A Prior History of Cryptozoospermia Is Associated with a Significantly Higher Chance of a Successful Microdissection Testicular Sperm Extraction Compared to Non-Obstructive Azoospermia
Abstract
1. Introduction
2. Methods
2.1. Patient Cohort
2.2. Surgical Procedure and Tissue Processing
2.3. Primary Outcome and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.M.; Spira, A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef]
- de Kretser, D.M. Male infertility. Lancet 1997, 349, 787–790. [Google Scholar] [CrossRef]
- Irvine, D.S. Epidemiology and aetiology of male infertility. Hum. Reprod. 1998, 13 (Suppl. 1), 33–44. [Google Scholar] [CrossRef] [PubMed]
- Acacio, B.D.; Gottfried, T.; Israel, R.; Sokol, R.Z. Evaluation of a large cohort of men presenting for a screening semen analysis. Fertil. Steril. 2000, 73, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Macleod, J. Reprint of: Semen Quality in One Thousand Men of Known Fertility and in Eight Hundred Cases of Infertile Marriage. Fertil. Steril. 2019, 112 (Suppl. 1), e28–e52. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Huang, Y.F.; Lu, N.Q. WHO Laboratory Manual for the Examination and Processing of Human Semen: Its applicability to andrology laboratories in China. Zhonghua Nan Ke Xue 2010, 16, 867–871. [Google Scholar] [PubMed]
- Bendikson, K.A.; Neri, Q.V.; Takeuchi, T.; Toschi, M.; Schlegel, P.N.; Rosenwaks, Z.; Palermo, G.D. The outcome of intracytoplasmic sperm injection using occasional spermatozoa in the ejaculate of men with spermatogenic failure. J. Urol. 2008, 180, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, S.; Keskin, I.; Kutlu, P.; Delikara, N.; Atvar, O.; Ozturk, M.I. Male infertility, azoozpermia and cryptozoospermia incidence among three infertility clinics in Turkey. Turk. J. Urol. 2018, 44, 109–113. [Google Scholar] [CrossRef]
- Tournaye, H.; Camus, M.; Goossens, A.; Liu, J.; Nagy, P.; Silber, S.; Van Steirteghem, A.C.; Devroey, P. Recent concepts in the management of infertility because of non-obstructive azoospermia. Hum. Reprod. 1995, 10 (Suppl. 1), 115–119. [Google Scholar] [CrossRef]
- Wosnitzer, M.; Goldstein, M.; Hardy, M.P. Review of Azoospermia. Spermatogenesis 2014, 4, e28218. [Google Scholar] [CrossRef]
- Jarow, J.P.; Espeland, M.A.; Lipshultz, L.I. Evaluation of the azoospermic patient. J. Urol. 1989, 142, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology. Evaluation of the azoospermic male: A committee opinion. Fertil. Steril. 2018, 109, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, N.; Kathrins, M.; Niederberger, C. Use of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with cryptozoospermia: A meta-analysis. Fertil. Steril. 2016, 105, 1469–1475.e1. [Google Scholar] [CrossRef]
- Pabuccu, E.G.; Caglar, G.S.; Tangal, S.; Haliloglu, A.H.; Pabuccu, R. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia 2017, 49, e12609. [Google Scholar] [CrossRef]
- Esteves, S.C.; Roque, M.; Bradley, C.K.; Garrido, N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: Systematic review and meta-analysis. Fertil. Steril. 2017, 108, 456–467.e1. [Google Scholar] [CrossRef]
- Arafa, M.; AlMalki, A.; AlBadr, M.; Burjaq, H.; Majzoub, A.; AlSaid, S.; Elbardisi, H. ICSI outcome in patients with high DNA fragmentation: Testicular versus ejaculated spermatozoa. Andrologia 2018, 50, e12835. [Google Scholar] [CrossRef]
- Kaiyal, R.S.; Cannarella, R.; Kuroda, S.; Parekh, N.V.; Vij, S.C.; Lundy, S.D. Clinical outcomes of cryptozoospermic patients undergoing surgical sperm retrieval. Front. Urol. 2023, 3, 1160122. [Google Scholar] [CrossRef]
- Abdel Raheem, A.; Garaffa, G.; Rushwan, N.; De Luca, F.; Zacharakis, E.; Abdel Raheem, T.; Freeman, A.; Serhal, P.; Harper, J.C.; Ralph, D. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. BJU Int. 2013, 111, 492–499. [Google Scholar] [CrossRef]
- Tsai, M.C.; Cheng, Y.S.; Lin, T.Y.; Yang, W.H.; Lin, Y.M. Clinical characteristics and reproductive outcomes in infertile men with testicular early and late maturation arrest. Urology 2012, 80, 826–832. [Google Scholar] [CrossRef]
- Weedin, J.W.; Bennett, R.C.; Fenig, D.M.; Lamb, D.J.; Lipshultz, L.I. Early versus late maturation arrest: Reproductive outcomes of testicular failure. J. Urol. 2011, 186, 621–626. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Schlegel, P.N. Microdissection testicular sperm extraction: An update. Asian J. Androl. 2013, 15, 35–39. [Google Scholar] [CrossRef]
- Alrabeeah, K.; Yafi, F.; Flageole, C.; Phillips, S.; Wachter, A.; Bissonnette, F.; Kadoch, I.J.; Zini, A. Testicular sperm aspiration for nonazoospermic men: Sperm retrieval and intracytoplasmic sperm injection outcomes. Urology 2014, 84, 1342–1346. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Palermo, G.D.; Goldstein, M.; Menendez, S.; Zaninovic, N.; Veeck, L.L.; Rosenwaks, Z. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology 1997, 49, 435–440. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Liu, J.; Joris, H.; Verheyen, G.; Tournaye, H.; Camus, M.; Derde, M.C.; Devroey, P.; Van Steirteghem, A.C. The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Hum. Reprod. 1995, 10, 1123–1129. [Google Scholar] [CrossRef]
- Hauser, R.; Bibi, G.; Yogev, L.; Carmon, A.; Azem, F.; Botchan, A.; Yavetz, H.; Klieman, S.E.; Lehavi, O.; Amit, A.; et al. Virtual azoospermia and cryptozoospermia--fresh/frozen testicular or ejaculate sperm for better IVF outcome? J. Androl. 2011, 32, 484–490. [Google Scholar] [CrossRef]
- Alkandari, M.H.; Moryousef, J.; Phillips, S.; Zini, A. Testicular Sperm Aspiration (TESA) or Microdissection Testicular Sperm Extraction (Micro-tese): Which Approach is better in Men with Cryptozoospermia and Severe Oligozoospermia? Urology 2021, 154, 164–169. [Google Scholar] [CrossRef]
- Almajed, W.; Alharbi, M.; Zini, A. Use of mini-incision microdissection testicular sperm extraction in men with cryptozoospermia and non-obstructive azoospermia. Andrology 2020, 8, 1136–1142. [Google Scholar] [CrossRef]
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 1999, 14, 131–135. [Google Scholar] [CrossRef]
- Vernaeve, V.; Verheyen, G.; Goossens, A.; Van Steirteghem, A.; Devroey, P.; Tournaye, H. How successful is repeat testicular sperm extraction in patients with azoospermia? Hum. Reprod. 2006, 21, 1551–1554. [Google Scholar] [CrossRef]
- Hussein, A. Evaluation of diagnostic testis biopsy and the repetition of testicular sperm extraction surgeries in infertility patients. Fertil. Steril. 2013, 100, 88–93. [Google Scholar] [CrossRef]
- Bromage, S.J.; Falconer, D.A.; Lieberman, B.A.; Sangar, V.; Payne, S.R. Sperm retrieval rates in subgroups of primary azoospermic males. Eur. Urol. 2007, 51, 534–539, discussion 539–540. [Google Scholar] [CrossRef]
- Zitzmann, M.; Nordhoff, V.; von Schonfeld, V.; Nordsiek-Mengede, A.; Kliesch, S.; Schuring, A.N.; Luetjens, C.M.; Kamischke, A.; Cooper, T.; Simoni, M.; et al. Elevated follicle-stimulating hormone levels and the chances for azoospermic men to become fathers after retrieval of elongated spermatids from cryopreserved testicular tissue. Fertil. Steril. 2006, 86, 339–347. [Google Scholar] [CrossRef]
- Souza, C.A.; Cunha Filho, J.S.; Santos, D.; Gratao, A.; Freitas, F.M.; Passos, E.P. Predictive factors for motile sperm recovery using testicular biopsy in nonobstructive azoospermic patients. Int. Urol. Nephrol. 2003, 35, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H.; Verheyen, G.; Nagy, P.; Ubaldi, F.; Goossens, A.; Silber, S.; Van Steirteghem, A.C.; Devroey, P. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum. Reprod. 1997, 12, 80–86. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Induction of acrosome reaction in human spermatozoa used for subzonal insemination. Hum. Reprod. 1992, 7, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ng, S.C. Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool. 1999, 284, 696–704. [Google Scholar] [CrossRef]
- Evenson, D.P.; Jost, L.K.; Marshall, D.; Zinaman, M.J.; Clegg, E.; Purvis, K.; de Angelis, P.; Claussen, O.P. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum. Reprod. 1999, 14, 1039–1049. [Google Scholar] [CrossRef]
- Tesarik, J. Paternal effects on cell division in the human preimplantation embryo. Reprod. Biomed. Online 2005, 10, 370–375. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Pourmasumi, S.; Sabeti, P.; Rahiminia, T.; Mangoli, E.; Tabibnejad, N.; Talebi, A.R. The etiologies of DNA abnormalities in male infertility: An assessment and review. Int. J. Reprod. Biomed. 2017, 15, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Moskovtsev, S.I.; Alladin, N.; Lo, K.C.; Jarvi, K.; Mullen, J.B.; Librach, C.L. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst. Biol. Reprod. Med. 2012, 58, 142–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, R.H.; Greene, C.; Rademaker, A.; Barclay, L.; Ko, E.; Chernos, J. Chromosome analysis of spermatozoa extracted from testes of men with non-obstructive azoospermia. Hum. Reprod. 2000, 15, 1121–1124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colaco, S.; Sakkas, D. Paternal factors contributing to embryo quality. J. Assist. Reprod. Genet. 2018, 35, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.N.; Hsiao, Y.W.; Chen, C.Y.; Wu, C.C. Testicular sperm is superior to ejaculated sperm for ICSI in cryptozoospermia: An update systematic review and meta-analysis. Sci. Rep. 2018, 8, 7874. [Google Scholar] [CrossRef] [PubMed]
- Ku, F.Y.; Wu, C.C.; Hsiao, Y.W.; Kang, Y.N. Association of sperm source with miscarriage and take-home baby after ICSI in cryptozoospermia: A meta-analysis of testicular and ejaculated sperm. Andrology 2018, 6, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, J.A.; Brant, A.; Kang, C.; Punjani, N.; Xie, P.; Zaninovic, N.; Palermo, G.D.; Rosenwaks, Z.; Schlegel, P.N. Successful cryptozoospermia management with multiple semen specimen collection. Fertil. Steril. 2023, 120, 996–1003. [Google Scholar] [CrossRef]
- Schoor, R.A.; Elhanbly, S.; Niederberger, C.S.; Ross, L.S. The role of testicular biopsy in the modern management of male infertility. J. Urol. 2002, 167, 197–200. [Google Scholar] [CrossRef]
- Ramasamy, R.; Lin, K.; Gosden, L.V.; Rosenwaks, Z.; Palermo, G.D.; Schlegel, P.N. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil. Steril. 2009, 92, 590–593. [Google Scholar] [CrossRef]
- Reifsnyder, J.E.; Ramasamy, R.; Husseini, J.; Schlegel, P.N. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J. Urol. 2012, 188, 532–536. [Google Scholar] [CrossRef]
- Pozzi, E.; Raffo, M.; Negri, F.; Boeri, L.; Sacca, A.; Belladelli, F.; Cilio, S.; Ventimiglia, E.; d’Arma, A.; Pagliardini, L.; et al. Anti-Mullerian hormone predicts positive sperm retrieval in men with idiopathic non-obstructive azoospermia-findings from a multi-centric cross-sectional study. Hum. Reprod. 2023, 38, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
| Retrieval Successful | Retrieval Not Successful | Total | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p Value | |
| Age | 0.3346 | ||||||
| Median | 34 | 34 | 34 | ||||
| IQR | (31–40) | (31–36) | (31–38) | ||||
| Semen Analysis | <0.0001 | ||||||
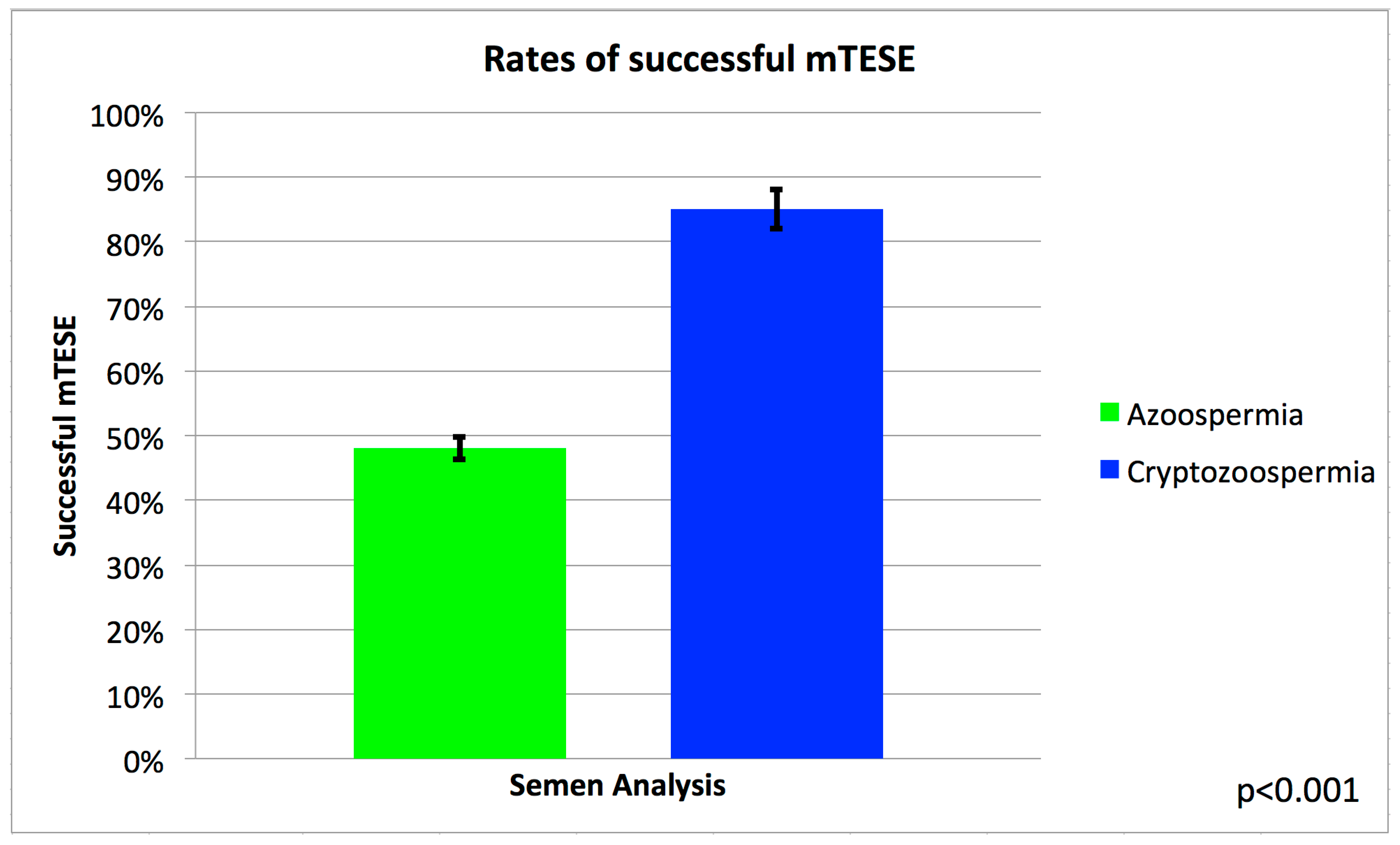
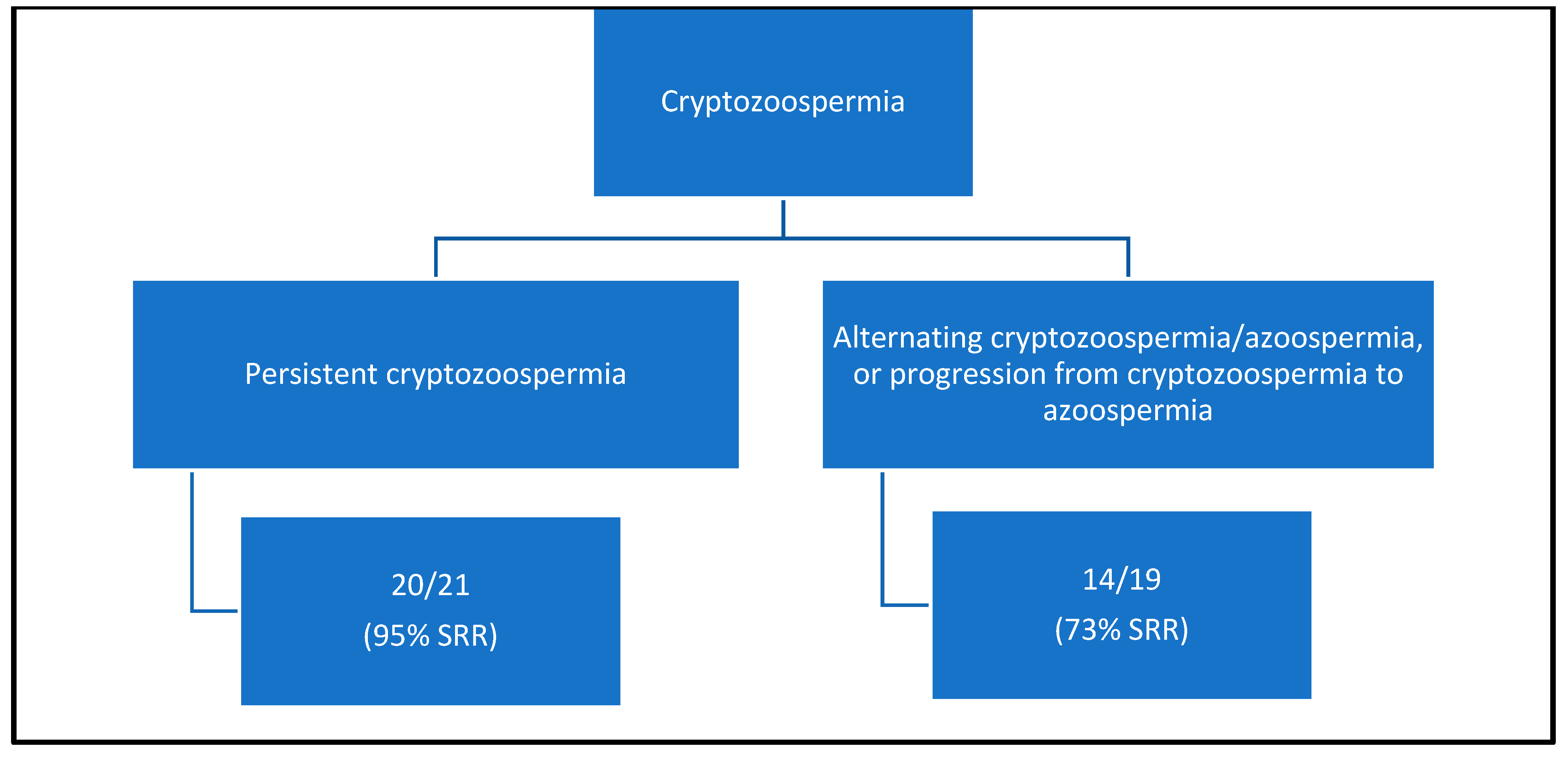
| Cryptozoospermia | 34 | (24.6) | 6 | (4.9) | 40 | (15.3) | |
| Azoospermia | 104 | (75.4) | 117 | (95.1) | 221 | (84.7) | |
| Genetic Diagnosis | 0.3975 | ||||||
| Yes | 12 | (12.2) | 16 | (16.5) | 28 | (14.4) | |
| No | 86 | (87.8) | 81 | (83.5) | 167 | (85.6) | |
| Varicocele | 0.7010 | ||||||
| Yes | 19 | (13.8) | 19 | (15.4) | 38 | (14.6) | |
| No | 119 | (86.2) | 104 | (84.6) | 223 | (85.4) | |
| Cryptorchidism | 0.6489 | ||||||
| Yes | 11 | (8.0) | 8 | (6.5) | 19 | (7.3) | |
| No | 127 | (92.0) | 115 | (93.5) | 242 | (92.7) | |
| Cancer/chemotherapy | 0.9901 | ||||||
| Yes | 19 | (13.8) | 17 | (13.8) | 36 | (13.8) | |
| No | 119 | (86.2) | 106 | (86.2) | 225 | (86.2) | |
| Idiopathic | 0.4836 | ||||||
| Yes | 80 | (58.0) | 66 | (53.7) | 146 | (55.9) | |
| No | 58 | (42.0) | 57 | (46.3) | 115 | (44.1) | |
| Sertoli Cell Only | <0.0001 | ||||||
| Yes | 29 | (22.7) | 90 | (75.0) | 119 | (48.0) | |
| No | 99 | (77.3) | 30 | (25.0) | 129 | (52.0) | |
| Hypospermatogenesis | <0.0001 | ||||||
| Yes | 39 | (30.5) | 3 | (2.5) | 42 | (16.9) | |
| No | 89 | (69.5) | 117 | (97.5) | 206 | (83.1) | |
| Maturation Arrest | 0.1375 | ||||||
| Yes | 17 | (13.3) | 9 | (7.5) | 26 | (10.5) | |
| No | 111 | (86.7) | 111 | (92.5) | 222 | (89.5) | |
| Active Spermatogenesis | <0.0001 | ||||||
| Yes | 25 | (19.5) | 0 | (0.0) | 25 | (10.1) | |
| No | 103 | (80.5) | 120 | (100.0) | 223 | (89.9) | |
| Tubular Atrophy | 0.1951 | ||||||
| Yes | 8 | (6.3) | 13 | (10.8) | 21 | (8.5) | |
| No | 120 | (93.8) | 107 | (89.2) | 227 | (91.5) | |
| Hyalinization | 0.4619 | ||||||
| Yes | 8 | (6.3) | 5 | (4.2) | 13 | (5.2) | |
| No | 120 | (93.8) | 115 | (95.8) | 235 | (94.8) | |
| Fibrosis | 0.4985 | ||||||
| Yes | 2 | (1.6) | 0 | (0.0) | 2 | (0.8) | |
| No | 126 | (98.4) | 120 | (100.0) | 246 | (99.2) | |
| Preoperative Testosterone | 0.0017 | ||||||
| <300 | 25 | (18.8) | 43 | (36.4) | 68 | (27.1) | |
| ≥300 | 108 | (81.2) | 75 | (63.6) | 183 | (72.9) | |
| Preoperative FSH | 0.0002 | ||||||
| <7.6 | 38 | (29.5) | 12 | (10.2) | 50 | (20.2) | |
| ≥7.6 | 91 | (70.5) | 106 | (89.8) | 197 | (79.8) | |
| Preoperative Testosterone | 0.0877 | ||||||
| Median | 375 | 347 | 364 | ||||
| IQR | (321–459) | (258–467) | (293–463) | ||||
| Preoperative FSH | 0.0008 | ||||||
| Median | 15.6 | 22.1 | 19.3 | ||||
| IQR | (5.9–27.1) | (14.3–27.1) | (9.7–27.1) | ||||
| Total Testicular Volume | 0.1608 | ||||||
| Median | 24 | 20 | 22 | ||||
| IQR | (16–36) | (14–32) | (15–32) | ||||
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Age | 1.01 | (0.96–1.06) | 0.6573 |
| Semen Analysis | |||
| Cryptozoospermia | 5.56 | (1.79–17.29) | 0.003 |
| Azoospermia | Ref | -- | -- |
| Genetic Diagnosis | |||
| Yes | 0.79 | (0.35–1.79) | 0.5726 |
| No | Ref | -- | -- |
| Varicocele | |||
| Yes | 1.45 | (0.63–3.3) | 0.3804 |
| No | Ref | -- | -- |
| Cryptorchidism | |||
| Yes | 1.1 | (0.34–3.56) | 0.8717 |
| No | Ref | -- | -- |
| Cancer/chemotherapy | |||
| Yes | 1.11 | (0.42–2.93) | 0.8403 |
| No | Ref | -- | -- |
| Idiopathic | |||
| Yes | 0.93 | (0.52–1.68) | 0.8098 |
| No | Ref | -- | -- |
| Sertoli Cell Only | |||
| Yes | 0.08 | (0.04–0.16) | <0.0001 |
| No | Ref | -- | -- |
| Hypospermatogenesis | |||
| Yes | 24.81 | (5.71–107.87) | <0.0001 |
| No | Ref | -- | -- |
| Maturation Arrest | |||
| Yes | 2.08 | (0.67–6.49) | 0.2049 |
| No | Ref | -- | -- |
| Tubular Atrophy | |||
| Yes | 0.53 | (0.15–1.81) | 0.3074 |
| No | Ref | -- | -- |
| Hyalinization | |||
| Yes | 1.34 | (0.39–4.55) | 0.6425 |
| No | Ref | -- | -- |
| Preoperative Testosterone | |||
| <300 | 0.3 | (0.15–0.61) | 0.0008 |
| ≥300 | Ref | -- | -- |
| Preoperative FSH | |||
| <7.6 | Ref | -- | -- |
| ≥7.6 | 0.27 | (0.11–0.65) | 0.0032 |
| Preoperative Testosterone | 1 | (1–1) | 0.1967 |
| Preoperative FSH | 1 | (0.99–1.01) | 0.9029 |
| Total Testicular Volume | 1.01 | (0.98–1.03) | 0.7157 |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Semen Analysis | |||
| Cryptozoospermia | 5.11 | (1.79–14.60) | 0.0024 |
| Azoospermia | ref | -- | -- |
| Sertoli Cell Only | |||
| Yes | 0.18 | (0.09–0.35) | <0.0001 |
| No | ref | -- | -- |
| Hypospermatogenesis | |||
| Yes | 6.16 | (1.68–22.65) | 0.0062 |
| No | ref | -- | -- |
| Preoperative Testosterone | |||
| <300 | 0.4 | (0.19–0.85) | 0.0165 |
| ≥300 | ref | -- | -- |
| Preoperative FSH | |||
| <7.6 | ref | -- | -- |
| ≥7.6 | 0.49 | (0.20–1.20) | 0.1204 |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Semen Analysis | |||
| Cryptozoospermia | 5.41 | (1.94–15.08) | 0.0013 |
| Azoospermia | ref | -- | -- |
| Sertoli Cell Only | |||
| Yes | 0.16 | (0.08–0.31) | <0.0001 |
| No | ref | -- | -- |
| Hypospermatogenesis | |||
| Yes | 5.82 | (1.61–21.05) | 0.0073 |
| No | ref | -- | -- |
| Preoperative Testosterone | |||
| <300 | 0.44 | (0.21–0.90) | 0.0251 |
| ≥300 | ref | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wren, J.; Hudnall, M.; Pham, M.; Darves-Bornoz, A.L.; Halpern, J.A.; Bennett, N.E.; Brannigan, R.E.; Hofer, M.D. A Prior History of Cryptozoospermia Is Associated with a Significantly Higher Chance of a Successful Microdissection Testicular Sperm Extraction Compared to Non-Obstructive Azoospermia. J. Clin. Med. 2023, 12, 7255. https://doi.org/10.3390/jcm12237255
Wren J, Hudnall M, Pham M, Darves-Bornoz AL, Halpern JA, Bennett NE, Brannigan RE, Hofer MD. A Prior History of Cryptozoospermia Is Associated with a Significantly Higher Chance of a Successful Microdissection Testicular Sperm Extraction Compared to Non-Obstructive Azoospermia. Journal of Clinical Medicine. 2023; 12(23):7255. https://doi.org/10.3390/jcm12237255
Chicago/Turabian StyleWren, James, Matthew Hudnall, Minh Pham, Anne L. Darves-Bornoz, Joshua A. Halpern, Nelson E. Bennett, Robert E. Brannigan, and Matthias D. Hofer. 2023. "A Prior History of Cryptozoospermia Is Associated with a Significantly Higher Chance of a Successful Microdissection Testicular Sperm Extraction Compared to Non-Obstructive Azoospermia" Journal of Clinical Medicine 12, no. 23: 7255. https://doi.org/10.3390/jcm12237255
APA StyleWren, J., Hudnall, M., Pham, M., Darves-Bornoz, A. L., Halpern, J. A., Bennett, N. E., Brannigan, R. E., & Hofer, M. D. (2023). A Prior History of Cryptozoospermia Is Associated with a Significantly Higher Chance of a Successful Microdissection Testicular Sperm Extraction Compared to Non-Obstructive Azoospermia. Journal of Clinical Medicine, 12(23), 7255. https://doi.org/10.3390/jcm12237255






