10 | 0.517 | 0.193 | 0.854 |
| Pearson’s r IgA-κ | Pearson’s r IgA-λ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | x | SD | n | x | SD | p-Value | n | x | SD | n | x | SD | p-Value | |
| Oxford Score | Score 0 | Score 1 (or T1/T2) (C1/C2) | Score 0 | Score 1 (or T1/T2) (C1/C2) | ||||||||||
| M | 7 | 0.756 | 0.131 | 12 | 0.799 | 0.077 | 0.385 | 7 | 0.843 | 0.043 | 12 | 0.849 | 0.054 | 0.829 |
| E | 10 | 0.787 | 0.115 | 9 | 0.779 | 0.085 | 0.881 | 10 | 0.850 | 0.064 | 9 | 0.843 | 0.027 | 0.751 |
| S | 7 | 0.782 | 0.072 | 12 | 0.784 | 0.115 | 0.978 | 7 | 0.851 | 0.030 | 12 | 0.844 | 0.059 | 0.784 |
| T | 13 | 0.800 | 0.076 | 5 | 0.797 | 0.071 | 0.951 | 13 | 0.847 | 0.051 | 5 | 0.845 | 0.054 | 0.939 |
| C | 9 | 0.783 | 0.122 | 10 | 0.784 | 0.081 | 0.983 | 9 | 0.867 | 0.044 | 10 | 0.829 | 0.049 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizk, D.V.; Novak, L.; Hall, S.D.; Moldoveanu, Z.; Julian, B.A.; Novak, J.; Haas, M. Colocalization of IgG and IgA Heavy Chains with Kappa and Lambda Light Chains in Glomerular Deposits of IgA Nephropathy Patients Using High-Resolution Confocal Microscopy and Correlation with Oxford MEST-C Scores. J. Clin. Med. 2023, 12, 7361. https://doi.org/10.3390/jcm12237361
Rizk DV, Novak L, Hall SD, Moldoveanu Z, Julian BA, Novak J, Haas M. Colocalization of IgG and IgA Heavy Chains with Kappa and Lambda Light Chains in Glomerular Deposits of IgA Nephropathy Patients Using High-Resolution Confocal Microscopy and Correlation with Oxford MEST-C Scores. Journal of Clinical Medicine. 2023; 12(23):7361. https://doi.org/10.3390/jcm12237361
Chicago/Turabian StyleRizk, Dana V., Lea Novak, Stacy D. Hall, Zina Moldoveanu, Bruce A. Julian, Jan Novak, and Mark Haas. 2023. "Colocalization of IgG and IgA Heavy Chains with Kappa and Lambda Light Chains in Glomerular Deposits of IgA Nephropathy Patients Using High-Resolution Confocal Microscopy and Correlation with Oxford MEST-C Scores" Journal of Clinical Medicine 12, no. 23: 7361. https://doi.org/10.3390/jcm12237361
APA StyleRizk, D. V., Novak, L., Hall, S. D., Moldoveanu, Z., Julian, B. A., Novak, J., & Haas, M. (2023). Colocalization of IgG and IgA Heavy Chains with Kappa and Lambda Light Chains in Glomerular Deposits of IgA Nephropathy Patients Using High-Resolution Confocal Microscopy and Correlation with Oxford MEST-C Scores. Journal of Clinical Medicine, 12(23), 7361. https://doi.org/10.3390/jcm12237361






