Multivisceral Resection for Locally Advanced Gastric Cancer: A Systematic Review and Evidence Quality Assessment
Abstract
1. Introduction
2. Materials and Methods
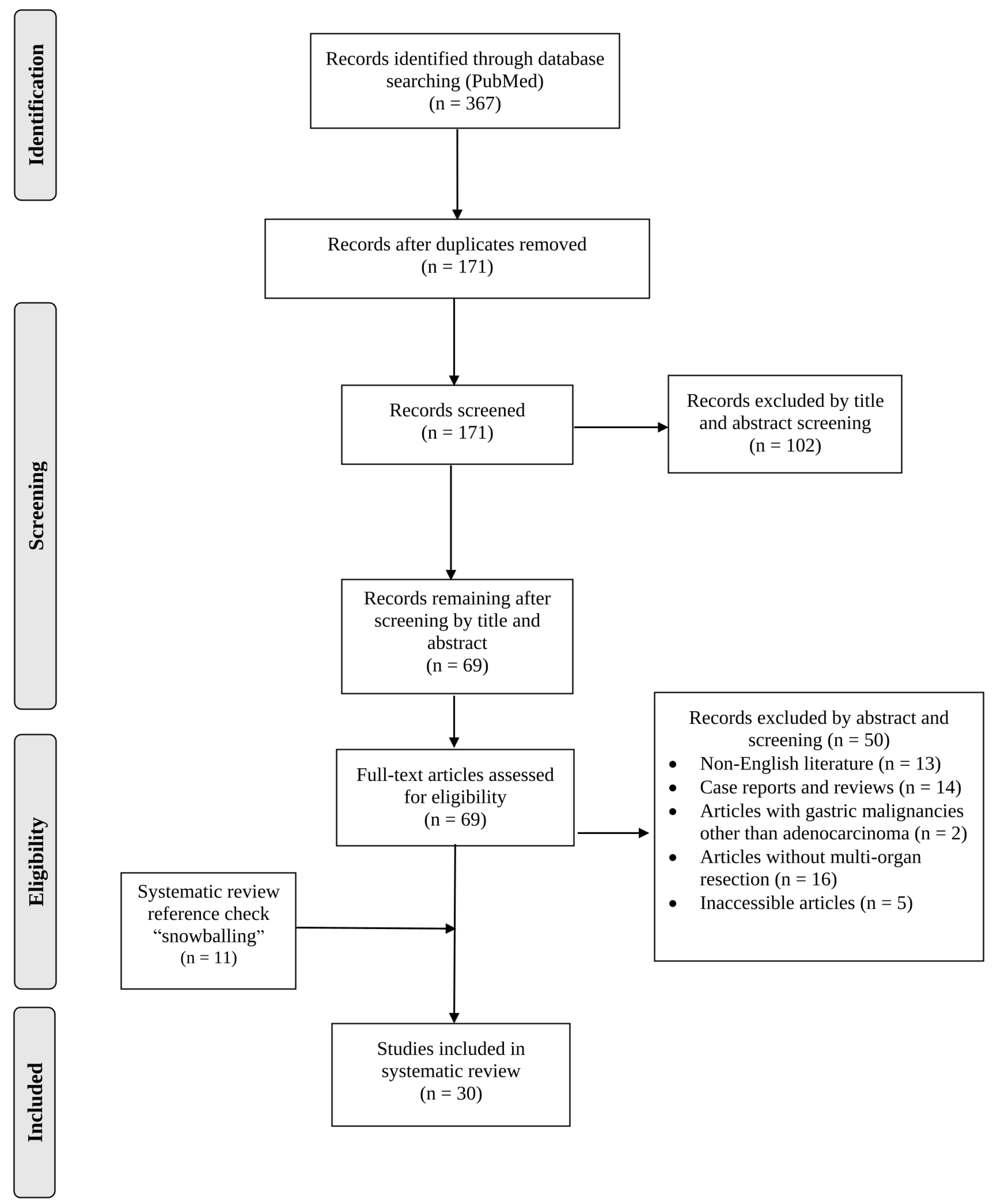
2.1. Literature Search and Inclusion Criteria
2.2. Data Extraction
2.3. Statistical Analyses
2.4. Assessment of Study Quality
3. Results
3.1. Article Selection and Patient Demographics
3.2. Quality of Evidence Assessment
3.3. Location and Characteristics of Gastric Tumors
3.4. Type of Gastrectomy
3.5. Incidence of Postoperative Complications
3.6. Survival of Patients Subjected to MVR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thrift, A.P.; Nguyen, T.H. Gastric Cancer Epidemiology. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 425–439. [Google Scholar] [CrossRef]
- Brar, S.S.; Seevaratnam, R.; Cardoso, R.; Yohanathan, L.; Law, C.; Helyer, L.; Coburn, N.G. Multivisceral resection for gastric cancer: A systematic review. Gastric Cancer 2012, 15 (Suppl. 1), 100–107. [Google Scholar] [CrossRef]
- Lyons, K.; Le, L.C.; Pham, Y.T.-H.; Borron, C.; Park, J.Y.; Tran, C.T.D.; Tran, T.V.; Tran, H.T.-T.; Vu, K.T.; Do, C.D.; et al. Gastric cancer: Epidemiology, biology, and prevention: A mini review. Eur. J. Cancer Prev. 2019, 28, 397–412. [Google Scholar] [CrossRef]
- Carboni, F.; Lepiane, P.; Santoro, R.; Lorusso, R.; Mancini, P.; Sperduti, I.; Carlini, M.; Santoro, E. Extended multiorgan resection for T4 gastric carcinoma: 25-year experience. J. Surg. Oncol. 2005, 90, 95–100. [Google Scholar] [CrossRef]
- Tawfik Amin, A.; Salem, A.A.S.; Ibrahim, A. Surgery for Locally Advanced GIT Cancers Has Potentially Good Postoperative Outcomes in a Tertiary Hospital. J. Gastrointest. Cancer 2020, 51, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Aversa, J.G.; Diggs, L.P.; Hagerty, B.L.; Dominguez, D.A.; Ituarte, P.H.G.; Hernandez, J.M.; Davis, J.L.; Blakely, A.M. Multivisceral Resection for Locally Advanced Gastric Cancer. J. Gastrointest. Surg. 2021, 25, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Devkota, M.; Sharma, A.; Chaudhary, M. Evidence Based Surgical Approach to Locally Advanced Gastric Cancer. J. Nepal Health Res. Counc. 2019, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, F.; Cusumano, G.; Rosa, F.; Marrelli, D.; Dicosmo, M.; Cipollari, C.; Marchet, A.; Scaringi, S.; Rausei, S.; di Leo, A.; et al. Multivisceral resection for locally advanced gastric cancer: An Italian multicenter observational study. JAMA Surg. 2013, 148, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.C.; Al-Hinai, A.; Gosseling-Tardif, A.; Bouchard, P.; Spicer, J.; Mulder, D.; Mueller, C.L.; Ferri, L.E. Multivisceral Resection for Locally Advanced Gastric and Gastroesophageal Junction Cancers—11-Year Experience at a High-Volume North American Center. J. Gastrointest. Surg. 2019, 23, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, M.; Xu, F.; Ye, H.; Wu, W.; Long, S.; Li, W.; He, Y. Extended multi-organ resection for cT4 gastric carcinoma: A retrospective analysis. Pak. J. Med. Sci. 2013, 29, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, M.A.; Gabrielyan, A.; Petrosyan, H.; Yesayan, S.; Shahbazyan, S.S.; Sahakyan, A.M. Extended Gastrectomy for T4b Gastric Adenocarcinoma: Single-Surgeon Experience. J. Gastrointest. Cancer 2020, 51, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ozer, I.; Bostanci, E.B.; Orug, T.; Ozogul, Y.B.; Ulas, M.; Ercan, M.; Kece, C.; Atalay, F.; Akoglu, M. Surgical outcomes and survival after multiorgan resection for locally advanced gastric cancer. Am. J. Surg. 2009, 198, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-G.; Pan, Z.-Y.; Zhang, S.; Lu, Y.-F.; Zhang, W.; Wang, L.; Meng, X.-Y.; Yu, W.-F. Living Donor Liver Transplantation in Children: Perioperative Risk Factors and a Nomogram for Prediction of Survival. Transplantation 2020, 104, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.T.; Tsai, C.Y.; Hsu, J.T.; Vinayak, R.; Liu, K.H.; Yeh, C.N.; Yeh, T.S.; Hwang, T.L.; Jan, Y.Y. Aggressive surgical approach for patients with T4 gastric carcinoma: Promise or myth? Ann. Surg. Oncol. 2011, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, H.; Tanaka, N.; Tanigawa, N.; Okajima, K. Prognostic factors in patients with advanced gastric cancer with macroscopic invasion to adjacent organs treated with radical surgery. Gastric Cancer 2000, 3, 202–210. [Google Scholar] [CrossRef]
- Jeong, O.; Choi, W.Y.; Park, Y.K. Appropriate selection of patients for combined organ resection in cases of gastric carcinoma invading adjacent organs. J. Surg. Oncol. 2009, 100, 115–120. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jang, Y.-J.; Park, S.-S.; Park, S.-H.; Kim, S.-J.; Mok, Y.-J.; Kim, C.-S. Surgical Outcomes and Prognostic Factors for T4 Gastric Cancers. Asian J. Surg. 2009, 32, 198–204. [Google Scholar] [CrossRef]
- Min, J.-S.; Jin, S.-H.; Park, S.; Kim, S.-B.; Bang, H.-Y.; Lee, J.-I. Prognosis of Curatively Resected pT4b Gastric Cancer with Respect to Invaded Organ Type. Ann. Surg. Oncol. 2012, 19, 494–501. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yang, L.-T.; Zhang, Z.-W.; Guo, J.-M.; Cheng, X.-D. Pancreaticoduodenectomy for advanced gastric cancer with pancreaticoduodenal region involvement. World J. Gastroenterol. 2008, 14, 3425–3429. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, J.; Ma, Y.; Chen, G.; Liu, Y. Multivisceral resection for locally advanced gastric cancer: A retrospective study. Am. J. Surg. 2021, 221, 1011–1017. [Google Scholar] [CrossRef]
- Mita, K.; Ito, H.; Katsube, T.; Tsuboi, A.; Yamazaki, N.; Asakawa, H.; Hayashi, T.; Fujino, K. Prognostic Factors Affecting Survival After Multivisceral Resection in Patients with Clinical T4b Gastric Cancer. J. Gastrointest. Surg. 2017, 21, 1993–1999. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, M.; Xiao, Y.; Ouyang, Y.; Tang, M.; Zhou, K.; Hong, Y.; Tang, B.; Zuo, C. Incomplete resection and linitis plastica are factors for poor survival after extended multiorgan resection in gastric cancer patients. Sci. Rep. 2017, 7, 15800. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Tsujitani, S.; Maeda, Y.; Fukuda, K.; Yamaguchi, K.; Ikeguchi, M.; Maeta, M.; Kaibara, N. Combined resection of invaded organs in patients with T4 gastric carcinoma. Gastric Cancer 2001, 4, 206–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colen, K.L.; Marcus, S.G.; Newman, E.; Berman, R.S.; Yee, H.; Hiotis, S.P. Multiorgan resection for gastric cancer: Intraoperative and computed tomography assessment of locally advanced disease is inaccurate. J. Gastrointest. Surg. 2004, 8, 899–902. [Google Scholar] [CrossRef]
- Dias, A.R.; Pereira, M.A.; Ramos, M.; Oliveira, R.J.; Ribeiro, U.; Zilberstein, B., Jr.; Cecconello, I. Prediction scores for complication and recurrence after multivisceral resection in gastric cancer. Eur. J. Surg. Oncol. 2020, 46, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Nakagohri, T.; Konishi, M.; Inoue, K.; Takahashi, S.; Itou, M.; Sugitou, M.; Ono, M.; Saito, N.; Kinoshita, T. Aggressive surgical treatment for T4 gastric cancer. J. Gastrointest. Surg. 2004, 8, 464–470. [Google Scholar] [CrossRef]
- Martin, R.C., 2nd; Jaques, D.P.; Brennan, M.F.; Karpeh, M. Achieving RO resection for locally advanced gastric cancer: Is it worth the risk of multiorgan resection? J. Am. Coll. Surg. 2002, 194, 568–577. [Google Scholar] [CrossRef]
- Shchepotin, I.B.; Chorny, V.A.; Nauta, R.J.; Shabahang, M.; Buras, R.R.; Evans, S.R. Extended surgical resection in T4 gastric cancer. Am. J. Surg. 1998, 175, 123–126. [Google Scholar] [CrossRef]
- Mita, K.; Ito, H.; Fukumoto, M.; Murabayashi, R.; Koizumi, K.; Hayashi, T.; Kikuchi, H. Surgical outcomes and survival after extended multiorgan resection for T4 gastric cancer. Am. J. Surg. 2012, 203, 107–111. [Google Scholar] [CrossRef]
- Tran, T.B.; Worhunsky, D.J.; Norton, J.A.; Squires, M.H., 3rd; Jin, L.X.; Spolverato, G.; Votanopoulos, K.I.; Schmidt, C.; Weber, S.; Bloomston, M.; et al. Multivisceral Resection for Gastric Cancer: Results from the US Gastric Cancer Collaborative. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), 840–847. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, D.; Okamura, T.; Baba, H.; Saito, A.; Sugimachi, K. Results of resection of gastric cancer extending to adjacent organs. Br. J. Surg. 1988, 75, 12–15. [Google Scholar] [CrossRef]
- Dhar, D.K.; Kubota, H.; Tachibana, M.; Kinugasa, S.; Masunaga, R.; Shibakita, M.; Kohno, H.; Nagasue, N. Prognosis of T4 gastric carcinoma patients: An appraisal of aggressive surgical treatment. J. Surg. Oncol. 2001, 76, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, S.; Nagasue, N.; Ogawa, Y.; Sasaki, Y.; Hirose, S.; Yukaya, H. The negative effect of splenectomy on the prognosis of gastric cancer. Am. J. Surg. 1984, 148, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lian, B.; Chen, J.; Song, D.; Zhao, Q. Systematic review and meta-analysis of splenectomy in gastrectomy for gastric carcinoma. Int. J. Surg. 2019, 68, 104–113. [Google Scholar] [CrossRef]
- Piso, P.; Bellin, T.; Aselmann, H.; Bektas, H.; Schlitt, H.; Klempnauer, J. Results of Combined Gastrectomy and Pancreatic Resection in Patients with Advanced Primary Gastric Carcinoma. Dig. Surg. 2002, 19, 281–285. [Google Scholar] [CrossRef]
- Radulescu, D.; Baleanu, V.D.; Padureanu, V.; Radulescu, P.M.; Bordu, S.; Patrascu, S.; Socea, B.; Bacalbasa, N.; Surlin, M.V.; Georgescu, I.; et al. Neutrophil/Lymphocyte Ratio as Predictor of Anastomotic Leak after Gastric Cancer Surgery. Diagnostics 2020, 10, 799. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Ma, Y.; Zhu, G.; Xue, Y. Prognosis and surgical treatment of gastric cancer invading adjacent organs. ANZ J. Surg. 2010, 80, 510–514. [Google Scholar] [CrossRef]
| Authors | Location n, % (95% CI). | |||
|---|---|---|---|---|
| Upper | Middle | Lower | Whole | |
| Aversa et al. [6] | 21, 11%, (7.24–16.29) | 46, 24% (18.55–30.6%) | 124, 64.9% (57.9–71.3) | NM |
| Carboni et al. [4] | 18, 29.51% (19.5–41.95) | 31, 50.8% (38.6–62.9%) | 12, 19.7% (11.5–31.5) | 2, 3.3% (0.25–11.85) |
| Cheng et al. [15] | 36, 39.56% (30.12–49.84) | 12, 13.2% (7.6–21.8%) | 35, 38.5% (29.1–48.7) | 8, 8.8% (4.3–16.6) |
| Isozaki et al. [16] | 26, 19.85% (13.87–27.55) | 45, 34.35% (26.8–42.8%) | 45, 34.35% (26.8–42.8) | 15, 11.45% (6.96–18.14) |
| Jeong et al. [17] | 20, 41.67% (28.8–55.7) | 11, 22.9% (13.15–36.7%) | 13, 27% (16.46–41.1) | 4, 8.33% (2.76–20.1) |
| Kim et al. [18] | 5, 14.7% (6–30.6) | 8, 23.5% (12.2–40.2%) | 13, 38.2% (23.9–55%) | 8, 23.5% (12.2–40.2) |
| Min et al. [19] | 24, 9.9% (6.7–14.3) | 58, 23.9% (18.9–29.6%) | 146, 60% (53.8–66%) | 15, 6.2% (3.7–10) |
| Pacelli et al. [8] | 30, 30.9% (22.6–40.7) | 45, 46.4% (36.8–56.3) | 22, 22.7% (15.4–32%) | NM |
| Molina et al. [9] | 16, 45.7% (30.46–61.8) | 13, 37.1% (23.1–53.7%) | 6, 17.1% (7.7–33.06%) | NM |
| Wang et al. [20] | NM | 12, 30% (18–45.5%) | 22, 55% (39.8–69.3%) | 6, 15% (6.7–29.5) |
| Yang et al. [21] | 48, 36.6% (28.9–45.2) | 40, 30.5% (23.3–38.9%) | 43 (32.8% (25.4–41.3%) | NM |
| Mita et al. [22] | 12, 29.3% (17.5–44.6) | 10, 24.4% (13.65–39.5%) | 15, 36.6% (23.55–51.9%) | 4, 9.8% (3.3–23.1) |
| Xiao et al. [10] | 24, 38.1% (27.1–50.5) | 17, 27% (17.5–39.1%) | 15, 23.8% (14.9–35.7) | 7, 11.1% (5.2–21.5) |
| Xiao et al. [23] | 18, 28.13% (18.5–40.2) | 20, 31.25% (21.2–43.4%) | 26, 40.6% (29.45–52.87) | NM |
| Ozer et al. [12] | 11, 19.6% (11.2–32) | 26, 46.4% (34–59.3) | 19, 33.93% (22.9–47.04%) | NM |
| Saito et al. [24] | 16, 29.1% (18.7–42.2) | 9, 16.36% (8.6–28.5) | 21, 38.2% (26.5–51.4) | 9, 16.4% (8.6–28.5) |
| Sahakyan et al. [11] | 15, 17.05% (10,5–26,35) | 28, 31.82% (23–42.16) | 30, 34.1% (25–44.5) | 14, 50% (39.8–60.2) |
| Total: | 340, 23.1% (21.04–25.35%) | 431, 29.4% (27.05–31.70%) | 607, 41.3% (38.8–43.83%) | 92, 6.3% (5.13–7.62%) |
| Authors | Organs Resected n, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| SP | C | L | P | SB | GB | O | |
| Amin et al. [5] | 12, 42.86% (26.49–60.95%) | 11, 39.29% (23.52–57.63%) | 3, 10.71% (2.9–28.01%) | 2, 7.14% (0.9–23.73%) | - | - | - |
| Carboni et al. [4] | 34, 33.01% (24.66–42.58%) | 16, 15.53%, (9.69–23.86%) | 12, 11.65% (6.65–19.41%) | 28, 27.18% (19.49–36.52%) | 1, 0.97% (0.01–5.83%) | 2, 1.94% (0.1–7.23%) | 10, 9.71% (5.19–17.13%) |
| Cheng et al. [15] | 46, 24.08% (18.55–30.64%) | 24, 12.57%, (8.53–18.07%) | 16, 8.38% (5.14–13.26%) | 54, 28.27%, (22.35–35.05%) | 12, 6.28% (3.53–10.76%) | 13, 6.81% (3.92–11.39%) | 6, 3.14% (1.29–6.84%) |
| Colen et al. [25] | 13, 37.14% (23.12–53.71%) | 5, 14.29% (5.78–29.85%) | 2, 5.71% (0.62–19.57%) | 12, 34.29% (20.76–50.92%) | 3, 8.57% (2.21–23.13%) | - | - |
| Dias et al. [26] | 32, 26.89% (19.71–35.52%) | 29, 24.37% (17.5–32.85%) | 14, 11.76% (7.02–18.9%) | 44, 36.97% (28.83–45.94%) | - | - | |
| Isozaki et al. [16] | 31, 41.33% (30.87–52.64%) | - | - | 31, 41.33% (30.87–52.64%) | - | - | 13, 17.33% (10.28–27.57%) |
| Jeong et al. [17] | 29, 35.37% (25.87–46.18%) | 9, 10.98% (5.67–19.77%) | 4, 4.88% (1.54–12.26%) | 37, 45.12% (34.81–55.87%) | - | - | 5, 6.1% (2.3–13.82%) |
| Kim et al. [18] | 13, 33.33% (20.56–49.09%) | 15, 38.46% (24.86–54.13%) | - | 10, 25.64% (14.41–41.24%) | - | 1, 2.56% (0.01–14.36%) | - |
| Kobayasbi et al. [27] | - | 35, 36.46% (27.51–46.45%) | 10, 10.42% (5.58–18.3%) | 36, 37.5% (28.46–47.5%) | - | - | 15, 15.63% (9.59–24.31%) |
| Martin et al. [28] | 251, 52.4% (47.93–56.84%) | 36, 7.52% (5.45–10.25%) | 65, 13.57% (10.78–16.94%) | 33, 6.89% (4.92–9.54%) | 27, 5.64% (3.87–8.11%) | 27, 5.64% (3.87–8.11%) | 40, 8.35% (6.17–11.19%) |
| Min et al. [19] | - | 169, 65.76% (59.76%, -71.29%) | 67, 26.07% (21.07–31.77%) | 21, 8.17% (5.35–12.22%) | - | - | - |
| Mita et al. [22] | 30, 27.03% (19.60–35.99%) | 14, 12.61% (7.54–20.18%) | 48, 43.24% (43.40–52.54%) | 12, 10.81% (6.15–18.09%) | - | 2, 1.80% (0.09–6.74%) | 7, 6.31% (2.87–12.66%) |
| Pacelli et al. [8] | 8, 5.16% (2.48–10.01%) | 43, 27.74% (21.28–35.28%) | 46, 29.68% (23.03–37.31%) | 17, 10.97% (6.87–16.95%) | - | - | 41, 26.45% (20.12–33.93%) |
| Molina et al. [9] | 12, 23.53% (13.87–36.9%) | 6, 11.76% (5.14–23.75%) | 17, 33.33% (21.92–47.08%) | 10, 19.61% (10.82–32.65%) | - | - | 6, 11.76% (5.14–23.75%) |
| Shchepotin et al. [29] | 150, 25.13% (21.81–28.76%) | 159 (26.63%, 23.24–30.32%) | 187 (31.32%, 27.73–35.16%) | 101 (16.92%, 14.12–20.14%) | - | - | - |
| Wang et al. [20] | - | 22 | - | - | - | - | |
| Yang et al. [21] | 86, 33.46% (27.97–39.44%) | 43, 16.73% (12.64–21.80%) | 81, 31.52% (26.14–37.44%) | 15, 5.84% (3.50–9.48%) | - | - | 32, 12.45% (8.92–17.09%) |
| Mita et al. [30] | 54, 48.21% (39.17–57.37%) | 10, 8.93% (4.76–15.82%) | 33, 29.46% (21.79–38.50%) | 4, 3.57% (1.10–9.12%) | 1, 0.89% (0.01–5.38%) | 1, 0.89% (0.01–5.38%) | 9, 8.04% (4.11–14.75%) |
| Xiao et al. [10] | 27, 28.13% (20.07–37.86%) | 23, 23.96% (16.47–33.45%) | 30, 31.25% (22.82–41.12%) | 16, 16.67% (10.42–25.48%) | - | - | - |
| Ozer et al. [12] | - | 18, 28.13% (18.53–40.20%) | 32, 50.00% (38.10–61.90%) | 8, 12.50% (6.22–23.03%) | - | 1, 1.56% (0.01–13.45%) | 5, 7.81% (3.00–17.40%) |
| Sahakyan et al. [11] | 34, 26.77% (19.81–35.10%) | 23, 18.11% (12.32–25.77%) | 30, 23.62% (17.04–31.76%) | 16, 12.60% (7.81–19.59%) | 8, 6.30% (3.05–12.12%) | - | 16, 12.60% (7.81–19.59%) |
| Tran et al. [31] | 76, 48.41% (40.72–56.17%) | 19 (12.10%, 7.81–18.21%) | 42 (26.75%, 20.42–34.19%) | 20 (12.74%, 8.33–18.93%) | - | - | - |
| Total: | 938, 28.98% (27.44–30.56%) | 729, 22.52% (21.11–23.99%) | 739, 22.83% (21.42–24.31%) | 527, 16.28% (15.05–17.59%) | 52, 1.61% (1.22–2.10%) | 47, 1.45% (1.09–1.93%) | 205, 6.33% (5.54–7.23%) |
| 5 | Survival after MVR | Survival after 1 Organ Resected | Survival after >1 Organs Resected | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Year | 3-Year | 5-Year | 1-Year | 3-Year | 5-Year | 1-Year | Year | 5-Year | |
| Carboni et al. [4] | NM | NM | 21.8% | NM | NM | NM | NM | NM | NM |
| Cheng et al. [15] | 55.2% | 22.4% | 12.2% | NM | NM | NM | NM | NM | NM |
| Dias et al. [26] | NM | NM | 53.4% | NM | NM | NM | NM | NM | NM |
| Isozaki et al. [16] | NM | NM | 35% | NM | NM | 40% | NM | NM | 10% |
| Jeong et al. [17] | 74.0% | 56.5% | 47.5% | NM | NM | NM | NM | NM | NM |
| Kim et al. [18] | NM | NM | 37.8% | NM | NM | NM | NM | NM | NM |
| Kobayasbi et al. [27] | 59.8% | 40.9% | 31.1% | NM | NM | NM | NM | NM | NM |
| Korenaga et al. [32] | NM | NM | NM | 78.2% | 54.2% | 39.5% | 42.9% | 21.4% | 21.4% |
| Min et al. [19] | NM | NM | 37% | NM | NM | NM | NM | NM | NM |
| Mita et al. [22] | NM | NM | NM | 82.5% | 47.4% | NM | 65.4% | 38.1% | NM |
| Pacelli et al. [8] | 60.7% | 30.3% | 27.2% | NM | NM | 32.5% | NM | NM | 17.2% |
| Molina et al. [9] | 88% | 51% | 34% | NM | NM | NM | NM | NM | NM |
| Shchepotin et al. [29] | NM | NM | 25% | NM | NM | NM | NM | NM | NM |
| Wang et al. [20] | 75% | 49.2% | 36.9% | NM | NM | NM | NM | NM | NM |
| Yang et al. [21] | 56.1% | 26.2% | 15.4% | 59.3% | 26.9% | 17.3% | 50% | 18.1% | 6.9% |
| Ozer et al. [12] | 53.3% | 36% | 28.1% | 62.3% | 40.8% | NM | 30.0% | 6.4% | NM |
| Sahakyan et al. [11] | NM | 18% | 10.8% | NM | NM | NM | NM | NM | NM |
| Total: | 65.2% (62.61–67.8%) | 33.05% (30.71–35.5%) | 30.21% (28.25–32.25%) | 64.46% (60.39–68.37%), | 42.33% (38.19–46.43%), | 32.33% (28.95–35.9%) | 47.1% (40.58–53.63%) | 21% (15.96–26.67%) | 15.17% (11.3–20%) |
| Authors | Univariate Analysis | Multivariate Analysis |
|---|---|---|
| Cheng et al. [15] | N3 status N ratio > 0.3 Bormann type IV R1 resection Lymphatic invasion Perineural invasion Pancreas invasion No liver invasion | Bormann type Curative resection Perineural invasion Nodal status No liver invasion |
| Isozaki et al. [16] | Bormann type 4 Whole stomach Upper-third stomach Dimension of tumor > 90 mm >2 invaded organs N3 status | Location of tumor Histological depth of invasion |
| Jeong et al. [17] | N3 status Lympho-vascular invasion | Lymphatic invasion |
| Kobayasbi et al. [27] | Poor differentiation Extensive vascular invasion Lymph vessel invasion Peritoneal dissemination | Peritoneal dissemination Lymph node ratio > 0.2 Poor differentiation |
| Min et al. [19] | Bormann IV Undifferentiated N3 status Pancreatic invasion | Pancreatic invasion |
| Mita et al. [22] | N3 status R1 resection Organs resected >= 2 Pancreatic resection Spleen resection | R1 resection |
| Pacelli et al. [8] | Peritoneal resection N + status R + resection | N + status Peritoneal resection R + resection |
| Molina et al. [9] | Lymph nodes involvement | Lymphatic invasion R1 resection |
| Wang et al. [20] | Tumor size (>9 cm) Advanced T stage (pT4b) Lymph node metastasis | Advanced T stage (pT4b) Lymph node metastasis |
| Yang et al. [21] | Pancreas resection Spleen resection Resection of >15 lymph nodes Vascular tumor emboli R+ resection | R+ resection Vascular tumor emboli Lymph nodes > 15 |
| Xiao et al. [10] | Total gastrectomy Whole gastric location R1 resection | R1 resection Linitis plastica |
| Xiao et al. [23] | Tumor > 7 cm R+ resection | Tumor > 7 cm Non-curative resection |
| Ozer et al. [12] | Age > 70 y >2 organs resected Positive lymph node metastasis Presence of comorbidities | Age older > 70 y Lymphatic invasion Number of organs resected >2 |
| Sahakyan et al. [11] | Total gastrectomy Obesity (BMI < 30) N3 status | Obesity (BMI < 30) Nodal stage (N3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schizas, D.; Giannakodimos, I.; Mylonas, K.S.; Kapetanakis, E.I.; Papavgeri, A.; Lianos, G.D.; Dellaportas, D.; Mastoraki, A.; Alexandrou, A. Multivisceral Resection for Locally Advanced Gastric Cancer: A Systematic Review and Evidence Quality Assessment. J. Clin. Med. 2023, 12, 7360. https://doi.org/10.3390/jcm12237360
Schizas D, Giannakodimos I, Mylonas KS, Kapetanakis EI, Papavgeri A, Lianos GD, Dellaportas D, Mastoraki A, Alexandrou A. Multivisceral Resection for Locally Advanced Gastric Cancer: A Systematic Review and Evidence Quality Assessment. Journal of Clinical Medicine. 2023; 12(23):7360. https://doi.org/10.3390/jcm12237360
Chicago/Turabian StyleSchizas, Dimitrios, Ilias Giannakodimos, Konstantinos S. Mylonas, Emmanouil I. Kapetanakis, Alexandra Papavgeri, Georgios D. Lianos, Dionysios Dellaportas, Aikaterini Mastoraki, and Andreas Alexandrou. 2023. "Multivisceral Resection for Locally Advanced Gastric Cancer: A Systematic Review and Evidence Quality Assessment" Journal of Clinical Medicine 12, no. 23: 7360. https://doi.org/10.3390/jcm12237360
APA StyleSchizas, D., Giannakodimos, I., Mylonas, K. S., Kapetanakis, E. I., Papavgeri, A., Lianos, G. D., Dellaportas, D., Mastoraki, A., & Alexandrou, A. (2023). Multivisceral Resection for Locally Advanced Gastric Cancer: A Systematic Review and Evidence Quality Assessment. Journal of Clinical Medicine, 12(23), 7360. https://doi.org/10.3390/jcm12237360







