Comparison of the iStent Inject® versus the iStent Inject® W—Both in Combination with Cataract Surgery—In Open-Angle Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Assessments
2.3. Cataract Surgery and iStent® Implantation
2.4. Outcome Measurements
2.5. Peri- and Postoperative Complications
2.6. Statistical Analysis
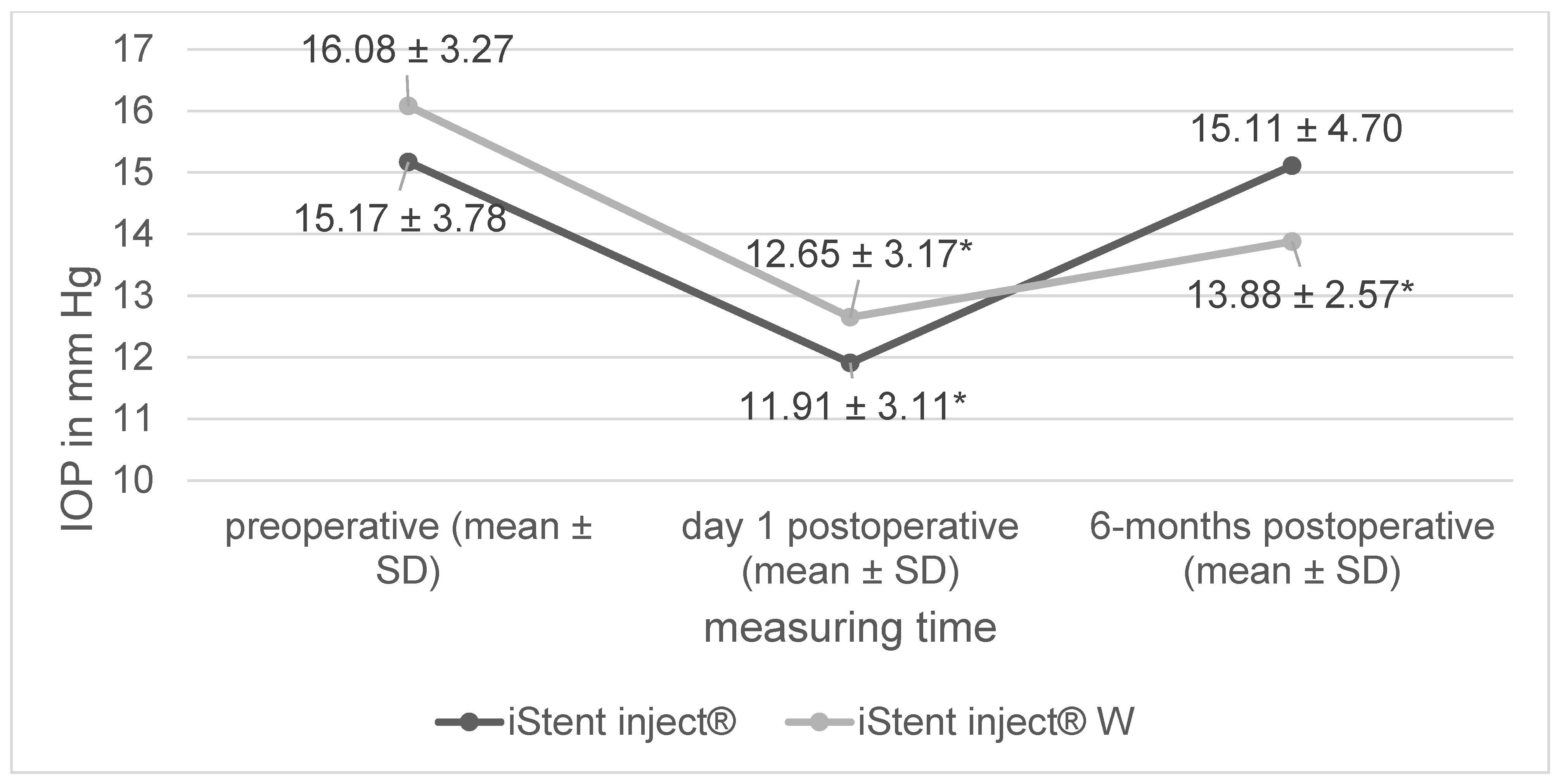
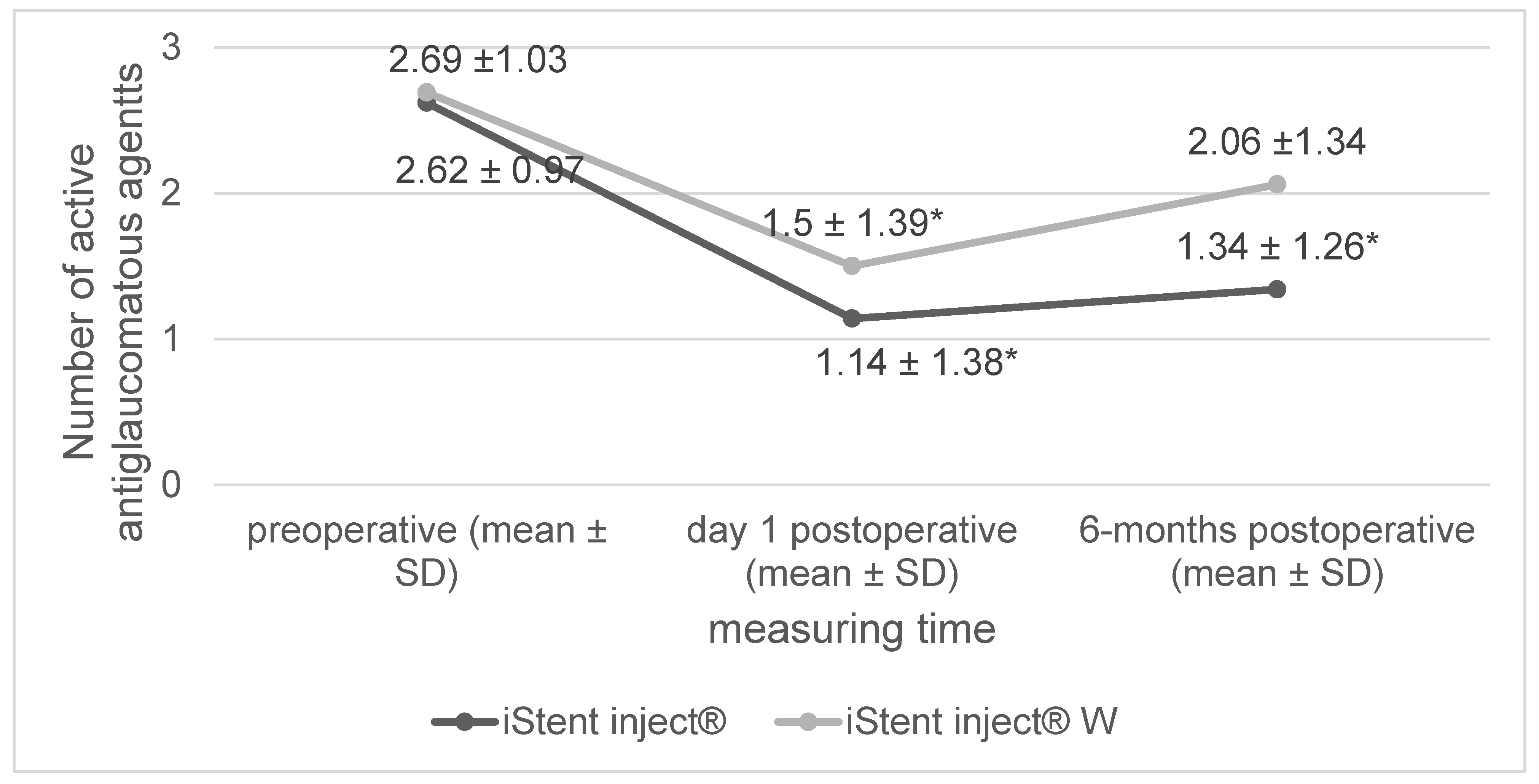
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caprioli, J.; Kim, J.H.; Friedman, D.S.; Kiang, T.; Moster, M.R.; Parrish, R.K., 2nd; Rorer, E.M.; Samuelson, T.; Tarver, M.E.; Singh, K.; et al. Special Commentary: Supporting Innovation for Safe and Effective Minimally Invasive Glaucoma Surgery: Summary of a Joint Meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, 26 February 2014. Ophthalmology 2015, 122, 1795–17801. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.A.; Singh, K.; Lin, S.C.; Hodapp, E.; Jampel, H.D.; Samples, J.R.; Smith, S.D. Novel glaucoma procedures: A report by the American Academy of Ophthalmology. Ophthalmology 2011, 118, 1466–1480. [Google Scholar] [CrossRef]
- Popovic, M.; Campos-Moller, X.; Saheb, H.; Ahmed, I.I.K. Efficacy and Adverse Event Profile of the iStent and iStent Inject Trabecular Micro-bypass for Open-angle Glaucoma: A Meta-analysis. J. Curr. Glaucoma Pract. 2018, 12, 67–84. [Google Scholar] [CrossRef]
- Resende, A.F.; Patel, N.S.; Waisbourd, M.; Katz, L.J. iStent® Trabecular Microbypass Stent: An Update. J. Ophthalmol. 2016, 2016, 2731856. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, R.D.; Voskanyan, L.; Vold, S.D.; Tetz, M.; Auffarth, G.; Masood, I.; Au, L.; Khouri, A.S.; Ahmed, I.I.K.; Saheb, H. Five-Year, Prospective, Randomized, Multi-Surgeon Trial of Two Trabecular Bypass Stents versus Prostaglandin for Newly Diagnosed Open-Angle Glaucoma. Ophthalmol. Glaucoma 2019, 2, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, S.R.; Grover, D.S.; Gallardo, M.J.; Brubaker, J.W.; Giamporcaro, J.E.; Hornbeak, D.M.; Katz, L.J.; Navratil, T.; iStent infinite Study Group. Effectiveness and Safety of iStent Infinite Trabecular Micro-Bypass for Uncontrolled Glaucoma. J. Glaucoma 2023, 32, 9–18. [Google Scholar] [CrossRef]
- Manning, D.K.; Haider, A.; Clement, C.; Viswanathan, D. Efficacy and Safety of iStent Inject Implantation in Manual and Femtosecond Laser-assisted Cataract Surgery before Lens Extraction. J. Curr. Glaucoma Pract. 2022, 16, 105–110. [Google Scholar] [CrossRef]
- Malvankar-Mehta, M.S.; Iordanous, Y.; Chen, Y.N.; Wang, W.W.; Patel, S.S.; Costella, J.; Hutnik, C.M.L. iStent with Phacoemulsification versus Phacoemulsification Alone for Patients with Glaucoma and Cataract: A Meta-Analysis. PLoS ONE 2015, 10, e0131770. [Google Scholar] [CrossRef] [PubMed]
- Glaukos Corporation. iStent Inject® W. 2022. Available online: https://www.glaukos.com/glaucoma/products/istent-inject-w/ (accessed on 5 May 2022).
- Bahler, C.K.; Hann, C.R.; Fjield, T.; Haffner, D.; Heitzmann, H.; Fautsch, M.P. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am. J. Ophthalmol. 2012, 153, 1206–1213. [Google Scholar] [CrossRef]
- Ferguson, T.J.; Swan, R.; Ibach, M.; Schweitzer, J.; Sudhagoni, R.; Berdahl, J.P. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J. Cataract. Refract. Surg. 2017, 43, 622–626. [Google Scholar] [CrossRef]
- Hengerer, F.H.; Auffarth, G.U.; Riffel, C.; Conrad-Hengerer, I. Prospective, Non-randomized, 36-Month Study of Second-Generation Trabecular Micro-Bypass Stents with Phacoemulsification in Eyes with Various Types of Glaucoma. Ophthalmol. Ther. 2018, 7, 405–415. [Google Scholar] [CrossRef]
- Shalaby, W.S.; Lam, S.S.; Arbabi, A.; Myers, J.S.; Moster, M.R.; Kolomeyer, N.N.; Razeghinejad, R.; Shukla, A.G.; Shukla, A.G.; Eid, T.M.; et al. iStent versus iStent inject implantation combined with phacoemulsification in open angle glaucoma. Indian J. Ophthalmol. 2021, 69, 2488–2495. [Google Scholar] [CrossRef]
- Guedes, R.A.P.; Gravina, D.M.; Lake, J.C.; Guedes, V.M.P.; Chaoubah, A. Intermediate Results of iStent or iStent inject Implantation Combined with Cataract Surgery in a Real-World Setting: A Longitudinal Retrospective Study. Ophthalmol. Ther. 2019, 8, 87–100. [Google Scholar] [CrossRef]
- Manning, D. Real-world Case Series of iStent or iStent inject Trabecular Micro-Bypass Stents Combined with Cataract Surgery. Ophthalmol. Ther. 2019, 8, 549–561. [Google Scholar] [CrossRef]
- Craven, E.R.; Katz, L.J.; Wells, J.M.; Giamporcaro, J.E. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J. Cataract. Refract. Surg. 2012, 38, 1339–1345. [Google Scholar] [CrossRef]
- Samuelson, T.W.; Katz, L.J.; Wells, J.M.; Duh, Y.-J.; Giamporcaro, J.E. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 2011, 118, 459–467. [Google Scholar] [CrossRef]
- Patel, I.; de Klerk, T.A.; Au, L. Manchester iStent study: Early results from a prospective UK case series. Clin. Exp. Ophthalmol. 2013, 41, 648–652. [Google Scholar] [CrossRef]
- Buchacra, O.; Duch, S.; Milla, E.; Stirbu, O. One-year analysis of the iStent trabecular microbypass in secondary glaucoma. Clin. Ophthalmol. 2011, 5, 321–326. [Google Scholar] [CrossRef][Green Version]
- Healey, P.R.; Clement, C.I.; Kerr, N.M.; Tilden, D.; Aghajanian, L. Standalone iStent Trabecular Micro-bypass Glaucoma Surgery: A Systematic Review and Meta-Analysis. J. Glaucoma 2021, 30, 606–620. [Google Scholar] [CrossRef]
- Holmes, D.P.; Clement, C.I.; Nguyen, V.; Healey, P.R.; Lim, R.; White, A.; Yuen, J.; Lawlor, M. Comparative study of 2-year outcomes for Hydrus or iStent inject microinvasive glaucoma surgery implants with cataract surgery. Clin. Exp. Ophthalmol. 2022, 50, 303–311. [Google Scholar] [CrossRef]
- Hamze, H.; Mohite, A.A.; Pandey, P.; Sung, V.C.T.; Masood, I. Comparison of 1-year surgical outcomes of combined cataract surgery and gonioscopy-assisted transluminal trabeculotomy (GATT) versus cataract surgery and iStent Inject. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3035–3044. [Google Scholar] [CrossRef]
- Gietzelt, C.; von Goscinski, C.; Lemke, J.; Schaub, F.; Hermann, M.M.; Dietlein, T.S.; Cursiefen, C.; Heindl, L.M.; Enders, P. Dynamics of structural reversal in Bruch’s membrane opening-based morphometrics after glaucoma drainage device surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1227–1236. [Google Scholar] [CrossRef]
- Sanchez, F.G.; Sanders, D.S.; Moon, J.J.; Gardiner, S.K.; Reynaud, J.; Fortune, B.; Mansberger, S.L. Effect of Trabeculectomy on OCT Measurements of the Optic Nerve Head Neuroretinal Rim Tissue. Ophthalmol. Glaucoma 2020, 3, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.F.; Hirneiss, C.W. Changes of Neuroretinal Rim and Retinal Nerve Fiber Layer Thickness Assessed by Optical Coherence Tomography After Filtration Surgery in Glaucomatous Eyes. Clin. Ophthalmol. 2021, 15, 2335–2344. [Google Scholar] [CrossRef]
| iStent Inject® (Mean ± SD) | iStent Inject® W (Mean ± SD) | p-Value | |
|---|---|---|---|
| n | 35 | 32 | 0.40 |
| age | 69.86 ± 6.85 | 70.20 ± 10.30 | 0.87 |
| sex | |||
| M | 17 (48.5%) | 13 (40.6%) | 0.62 |
| F | 18 (51.5%) | 19 (59.4%) | 0.63 |
| number of previous operations | selective laser trabeculoplasty: 2 (5.7%) | selective laser trabeculoplasty: 13 (40%) | <0.01 * |
| cyclophotocoagulation: 1 (2.9%) | cyclophotocoagulation: 0 | 0.93 | |
| trabeculectomy: 1 (2.9%) | trabeculectomy: 0 | 0.93 | |
| visual field (MD) | 11.76 ± 2.3 dB | 6.15 ± 5.32 dB | 0.04 * |
| RNFL-OCT | 175.9 ± 53.1 µm | 191.6 ± 39.6 µm | 0.25 |
| BMO-OCT | 187.6 ± 72.1 µm | 211.6 ± 79.9 µm | 0.41 |
| preoperative IOP | 15.17 ± 3.78 mm Hg | 16.08 ± 3.27 mm Hg | 0.29 |
| number of glaucoma agents | 2.63 ± 0.97 | 2.69 ± 1.03 | 0.73 |
| Postoperative Complications | iStent Inject® | iStent Inject® W | p-Value |
|---|---|---|---|
| anterior chamber hemorrhage | 0 | 2 (6.2%) | 0.13 |
| dislocation of iStent® | 0 | 1 (3.1%) | 0.29 |
| postoperative hypotony (<5 mm Hg) | 0 | 0 | >0.99 |
| Irvine–Gass syndrome | 0 | 1 (3.1%) | 0.48 |
| iStent Inject® (Mean ± SD) | iStent Inject® W (Mean ± SD) | p-Value (Difference between Groups) | |
|---|---|---|---|
| Perimetry: preoperative (MD) | −11.75 ± 11.02 dB | −6.15 ± 5.32 dB | |
| Perimetry: 6 months post operation (MD) | −11.56 ± 11.58 dB | −6.37 ± 6.34 dB | 0.67 |
| BMO: preoperative | 188 ± 71 µm | 212 ± 80 µm | |
| BMO: 6 months post operation | 183 ± 67 µm | 218 ± 92 µm | 0.24 |
| RNFL: preoperative (global) | 176 ± 53 µm | 192 ± 40 µm | |
| RNFL: 6 months post operation (global) | 184 ± 53 µm | 200 ± 37 µm | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deneri, S.; Merté, R.-L.; Eter, N.; Brücher, V.C. Comparison of the iStent Inject® versus the iStent Inject® W—Both in Combination with Cataract Surgery—In Open-Angle Glaucoma. J. Clin. Med. 2023, 12, 7259. https://doi.org/10.3390/jcm12237259
Deneri S, Merté R-L, Eter N, Brücher VC. Comparison of the iStent Inject® versus the iStent Inject® W—Both in Combination with Cataract Surgery—In Open-Angle Glaucoma. Journal of Clinical Medicine. 2023; 12(23):7259. https://doi.org/10.3390/jcm12237259
Chicago/Turabian StyleDeneri, Steffen, Ralph-Laurent Merté, Nicole Eter, and Viktoria C. Brücher. 2023. "Comparison of the iStent Inject® versus the iStent Inject® W—Both in Combination with Cataract Surgery—In Open-Angle Glaucoma" Journal of Clinical Medicine 12, no. 23: 7259. https://doi.org/10.3390/jcm12237259
APA StyleDeneri, S., Merté, R.-L., Eter, N., & Brücher, V. C. (2023). Comparison of the iStent Inject® versus the iStent Inject® W—Both in Combination with Cataract Surgery—In Open-Angle Glaucoma. Journal of Clinical Medicine, 12(23), 7259. https://doi.org/10.3390/jcm12237259






