Pulmonary Rehabilitation with and without a Cognitive Behavioral Intervention for Breathlessness in People Living with Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Interventions
2.2. Cognitive Behavioral Therapy Group (CPRP + CBT)
2.3. Social Group (CPRP + SC)
2.4. Outcomes
2.5. Sample Size Estimate
2.6. Data Management and Analysis
2.7. Changes to Trial Outcomes after Protocol Pre-Registered
3. Results
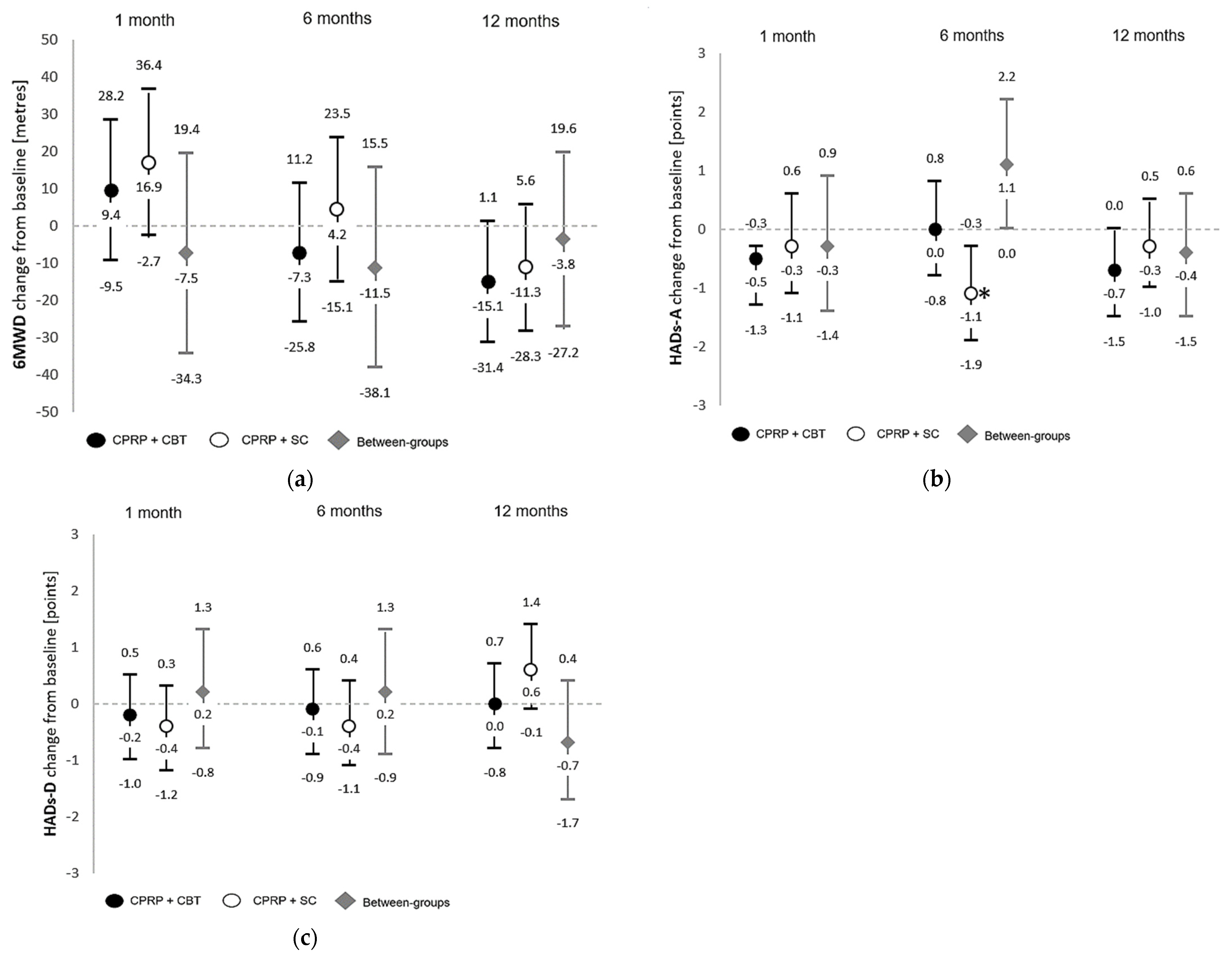
3.1. Primary Outcomes
3.2. Secondary Outcomes
3.3. Participant Feedback
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, S.; Johnson, M.J. Improving the quality of life of people with advanced respiratory disease and severe breathlessness. Breathe 2019, 15, 198–215. [Google Scholar] [CrossRef]
- Faull, O.K.; Hayen, A.; Pattinson, K.T. Breathlessness and the body: Neuroimaging clues for the inferential leap. Cortex 2017, 95, 211–221. [Google Scholar] [CrossRef]
- Mullerova, H.; Lu, C.; Li, H.; Tabberer, M. Prevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary care. PLoS ONE 2014, 9, e85540. [Google Scholar] [CrossRef]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.C.; et al. An Official Thoracic Society Statement: Update on the mechanism, assessment and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Başoğlu, M. Effective management of breathlessness: A review of potential human rights issues. Eur. Respir. J. 2017, 49, 1602099. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; O’Donnell, D.E. Activity-related dyspnea in chronic obstructive pulmonary disease: Physical and psychological consequences, unmet needs, and future directions. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Herigstad, M.; Hayen, A.; Reinecke, A.; Pattinson, K.T. Development of a dyspnoea word cue set for studies of emotional processing in COPD. Respir. Physiol. Neurobiol. 2016, 223, 37–42. [Google Scholar] [CrossRef]
- Herigstad, M.; Faull, O.K.; Hayen, A.; Evans, E.; Hardinge, F.M.; Wiech, K.; Pattinson, K.T.S. Treating breathlessness via the brain: Changes in brain activity over a course of pulmonary rehabilitation. Eur. Respir. J. 2017, 50, 1701029. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2015, CD003793. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, C.R.; Singh, S. Pulmonary rehabilitation for obstructive lung disease. Respirology 2019, 24, 871–878. [Google Scholar] [CrossRef]
- Williams, M.T.; Johnston, K.N.; Paquet, C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: Rapid review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 903–919. [Google Scholar] [CrossRef] [PubMed]
- von Leupoldt, A.; Dahme, B. Psychological aspects in the perception of dyspnea in obstructive pulmonary diseases. Respir. Med. 2007, 101, 411–422. [Google Scholar] [CrossRef] [PubMed]
- von Leupoldt, A.; Fritzsche, A.; Trueba, A.F.; Meuret, A.E.; Ritz, T. Behavioral medicine approaches to chronic obstructive pulmonary disease. Ann. Behav. Med. 2012, 44, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, A.; Sheffield, D. The efficacy of psychologically based interventions to improve anxiety, depression and quality of life in COPD: A systematic review and meta-analysis. Patient Educ. Couns. 2011, 83, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, A.; Clamor, A.; von Leupoldt, A. Effects of medical and psychological treatment on depression in patients with COPD—A review. Respir. Med. 2011, 105, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Coventry, P.A.; Bower, P.; Keyworth, C.; Kenning, C.; Knopp, J.; Garrett, C.; Hind, D.; Malpass, A.; Dickens, C. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: Systematic review and meta-analysis. PLoS ONE 2013, 8, e60532. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.S.; Sonego, S.; Ketcheson, L.; Larson, J.L. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2014, 1, e000042. [Google Scholar] [CrossRef]
- Usmani, Z.A.; Carson, K.V.; Heslop, K.; Esterman, A.J.; De Soyza, A.; Smith, B.J. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 2017, CD010673. [Google Scholar] [CrossRef]
- Pollok, J.; van Agteren, J.E.M.; Esterman, A.J.; Carson-Chahhoud, K.V. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 2019, CD012347. [Google Scholar] [CrossRef]
- Wiles, L.; Cafarella, P.; Williams, M.T. Exercise training combined with psychological interventions for people with chronic obstructive pulmonary disease: Systematic review. Respirology 2015, 20, 46–55. [Google Scholar] [CrossRef]
- Farver-Vestergaard, I.; Jacobsen, D.; Zachariae, R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Psychother. Psychosom. 2015, 84, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Junkes-Cunha, M.; Smith, J.; Vestbo, J. Management of dyspnea and anxiety in chronic obstructive pulmonary disease: A critical review. J. Am. Med. Dir. Assoc. 2017, 18, 1096.e1–1096.e17. [Google Scholar] [CrossRef] [PubMed]
- Volpato, E.; Farver-Vestergaard, I.; Brighton, L.J.; Peters, J.; Verkleij, M.; Hutchinson, A.; Heijmans, M.; von Leupoldt, A. Nonpharmacological management of psychological distress in people with COPD. Eur. Respir. Rev. 2023, 32, 220170. [Google Scholar] [CrossRef] [PubMed]
- Rzadkiewicz, M.; Nasiłowski, J. Psychosocial interventions for patients with severe COPD-An up-to-date literature review. Medicina 2019, 55, 597. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence [NICE]. Generalised Anxiety Disorder and Panic Disorder in Adults: Management. In Clinical Guideline; NICE: London, UK, 2011. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 340, c332. [Google Scholar] [CrossRef]
- Montgomery, P.; Grant, S.; Mayo-Wilson, E.; Michie, S.; Hopewell, S.; Moher, D.; CONSORT-SPI Group. Reporting randomised trials of social and psychological interventions: The CONSORT-SPI 2018 Extension. Trials 2018, 19, 407. [Google Scholar] [CrossRef]
- Williams, M.T.; John, D.; Frith, P. Comparison of the Dyspnoea-12 and Multidimensional Dyspnoea Profile in people with COPD. Eur. Respir. J. 2017, 49, 1600773. [Google Scholar] [CrossRef]
- Hunt, T.; Williams, M.T.; Olds, T.S.; Dumuid, D. Patterns of time use across the chronic obstructive pulmonary disease severity spectrum. Int. J. Environ. Res. Public Health 2018, 15, 533. [Google Scholar] [CrossRef]
- Lewthwaite, H.; Olds, T.; Williams, M.T.; Effing, T.W.; Dumuid, D. Use of time in chronic obstructive pulmonary disease: Longitudinal associations with symptoms and quality of life using a compositional analysis approach. PLoS ONE 2019, 14, e0214058. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; Global Initiative for Chronic Obstructive Lung Disease: Deer Park, IL, USA, 2011. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- American Thoracic Society. Surveillance for respiratory hazards in the occupational setting. Am. Rev. Respir. Dis. 1982, 126, 952–956. [Google Scholar]
- Williams, M.T.; Cafarella, P.; Paquet, C.; Frith, P. Cognitive behavioral therapy for management of dyspnea: A pilot study. Respir. Care 2015, 60, 1303–1313. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Smid, D.E.; Franssen, F.M.; Houben-Wilke, S.; Vanfleteren, L.E.; Janssen, D.J.; Wouters, E.F.; Spruit, M.A. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: A prospective analysis. J. Am. Med. Dir. Assoc. 2017, 18, 53–58. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Yorke, J.; Moosavi, S.H.; Shuldham, C.; Jones, P.W. Quantification of dyspnoea using descriptors: Development and initial testing of the Dyspnoea-12. Thorax 2010, 65, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Banzett, R.B.; O’Donnell, C.R.; Guilfoyle, T.E.; Parshall, M.B.; Schwartzstein, R.M.; Meek, P.M.; Gracely, R.H.; Lansing, R.W. Multidimensional Dyspnea Profile: An instrument for clinical and laboratory research. Eur. Respir. J. 2015, 45, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, M.P.; Bornefalk, H.; Skold, C.M.; Janson, C.; Blomberg, A.; Bornefalk-Hermansson, A.; Igelström, H.; Sandberg, J.; Sundh, J. Minimal clinically important differences and feasibility of Dyspnea-12 and the Multidimensional Dyspnea Profile in cardiorespiratory disease. J. Pain Symptom Manag. 2020, 60, 968–975.e1. [Google Scholar] [CrossRef]
- Williams, J.E.A.; Singh, S.J.; Sewell, L.; Guyatt, G.H.; Morgan, M.D. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax 2001, 56, 954–959. [Google Scholar] [CrossRef]
- Schünemann, H.; Puhan, M.; Goldstein, R.; Jaeschke, R.; Guyatt, G. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). COPD J. Chronic Obstr. Pulm. Dis. 2005, 2, 81–89. [Google Scholar] [CrossRef]
- Hunt, T.; Williams, M.T.; Olds, T.S. Reliability and validity of the multimedia activity recall in children and adults (MARCA) in people with chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e81274. [Google Scholar] [CrossRef][Green Version]
- Gomersall, S.R.; Olds, T.S.; Ridley, K. Development and evaluation of an adult use-of-time instrument with an energy expenditure focus. J. Sci. Med. Sport 2011, 14, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Effing, T.; Kerstjens, H.; van der Valk, P.; Zielhuis, G.; van der Palen, J. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: The COPE II study. Thorax 2009, 64, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. SAS System for Mixed Models; SAS Institute: Cary, NC, USA, 1996. [Google Scholar]
- Francis, D.J.; Fletcher, J.M.; Stuebing, K.K.; Davidson, K.C.; Thompson, N.M. Analysis of change: Modelling individual growth. J. Consult. Clin. Psychol. 1991, 59, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Mackinnon, A.; Christensen, H.; Griffiths, K. Comparison of data analysis strategies for intent-to-treat analysis in pre-test-post-test designs with substantial dropout rates. Psychiatry Res. 2008, 160, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Alison, P.D. Handling missing data by maximum likelihood, Paper 312-2012. In Proceedings of the SAS Global Forum, Orlando, FL, USA, 22–25 April 2012. [Google Scholar]
- Taylor, S.J.; Sohanpal, R.; Steed, L.; Marshall, K.; Chan, C.; Yaziji, N.; Barradell, A.C.; Font-Gilabert, P.; Healey, A.; Hooper, R.; et al. Tailored psychological intervention for anxiety or depression in COPD (TANDEM): A randomised controlled trial. Eur. Respir. J. 2023, 62, 2300432. [Google Scholar] [CrossRef]
- Luk, E.K.; Gorelik, A.; Louis, I.; Fary, K. Effectiveness of cognitive behavioral therapy in a community-based pulmonary rehabilitation programme: A controlled clinical trial. J. Rehabil. Med. 2017, 49, 264–269. [Google Scholar] [CrossRef]
- Pumar, M.I.; Roll, M.; Fung, P.; Rolls, T.A.; Walsh, J.R.; Bowman, R.V.; Fong, K.M.; Yang, I.A. A cognitive behavioural therapy (CBT) for patients with chronic lung disease and psychological comorbidities undergoing pulmonary rehabilitation. J. Thorac. Dis. 2019, 11, S2238–S2253. [Google Scholar] [CrossRef]
- Farver-Vestergaard, I.; O’Toole, M.S.; O’Connor, M.; Løkke, A.; Bendstrup, E.; Basdeo, S.A.; Cox, D.J.; Dunne, P.J.; Ruggeri, K.; Early, F.; et al. Mindfulness-based cognitive therapy in COPD: A cluster randomised controlled trial. Eur. Respir. J. 2018, 51, 1702082. [Google Scholar] [CrossRef]
- de Godoy, D.V.; de Godoy, R.F. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2003, 84, 1154–1157. [Google Scholar] [CrossRef]
- de Godoy, D.V.; de Godoy, R.F.; Becker Júnior, B.; Vaccari, P.F.; Michelli, M.; Teixeira, P.J.Z.; Palombini, B.C. The effect of psychotherapy provided as part of a pulmonary rehabilitation program for the treatment of patients with chronic obstructive pulmonary disease. J. Bras. Pneumol. 2005, 31, 499–505. [Google Scholar]
- Emery, C.F.; Schein, R.L.; Hauck, E.R.; MacIntyre, N.R. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998, 17, 232–240. [Google Scholar] [CrossRef]
- Williams, M.T.; Effing, T.W.; Paquet, C.; Gibbs, C.A.; Lewthwaite, H.; Li, L.S.K.; Phillips, A.C.; Johnston, K.N. Counseling for health behavior change in people with COPD: Systematic review. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Erueti, C.; Glasziou, P.P. Poor description of non-pharmacological interventions: Analysis of consecutive sample of randomised trials. BMJ 2013, 347, f3755. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.; Hume, E.; McNeillie, L.; Chambers, F.; Wakenshaw, L.; Burns, G.; Heslop Marshall, K.; Vogiatzis, U. Cognitive behavioural therapy combined with physical activity behavioural modification strategies during pulmonary rehabilitation in patients with COPD. ERJ Open Res. 2023, 9, 00074-2023. [Google Scholar] [CrossRef] [PubMed]
- Benzo, R.; Hoult, J.; McEvoy, C.; Clark, M.; Benzo, M.; Johnson, M.; Novotny, P. Promoting Chronic Obstructive Pulmonary Disease wellness through remote monitoring and health coaching: A clinical trial. Ann. Am. Thorac. Soc. 2022, 19, 1808–1817. [Google Scholar] [CrossRef]
- Simonÿ, C.; Andersen, I.C.; Bodtger, U.; Birkelund, R. Breathing through a troubled life—A phenomenological-hermeneutic study of chronic obstructive pulmonary disease patients’ lived experiences during the course of pulmonary rehabilitation. Int. J. Qual. Stud. Health Well-Being 2019, 14, 1647401. [Google Scholar] [CrossRef]
- Christiansen, C.F.; Løkke, A.; Bregnballe, V.; Prior, T.S.; Farver-Vestergaard, I. COPD-Related Anxiety: A systematic review of patient perspectives. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 1031–1046. [Google Scholar] [CrossRef]
- Holland, A.E.; Mahal, A.; Hill, C.J.; Lee, A.L.; Burge, A.T.; Cox, N.S.; Moore, R.; Nicolson, C.; O’Halloran, P.; Lahham, A.; et al. Home-based rehabilitation for COPD using minimal resources: A randomised, controlled equivalence trial. Thorax 2017, 72, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Farquhar, M.; Gysels, M.; Bausewein, C.; Higginson, I.J. The impact of a breathlessness intervention service (BIS) on the lives of patients with intractable dyspnea: A qualitative phase 1 study. Palliat. Support. Care 2006, 4, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Moffat, C.; Farquhar, M.; Higginson, I.J.; Burkin, J. Developing a breathlessness intervention service for patients with palliative and supportive care needs, irrespective of diagnosis. J. Palliat. Care 2011, 27, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Spathis, A.; Booth, S.; Moffat, C.; Hurst, R.; Ryan, R.; Chin, C.; Burkin, J. The Breathing, Thinking, Functioning clinical model: A proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim. Care Respir. Med. 2017, 27, 27. [Google Scholar] [CrossRef]
- Spathis, A.; Burkin, J.; Moffat, C.; Tuffnell, R.; Barclay, S.; Mant, J.; Booth, S. Cutting through complexity: The Breathing, Thinking, Functioning clinical model is an educational tool that facilitates chronic breathlessness management. NPJ Prim. Care Respir. Med. 2021, 31, 25. [Google Scholar] [CrossRef]
- Palmer, T.; Obst, S.J.; Aitken, C.R.; Walsh, J.; Sabapathy, S.; Adams, L.; Morris, N.R. Fixed-intensity exercise tests to measure exertional dyspnoea in chronic heart and lung populations: A systematic review. Eur. Respir. Rev. 2023, 32, 230016. [Google Scholar] [CrossRef]


| CPRP + CBT n = 52 | CPRP + SC n = 49 | |||
|---|---|---|---|---|
| Age (years) | 71 ± 6 | 69 ± 10 | ||
| Female: Male n= | 28: 24 | 19: 30 | ||
| Body mass index kg/m2 | 28 ± 7 | 27 ± 7 | ||
| English spoken at home n (%) | 52 (100) | 46 (94) | ||
| Current smoker n (%) | 10 (19) | 6 (12) | ||
| Mini Mental State Examination | 29.4 ± 1.3 | 29.0 ± 1.8 | ||
| COTE-Index score | 1.79 (2.55) | 1.92 (2.77) | ||
| COTE score ≥ 4 n (%) | 11 (21) | 9 (18) | ||
| FEV1 percent predicted | 48 ± 14 | 47 ± 19 | ||
| FEV1/FVC | 43 ± 13 | 42 ± 16 | ||
| PaCO2 mmHg | 40.5 ± 6.0 | 40.5 ± 6.0 | ||
| PaO2 mmHg | 73.8 ± 9.6 | 73.9 ± 11.7 | ||
| GOLD Stage n (%) | 2 | 25 (48) | 22 (45) | |
| 3 | 21 (40) | 16 (33) | ||
| 4 | 6 (12) | 11 (22) | ||
| Modified Medical Research Council dyspnea scale n (%) * | 0 | 1 (2) | 4 (8) | |
| 1 | 27 (52) | 15 (31) | ||
| 2 | 11 (21) | 6 (12) | ||
| 3 | 11 (21) | 12 (24) | ||
| 4 | 2 (4) | 12 (24) | ||
| n = 51 | n = 46 | |||
| Maximum distance 6MWD (m) | 375 ± 127 | 374 ± 146 | ||
| Before 6MWD | Perceived exertion | 0.8 ± 1.0 | 1.0 ± 1.1 | |
| MDP-A1 daily life | 4.7 ± 2.1 | 4.8 ± 2.7 | ||
| End 6MWD | Perceived exertion | 3.4 ± 1.4 | 3.7 ± 2.0 | |
| MDP-A1 | 4.3 ± 2.6 | 4.2 ± 2.9 | ||
| n = 49 | n = 47 | |||
| HADs-Anxiety | Score | 7.1 ± 4.5 | 6.8 ± 4.5 | |
| No case n (%) | 28 (57) | 27 (57) | ||
| Borderline | 10 (20) | 7 (15) | ||
| Case | 11 (22) | 13 (28) | ||
| HADs-Depression | Score | 5.9 ± 4.0 | 6.6 ± 4.2 | |
| No case n (%) * | 33 (67) | 27 (57) | ||
| Borderline * | 8 (16) | 12 (26) | ||
| Case * | 1 (2) | 8 (17) | ||
| Chronic Respiratory Questionnaire | n = 48 | n = 46 | ||
| Dyspnea | 4.5 ± 1.3 | 4.7 ± 1.5 | ||
| Fatigue | 3.8 ± 1.2 | 3.9 ± 1.3 | ||
| Emotion | 4.6 ± 1.2 | 4.8 ± 1.2 | ||
| Mastery | 4.7 ± 1.3 | 4.9 ± 1.4 | ||
| Habitual activity (accelerometry) | n = 43 | n = 38 | ||
| Mean minutes per day (awake time, excluding non-wear) | Sedentary | 713.8 ± 111.6 | 726.2 ± 154.1 | |
| Light | 257.7 ± 94.4 | 244.3 ± 115.5 | ||
| MVPA# | 7.1 ± 10.2 | 7.8 ± 9.5 | ||
| Session attendance | Education sessions (max = 8) | 6 ± 3 | 6 ± 3 | |
| Exercise sessions (max = 16) | 11 ± 5 | 11 ± 5 | ||
| CBT/social sessions (max = 8) | 5 ± 3 | 5 ± 3 | ||
| Total sessions attended (max = 32) | 21 ± 10 | 21 ± 9 | ||
| Within-Group Differences from Baseline Mean (SE) [95% CI] | Between-Group Differences Mean (SE) [95% CI] | |||
|---|---|---|---|---|
| Months Post Intervention | CPRP + CBT n = 52 | CPRP + SC n = 49 | CPRP + CBT vs. CPRP + SC [95% CI] | |
| CRQ—Dyspnea MID 0.5 [43] | 1 | 0.2 ± 0.2 [−0.3 to 0.6] | −0.4 ± 0.2 [−0.9 to 0.1] | 0.6 ± 0.3 [−0.1 to 1.2] |
| 6 | 0.0 ± 0.2 [−0.4 to 0.5] | −0.3 ± 0.2 [−0.8 to 0.1] | 0.3 ± 0.3 [−0.1 to 1.0] | |
| 12 | 0.1 ± 0.2 [−0.3 to 0.5] | −0.1 ± 0.2 [−0.5 to 0.3] | 0.2 ± 0.3 [−0.4 to 0.8] | |
| CRQ—Emotion MID 0.5 [43] | 1 | 0.1 ± 0.2 [−0.2 to 0.5] | 0.3 ± 0.2 [0.0 to 0.7] | −0.2 ± 0.2 [−0.7 to 0.3] |
| 6 | 0.2 ± 0.2 [−0.1 to 0.6] | 0.2 ± 0.2 [−0.2 to 0.6] | 0.0 ± 0.2 [−0.5 to 0.5] | |
| 12 | 0.0 ± 0.2 [−0.3 to 0.3] | −0.1 ± 0.2 [−0.5 to 0.2] | 0.1 ± 0.2 [−0.3 to 0.6] | |
| CRQ—Fatigue MID 0.5 [43] | 1 | 0.1 ± 0.2 [−0.3 to 0.5] | 0.2 ± 0.2 [−0.2 to 0.6] | −0.1 ± 0.3 [−0.7 to 0.4] |
| 6 | 0.1 ± 0.2 [−0.3 to 0.5] | −0.1 ± 0.2 [−0.5 to 0.3] | 0.2 ± 0.3 [−0.4 to 0.8] | |
| 12 | 0.2 ± 0.2 [−0.2 to 0.6] | −0.2 ± 0.0 [−0.6 to 0.2] | 0.3 ± 0.3 [−0.2 to 0.9] | |
| CRQ—Mastery MID 0.5 [43] | 1 | 0.2 ± 0.2 [−0.2 to 0.6] | 0.4 ± 0.2 [0.04 to 0.0] | −0.1 ± 0.3 [−0.7 to 0.4] |
| 6 | 0.3 ± 0.2 [0.0 to 0.7] | 0.5 ± 0.2 [0.2 to 0.9] p = 0.01 | −0.2 ± 0.3 [−0.7 to 0.3] | |
| 12 | 0.2 ± 0.2 [−0.2 to 0.5] | −0.2 ± 0.2 [−0.5 to 0.2] | 0.40 ± 0.3 [−0.1 to 0.9] | |
| Within-Group Differences from Baseline Mean ± SE [95% CI] | Between-Group Differences Mean (SE) [95% CI] | |||
|---|---|---|---|---|
| Mean Minutes Per Day (Awake Time, Excluding Non-Wear) | CPRP + CBT n = 43 | CPRP + SC n = 38 | CPRP + CBT vs. CPRP + SC [95% CI] | |
| Sedentary | Baseline | 713.8 ± 111.6 | 726.2 ± 154.1 | - |
| 1 | 39.3 ± 27.0 [−14.1 to 92.7] | −13.2 ± 30.0 [−72.7 to 46.3] | 52.5 ±40.0 [−26.8 to 131.7] | |
| 6 | 52.8 ± 28.1 [−2.8 to 108.4] | 59.8 ± 30.5 [−0.6 to 120.2] p = 0.05 | −7.0 ± 41.4 [−89.0 to 75.0] | |
| 12 | 53.3 ± 27.7 [−1.6 to 108.1] | 32.5 ± 28.9 [−24.8 to 89.8] | 20.8 ± 40.2 [−58.8 to 100.3] | |
| Light | Baseline | 257.7 ± 94.4 | 244.3 ± 115.5 | - |
| 1 | −19.3 ± 11.5 [−42.1 to 3.5] | 7.0 ± 12.9 [−18.5 to 32.5] | −26.3 ± 17.1 [−60.2 to 7.6] | |
| 6 | −26.9 ± 12.4 [−51.5 to −2.3] p = 0.03 | −4.0 ± 13.4 [−30.6 to 22.6] | −22.9 ± 18.3 [−59.1 to 13.3] | |
| 12 | −50.5 ± 13.0 [−76.3 to −24.7] p = 0.0002 | −35.1 ± 13.6 [−62.0 to −8.1] p = 0.01 | −15.4 ± 18.9 [−52.9 to 22.0] | |
| MVPA# | Baseline | 7.1 ± 10.2 | 7.8 ± 9.5 | - |
| 1 | 1.00 [0.78 to 1.29] | 0.88 [0.68 to 1.12] | 1.15 [0.81 to 1.62] | |
| 6 | 0.68 [0.49 to 0.95] p = 0.02 | 0.82 [0.63 to 1.06] | 0.83 [0.55 to 1.26] | |
| 12 | 0.63 [0.45 to 0.88] p = 0.01 | 0.78 [0.60 to 1.01] | 0.81 [0.53 to 1.23] | |
| Superdomains | Within-Group Differences from Baseline Mean ± SE [95% CI] | Between-Group Differences Mean (SE) [95% CI] | ||
|---|---|---|---|---|
| Mean Minutes Per Day unless Otherwise Stated | CPRP + CBT n = 49 | CPRP + SC n = 48 | CPRP + CBT vs. CPRP + SC [95% CI] | |
| Sleep | Baseline | 493 ± 77 | 482 ± 74 | - |
| 1 | 20.0 ± 12.8 [−5.3 to 45.3] | −11.8 ± 13.5 [−38.5 to 14.9] | 31.8 ± 18.5 [−4.6 to 68.3] | |
| 6 | 7.3 ± 13.3 [−18.9 to 33.5] | 19.6 ± 13.5 [−7.1 to 46.2] | −12.2 ± 18.9 [−49.5 to 25.0] | |
| 12 | 7.6 ± 13.7 [−19.4 to 34.6] | −5.5 ± 13.7 [−32.6 to 21.6] | 13.1 ± 19.3 [−25.0 to 51.2] | |
| Chores (indoor/outdoor) | Baseline | 192 ± 97 | 173 ± 104 | - |
| 1 | −8.2 ± 14.5 [−36.9 to 20.5] | −9.2 ± 15.3 [−39.4 to 21.1] | 0.9 ± 20.9 [−40.4 to 42.3] | |
| 6 | −13.6 ± 14.9 [−43.1 to 15.8] | −26.0 ± 15.2 [−56.0 to 4.0] | 12.4 ± 21.2 [−29.5 to 54.3] | |
| 12 | −9.2 ± 14.8 [−38.4 to 20.0] | −34.3 ± 14.8 [−63.5 to −5.0] p = 0.02 | 25.1 ± 20.8 [−16.1 to 66.3] | |
| Transport (passive, e.g., car) | Baseline | 60 ± 37 | 52 ±35 | - |
| 1 | 10.6 ± 6.7 [−2.6 to 23.8] | 7.3 ± 7.1 [−6.6 to 21.3] | 3.2 ±9.6 [−15.8 to 22.3] | |
| 6 | 9.9 ± 6.8 [−3.6 to 23.4] | 3.0 ± 7.0 [−10.7 to 16.8] | 6.8 ± 9.7 [−12.4 to 26.1] | |
| 12 | 1.0 ± 6.7 [−12.2 to 14.2] | 3.8 ± 6.7 [−9.4 to 17.1] | −2.8 ± 9.4 [−21.5 to 15.8] | |
| Screen time (television + computer use) | Baseline | 218 ± 116 | 244 ± 109 | |
| 1 | −7.5 ± 15.1 [37.2 to 22.2] | 23.6 ± 16.0 [−8.0 to 55.1] | −31.1 ± 21.8 [−74.0 to 11.9] | |
| 6 | 15.9 ± 15.7 [−15.0 to 46.8] | −9.2 ± 15.9 [−40.7 to 22.3] | 25.1 ± 22.3 [−18.9 to 69.1] | |
| 12 | 1.9 ± 16.9 [−31.5 to 35.3] | 34.9 ± 17.0 [1.4 to 68.5] | −33.0 ± 23.9 [−80.2 to 14.1] | |
| Quiet time (reading /non reading) | Baseline | 170 ± 86 | 158 ± 105 | |
| 1 | −25.4 ± 15.2 [−55.4 to 4.6] | −1.8 ± 16.1 [−33.6 to 30.0] | −23.6 ± 21.9 [−66.9 to 19.7] | |
| 6 | −10.9 ± 15.8 [−42.1 to 20.3] | 19.6 ± 16.1 [−12.2 to 51.4] | −30.5 ± 22.5 [−74.9 to 13.9] | |
| 12 | 0.2 ± 16.8 [−33.0 to 33.4] | 12.2 ± 16.8 [−21.0 to 45.5] | −12.0 ± 23.7 [−58.8 to 34.8] | |
| Self-care (grooming, bathing, eating) | Baseline | 138 ± 27 | 149 ± 26 | |
| 1 | 9.6 ± 6.4 [−2.9 to 22.1] | −12.7 ± 6.7 [−26.0 to 0.5] | 22.3 ± 9.1 [4.3 to 40.4] p = 0.02 | |
| 6 | 10.1 ± 6.6 [−2.8 to 23.0] | 18.0 ± 6.7 [−31.1 to −4.8] p = 0.01 | 28.1 ± 9.3 [9.7 to 46.5] p = 0.003 | |
| 12 | 0.4 ± 6.6 [−12.6 to 13.5] | −21.8 ± 6.6 [−34.9 to −8.8] p = 0.001 | 22.3 ± 9.3 [3.9 to 40.7] p = 0.02 | |
| Sociocultural (socializing, communicating, religious) | Baseline | 104 ± 76 | 108 ± 47 | |
| 1 | 1.3 ± 12.9 [−24.2 to 26.8] | −0.7 ± 13.6 [−27.6 to 26.2] | 2.0 ± 18.6 [−34.7 to 38.7] | |
| 6 | −5.9 ± 13.4 [−32.2 to 20.5] | 24.6 ± 13.6 [−2.3 to 51.5] | −30.4 ± 19.0 [−68.0 to 7.0] | |
| 12 | −2.0 ± 13.6 [−28.8 to 24.9] | 4.9 ± 13.6 [−22.1 to 31.8] | −6.8 ± 19.2 [−44.7 to 31.1] | |
| Physical activity (sports, exercise, active transport) #OR | Baseline | 6 ± 15 (min/day) | 9 ± 22 (min/day) | |
| 1 | 13.11 [4.40 to 39.11] p < 0.0001 | 1.13 [0.34 to 3.77] | 11.59 [2.37 to 56.65] p = 0.003 | |
| 6 | 1.92 [0.70 to 5.25] | 0.22 [0.06 to 0.80] p = 0.02 | 8.82 [1.71 to 45.52] p = 0.01 | |
| 12 | 2.55 [0.75 to 8.64] | 3.04 [0.78 to 11.81] | 0.84 [0.14 to 5.17] | |
| Work/study (occupational, non-screen) #OR | Baseline | 58 ± 80 (min/day) | 65 ± 86 (min/day) | |
| 1 | 0.39 [0.14 to 1.11] | 1.83 [0.60 to 5.53] | 0.22 [0.05 to 0.97] p = 0.05 | |
| 6 | 1.00 [0.33 to 3.05] | 0.72 [0.24 to 2.08] | 1.39 [0.30 to 6.47] | |
| 12 | 0.31 [0.11 to 0.93] p = 0.04 | 0.88 [0.30 to 2.57] | 0.36 [0.08 to 1.61] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, M.T.; Lewthwaite, H.; Paquet, C.; Cafarella, P.; Frith, P. Pulmonary Rehabilitation with and without a Cognitive Behavioral Intervention for Breathlessness in People Living with Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. J. Clin. Med. 2023, 12, 7286. https://doi.org/10.3390/jcm12237286
Williams MT, Lewthwaite H, Paquet C, Cafarella P, Frith P. Pulmonary Rehabilitation with and without a Cognitive Behavioral Intervention for Breathlessness in People Living with Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(23):7286. https://doi.org/10.3390/jcm12237286
Chicago/Turabian StyleWilliams, Marie T., Hayley Lewthwaite, Catherine Paquet, Paul Cafarella, and Peter Frith. 2023. "Pulmonary Rehabilitation with and without a Cognitive Behavioral Intervention for Breathlessness in People Living with Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 23: 7286. https://doi.org/10.3390/jcm12237286
APA StyleWilliams, M. T., Lewthwaite, H., Paquet, C., Cafarella, P., & Frith, P. (2023). Pulmonary Rehabilitation with and without a Cognitive Behavioral Intervention for Breathlessness in People Living with Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. Journal of Clinical Medicine, 12(23), 7286. https://doi.org/10.3390/jcm12237286






