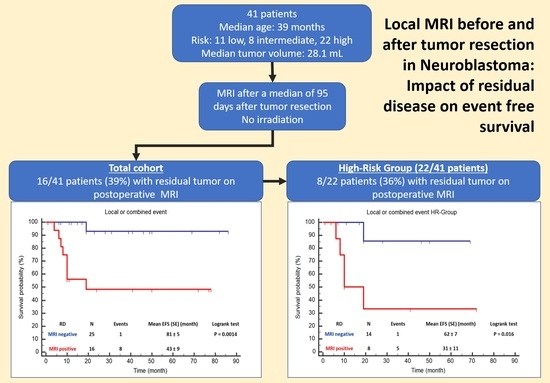
Local MRI before and after Tumor Resection in Neuroblastoma: Impact of Residual Disease on Event Free Survival
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ries, L.A.G.; Smith, M.A.; Gurney, J.; Linet, M.; Tamra, T.; Young, J.; Bunin, G. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; National Cancer Institute: Rockville, MD, USA, 1999.
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Bar-Sever, Z.; Biassoni, L.; Shulkin, B.; Kong, G.; Hofman, M.S.; Lopci, E.; Manea, I.; Koziorowski, J.; Castellani, R.; Boubaker, A.; et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2009–2024. [Google Scholar] [CrossRef]
- Brisse, H.J.; McCarville, M.B.; Granata, C.; Krug, K.B.; Wootton-Gorges, S.L.; Kanegawa, K.; Giammarile, F.; Schmidt, M.; Shulkin, B.L.; Matthay, K.K.; et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011, 261, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.L.; Alazraki, A.; Wasilewski-Masker, K.; Jones, R.A.; Porter, D.A.; Palasis, S. Diffusion-weighted Imaging Using Readout-segmented EPI Reveals Bony Metastases from Neuroblastoma. J. Pediatr. Hematol. Oncol. 2016, 38, e263–e266. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, N.D.; Gaze, M.N.; Sullivan, K.; Aldridge, M.; Waddington, W.; Almuhaideb, A.; Bomanji, J.B. 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: Diagnostic comparison and survival analysis. J. Nucl. Med. 2011, 52, 519–525. [Google Scholar] [CrossRef]
- Matthay, K.K.; Shulkin, B.; Ladenstein, R.; Michon, J.; Giammarile, F.; Lewington, V.; Pearson, A.D.; Cohn, S.L. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) Task Force. Br. J. Cancer 2010, 102, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Avanzini, S.; Pio, L.; Erminio, G.; Granata, C.; Holmes, K.; Gambart, M.; Buffa, P.; Castel, V.; Valteau Couanet, D.; Garaventa, A.; et al. Image-defined risk factors in unresectable neuroblastoma: SIOPEN study on incidence, chemotherapy-induced variation, and impact on surgical outcomes. Pediatr. Blood Cancer 2017, 64, e26605. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Trout, A.T.; Towbin, A.J. A review of neuroblastoma image-defined risk factors on magnetic resonance imaging. Pediatr. Radiol. 2018, 48, 1337–1347. [Google Scholar] [CrossRef]
- Irtan, S.; Brisse, H.J.; Minard-Colin, V.; Schleiermacher, G.; Galmiche-Rolland, L.; Le Cossec, C.; Elie, C.; Canale, S.; Michon, J.; Valteau-Couanet, D.; et al. Image-defined risk factor assessment of neurogenic tumors after neoadjuvant chemotherapy is useful for predicting intra-operative risk factors and the completeness of resection. Pediatr. Blood Cancer 2015, 62, 1543–1549. [Google Scholar] [CrossRef]
- Berthold, F.; Faldum, A.; Ernst, A.; Boos, J.; Dilloo, D.; Eggert, A.; Fischer, M.; Frühwald, M.; Henze, G.; Klingebiel, T.; et al. Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: Results of the randomized open-label GPOH trial NB2004-HR. Ann. Oncol. 2020, 31, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; Bernardi, B.D.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, S.M.; Douglas, J.G.; Hawkins, D.S.; Sanders, J.E.; Park, J.R. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer 2004, 100, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Gatcombe, H.G.; Marcus, R.B., Jr.; Katzenstein, H.M.; Tighiouart, M.; Esiashvili, N. Excellent local control from radiation therapy for high-risk neuroblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Haas-Kogan, D.A.; Swift, P.S.; Selch, M.; Haase, G.M.; Seeger, R.C.; Gerbing, R.B.; Stram, D.O.; Matthay, K.K. Impact of radiotherapy for high-risk neuroblastoma: A Children’s Cancer Group study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Wolden, S.; LaQuaglia, M.P.; Kramer, K.; Verbel, D.; Heller, G.; Cheung, N.K. Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J. Clin. Oncol. 2001, 19, 2821–2828. [Google Scholar] [CrossRef]
- Liu, K.X.; Naranjo, A.; Zhang, F.F.; DuBois, S.G.; Braunstein, S.E.; Voss, S.D.; Khanna, G.; London, W.B.; Doski, J.J.; Geiger, J.D.; et al. Prospective Evaluation of Radiation Dose Escalation in Patients With High-Risk Neuroblastoma and Gross Residual Disease After Surgery: A Report From the Children’s Oncology Group ANBL0532 Study. J. Clin. Oncol. 2020, 38, 2741–2752. [Google Scholar] [CrossRef]
- Robbins, J.R.; Krasin, M.J.; Pai Panandiker, A.S.; Watkins, A.; Wu, J.; Santana, V.M.; Furman, W.L.; Davidoff, A.M.; McGregor, L.M. Radiation therapy as part of local control of metastatic neuroblastoma: The St Jude Children’s Research Hospital experience. J. Pediatr. Surg. 2010, 45, 678–686. [Google Scholar] [CrossRef]
- Lucas, J.T., Jr.; McCarville, M.B.; Cooper, D.A.; Doubrovin, M.; Wakefield, D.; Santiago, T.; Li, Y.; Li, X.; Krasin, M.; Santana, V.; et al. Implications of Image-Defined Risk Factors and Primary-Site Response on Local Control and Radiation Treatment Delivery in the Management of High-Risk Neuroblastoma: Is There a Role for De-escalation of Adjuvant Primary-Site Radiation Therapy? Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 869–877. [Google Scholar] [CrossRef]
- Liu, K.X.; Naranjo, A.; Zhang, F.F.; Dubois, S.G.; Braunstein, S.E.; Voss, S.D.; Khanna, G.; London, W.B.; Doski, J.; Geiger, J.; et al. Role of Radiotherapy Dose-Escalation for High-Risk Neuroblastoma with Post-Surgical Primary Site Gross Residual Disease: A Report from the COG ANBL0532 Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S3. [Google Scholar] [CrossRef]
- Holmes, K.; Potschger, U.; Pearson, A.D.J.; Sarnacki, S.; Cecchetto, G.; Gomez-Chacon, J.; Squire, R.; Freud, E.; Bysiek, A.; Matthyssens, L.E.; et al. Influence of Surgical Excision on the Survival of Patients With Stage 4 High-Risk Neuroblastoma: A Report From the HR-NBL1/SIOPEN Study. J. Clin. Oncol. 2020, 38, 2902–2915. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.C.; Mayr, N.A.; Simon, J.H.; Buatti, J.M. Locoregional control in infants with neuroblastoma: Role of radiation therapy and late toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Tsiflikas, I.; Maennlin, S.; Urla, C.; Warmann, S.W.; Schaefer, J.F. Retrospective accuracy analysis of MRI based lesion size measurement in neuroblastic tumors: Which sequence should we choose? BMC Med. Imaging 2020, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- Matthyssens, L.E.; Nuchtern, J.G.; Van De Ven, C.P.; Gabra, H.O.S.; Bjornland, K.; Irtan, S.; Stenman, J.; Pio, L.; Cross, K.M.; Avanzini, S.; et al. A Novel Standard for Systematic Reporting of Neuroblastoma Surgery: The International Neuroblastoma Surgical Report Form (INSRF): A Joint Initiative by the Pediatric Oncological Cooperative Groups SIOPEN*, COG**, and GPOH***. Ann. Surg. 2022, 275, e575–e585. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Boterberg, T.; Lucas, J.; Panoff, J.; Valteau-Couanet, D.; Hero, B.; Bagatell, R.; Hill-Kayser, C.E. Neuroblastoma. Pediatr. Blood Cancer 2021, 68 (Suppl. S2), e28473. [Google Scholar] [CrossRef]
- Applebaum, M.A.; Henderson, T.O.; Lee, S.M.; Pinto, N.; Volchenboum, S.L.; Cohn, S.L. Second malignancies in patients with neuroblastoma: The effects of risk-based therapy. Pediatr. Blood Cancer 2015, 62, 128–133. [Google Scholar] [CrossRef]
- Callahan, M.J.; MacDougall, R.D.; Bixby, S.D.; Voss, S.D.; Robertson, R.L.; Cravero, J.P. Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: Patient safety considerations. Pediatr. Radiol. 2018, 48, 21–30. [Google Scholar] [CrossRef]
- Owens, C.; Li, B.K.; Thomas, K.E.; Irwin, M.S. Surveillance imaging and radiation exposure in the detection of relapsed neuroblastoma. Pediatr. Blood Cancer 2016, 63, 1786–1793. [Google Scholar] [CrossRef]
- Casey, D.L.; Kushner, B.H.; Cheung, N.V.; Modak, S.; Basu, E.M.; Roberts, S.S.; LaQuaglia, M.P.; Wolden, S.L. Reduced-Dose Radiation Therapy to the Primary Site is Effective for High-Risk Neuroblastoma: Results From a Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Jazmati, D.; Brualla, L.; Littooij, A.S.; Webber, B.; Dieckmann, K.; Janssens, G.O.; Simon, T.; Gaze, M.N.; Merta, J.; Serrano, A.; et al. Overcoming inter-observer planning variability in target volume contouring and dose planning for high-risk neuroblastoma—A European multicenter effort of the SIOPEN radiotherapy committee. Radiother. Oncol. 2023, 181, 109464. [Google Scholar] [CrossRef] [PubMed]
- Beltran, C.; Pai Panandiker, A.S.; Krasin, M.J.; Merchant, T.E. Daily image-guided localization for neuroblastoma. J. Appl. Clin. Med. Phys. 2010, 11, 3388. [Google Scholar] [CrossRef] [PubMed]
- Pai Panandiker, A.S.; Beltran, C.; Gray, J.; Hua, C. Methods for image guided and intensity modulated radiation therapy in high-risk abdominal neuroblastoma. Pract. Radiat. Oncol. 2013, 3, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Teo, B.K.; Solberg, T.; Hill-Kayser, C. Organ motion in pediatric high-risk neuroblastoma patients using four-dimensional computed tomography. J. Appl. Clin. Med. Phys. 2017, 18, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Ricciardi, G.K.; Longhi, M.; Nicolato, A. Radiosurgical options in neuro-oncology: A review on current tenets and future opportunities. Part II: Adjuvant radiobiological tools. Tumori 2015, 101, 57–63. [Google Scholar] [CrossRef]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Zivelonghi, E.; Longhi, M.; Nicolato, A. Radiosurgical options in neuro-oncology: A review on current tenets and future opportunities. Part I: Therapeutic strategies. Tumori 2014, 100, 459–465. [Google Scholar] [CrossRef]
- Schmitt, J.; Schwenck, J.; Maurer, A.; Przybille, M.; Sonanini, D.; Reischl, G.; Wehrmüller, J.E.; Quintanilla-Martinez, L.; Gillies, S.D.; Krueger, M.A.; et al. Translational immunoPET imaging using a radiolabeled GD2-specific antibody in neuroblastoma. Theranostics 2022, 12, 5615–5630. [Google Scholar] [CrossRef]
- Maric, I.; Weber, M.; Prochnow, A.; Schmitz, J.; Unger, N.; Schaarschmidt, B.M.; Poeppel, T.D.; Rischpler, C.; Bockisch, A.; Herrmann, K.; et al. Efficacy and Safety of (124)I-MIBG Dosimetry-Guided High-Activity (131)I-MIBG Therapy of Advanced Pheochromocytoma or Neuroblastoma. J. Nucl. Med. 2023, 64, 885–891. [Google Scholar] [CrossRef]
- Yang, D.D.; Liu, C.; Gao, J.; Hu, Q.J.; Liang, Y.; Liu, J. Association of image-defined risk factors with clinical features, tumor biology, and outcomes in neuroblastoma: A single-center retrospective study. Eur. J. Pediatr. 2023. [Google Scholar] [CrossRef]
- Alhilali, L.M.; Little, A.S.; Yuen, K.C.J.; Lee, J.; Ho, T.K.; Fakhran, S.; White, W.L. Early postoperative MRI and detection of residual adenoma after transsphenoidal pituitary surgery. J. Neurosurg. 2020, 134, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Marner, L.; Nysom, K.; Sehested, A.; Borgwardt, L.; Mathiasen, R.; Henriksen, O.M.; Lundemann, M.; Munck Af Rosenschold, P.; Thomsen, C.; Bogeskov, L.; et al. Early Postoperative (18)F-FET PET/MRI for Pediatric Brain and Spinal Cord Tumors. J. Nucl. Med. 2019, 60, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Olesrud, I.C.; Schulz, M.K.; Marcovic, L.; Kristensen, B.W.; Pedersen, C.B.; Kristiansen, C.; Poulsen, F.R. Early postoperative MRI after resection of brain metastases-complete tumour resection associated with prolonged survival. Acta Neurochir. 2019, 161, 555–565. [Google Scholar] [CrossRef]
- Hassan, H.A.; Bessar, M.A.; Herzallah, I.R.; Laury, A.M.; Arnaout, M.M.; Basha, M.A.A. Diagnostic value of early postoperative MRI and diffusion-weighted imaging following trans-sphenoidal resection of non-functioning pituitary macroadenomas. Clin. Radiol. 2018, 73, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Tsiflikas, I.; Fuchs, J.; Grimm, R.; Urla, C.; Esser, M.; Maennlin, S.; Ebinger, M.; Warmann, S.W.; Schafer, J.F. Feasibility and possible value of quantitative semi-automated diffusion weighted imaging volumetry of neuroblastic tumors. Cancer Imaging 2020, 20, 89. [Google Scholar] [CrossRef]
- Peschmann, A.L.; Beer, M.; Ammann, B.; Dreyhaupt, J.; Kneer, K.; Beer, A.J.; Beltinger, C.; Steinbach, D.; Cario, H.; Neubauer, H. Quantitative DWI predicts event-free survival in children with neuroblastic tumours: Preliminary findings from a retrospective cohort study. Eur. Radiol. Exp. 2019, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.; Li, M.; Muller, V.R.; Pabst, T.; Beer, M. Diagnostic Value of Diffusion-Weighted MRI for Tumor Characterization, Differentiation and Monitoring in Pediatric Patients with Neuroblastic Tumors. Rofo 2017, 189, 640–650. [Google Scholar] [CrossRef]
- Burnand, K.; Barone, G.; McHugh, K.; Cross, K. Preoperative computed tomography scanning for abdominal neuroblastomas is superior to magnetic resonance imaging for safe surgical planning. Pediatr. Blood Cancer 2019, 66, e27955. [Google Scholar] [CrossRef]
- Trout, A.T.; Towbin, A.J.; Klingbeil, L.; Weiss, B.D.; von Allmen, D. Single and multidimensional measurements underestimate neuroblastoma response to therapy. Pediatr. Blood Cancer 2017, 64, 18–24. [Google Scholar] [CrossRef]
- Orsatti, G.; Morosi, C.; Giraudo, C.; Varotto, A.; Crimì, F.; Bonzini, M.; Minotti, M.; Frigo, A.C.; Zanetti, I.; Chiaravalli, S.; et al. Pediatric Rhabdomyosarcomas: Three-Dimensional Radiological Assessments after Induction Chemotherapy Predict Survival Better than One-Dimensional and Two-Dimensional Measurements. Cancers 2020, 12, 3808. [Google Scholar] [CrossRef]
- Littooij, A.S.; de Keizer, B. Imaging in neuroblastoma. Pediatr. Radiol. 2023, 53, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.F.; Gatidis, S.; Schmidt, H.; Guckel, B.; Bezrukov, I.; Pfannenberg, C.A.; Reimold, M.; Ebinger, M.; Fuchs, J.; Claussen, C.D.; et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: Initial results. Radiology 2014, 273, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Zanzonico, P.; Staton, K.D.; Carrasquillo, J.A.; Reidy-Lagunes, D.; Lyashchenko, S.; Burnazi, E.; Zhang, H.; Lewis, J.S.; Blasberg, R.; et al. Biodistribution and Dosimetry of (18)F-Meta-Fluorobenzylguanidine: A First-in-Human PET/CT Imaging Study of Patients with Neuroendocrine Malignancies. J. Nucl. Med. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Values |
|---|---|
| Number of patients (female) | 41 (20) |
| Median age (months) | 39 (24–71) |
| Neuroblastoma (N) | 23 |
| Ganglioneuroblastoma (N) | 15 |
| Ganglioneuroma (N) | 3 |
| High risk (N) | 22 |
| Intermediate risk (N) | 8 |
| Low risk (N) | 11 |
| N-MYC Amplification | |
| Positive (N) | 7 |
| Negative (N) | 34 |
| IDRFs | |
| Negative (N) | 5 |
| Positive (N) | 36 |
| CME (N) | 30 |
| Histological regression | |
| 1 | 0 |
| 2 | 2 |
| 3 | 2 |
| 4 | 37 |
| Median Time Span between MRI and Surgery | |
| Preoperative (days) | 12 (5–30) |
| Postoperative (days) | 95 (45–141) |
| Median preoperative tumor volume (mL) | 28.1 (8.5–76.6) |
| Median postoperative tumor volume (mL) | 2.2 (0.9–6.4) |
| In 16 patients |
| Sex | Age at OP (Months) | N-MYC | Stage (INSS) | Risk | IDRFs (Numbers) | IME/CME | RD in MRI | Localization | Volume Preop (mL) | Volume Postop (mL) | Histology | Percentage of Resection | EFS (Months) | Event Localization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f | 29 | - | III | i | 3 | IME | + | PT + LN | 92.4 | 15.9 | NB | 82.8 | 16 | L * |
| m | 75 | - | IV | h | 2 | IME | + | PT | 23.7 | 7.4 | NB | 68.8 | 14 | L + M |
| m | 65 | - | III | i | 4 | IME | + | PT | 18.5 | 0.3 | NB | 98.4 | 32 | L |
| m | 86 | - | IV | h | 1 | IME | - | 22.4 | NB | 100.0 | 16 | M * | ||
| f | 51 | - | IV | h | 2 | CME | - | 22.5 | NB | 100.0 | 20 | M * | ||
| f | 59 | - | IV | h | 1 | CME | + | LN | 15.1 | 0.2 | NB | 98.7 | 13 | L + M |
| f | 9 | + | III | h | 2 | CME | + | PT | 1109.8 | 1.2 | NB | 99.9 | 15 | L + M |
| m | 31 | + | IV | h | 2 | CME | - | 2.9 | NB | 100.0 | 24 | L + M * | ||
| f | 126 | - | IV | h | 2 | CME | + | LN | 293.2 | 1.8 | GNB | 99.4 | 12 | L * |
| m | 25 | - | IV | h | 4 | CME | + | LN | 31.6 | 3.6 | GNB | 88.6 | 37 | L + M * |
| m | 5 | - | III | l | 4 | CME | + | LN | 8.3 | 0.9 | GNB | 89.7 | 8 | L + M |
| Local or Combined Event Total Cohort (N = 41) | Local or Combined Event HR Group (N = 22) | |||||
|---|---|---|---|---|---|---|
| No (N = 32) | Yes (N = 9) | p | No (N= 16) | Yes (N =6) | p | |
| Number of IDRFs | 1 (1–2.75) | 2 (2–4) | 0.04 | 2 (1–3) | 2 (1.75–2.75) | >0.05 |
| N-MYC (N) −|+ | 27|5 | 7|2 | >0.05 | 11|5 | 4|2 | >0.05 |
| Preoperative Tumor Volume (mL) | 35.3 (7.5–73.2) | 23.7 (11.7–192.8) | >0.05 | 35.3 (11.0–60.2) | 27.7 (12.1–497.4) | >0.05 |
| CME|IME (N) | 24|8 | 6|3 | >0.05 | 12|4 | 5|1 | >0.05 |
| Histological regression (N) 1|2|3|4 | 0|2|2|28 | 0|0|0|9 | >0.05 | 0|1|2|13 | 0|0|0|6 | >0.05 |
| RD by MRI (N) −|+ | 24|8 | 1|8 | 0.0004 | 13|3 | 1|5 | 0.005 |
| Postoperative Tumor Volume (mL) RD by MRI −|+ | 0|2.7 (0)|(1.2–7.1) | 0|1.5 (0)|(0.4–6.4) | >0.05 | 0|1.4 (0)|(0.5–8.4) | 0|1.8 (0)|(0.7–5.5) | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, J.F.; Gassenmaier, S.; Warmann, S.; Urla, C.; Frauenfeld, L.; Flaadt, T.; Chaika, M.; Esser, M.; Tsiflikas, I.; Timmermann, B.; et al. Local MRI before and after Tumor Resection in Neuroblastoma: Impact of Residual Disease on Event Free Survival. J. Clin. Med. 2023, 12, 7297. https://doi.org/10.3390/jcm12237297
Schäfer JF, Gassenmaier S, Warmann S, Urla C, Frauenfeld L, Flaadt T, Chaika M, Esser M, Tsiflikas I, Timmermann B, et al. Local MRI before and after Tumor Resection in Neuroblastoma: Impact of Residual Disease on Event Free Survival. Journal of Clinical Medicine. 2023; 12(23):7297. https://doi.org/10.3390/jcm12237297
Chicago/Turabian StyleSchäfer, Jürgen F., Sebastian Gassenmaier, Steven Warmann, Cristian Urla, Leonie Frauenfeld, Tim Flaadt, Maryanna Chaika, Michael Esser, Ilias Tsiflikas, Beate Timmermann, and et al. 2023. "Local MRI before and after Tumor Resection in Neuroblastoma: Impact of Residual Disease on Event Free Survival" Journal of Clinical Medicine 12, no. 23: 7297. https://doi.org/10.3390/jcm12237297
APA StyleSchäfer, J. F., Gassenmaier, S., Warmann, S., Urla, C., Frauenfeld, L., Flaadt, T., Chaika, M., Esser, M., Tsiflikas, I., Timmermann, B., & Fuchs, J. (2023). Local MRI before and after Tumor Resection in Neuroblastoma: Impact of Residual Disease on Event Free Survival. Journal of Clinical Medicine, 12(23), 7297. https://doi.org/10.3390/jcm12237297