Sex-Specific Disparities in Outcomes of Transcatheter Edge-to-Edge Repair for Mitral Regurgitation: A Multicenter “Real-World” Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Data Collection and Definitions
2.2. Statistical Analyses
2.3. Missing Data
3. Results
3.1. Baseline Characteristics
3.2. Complications and Short-Term Outcome
3.3. Long-Term Outcome
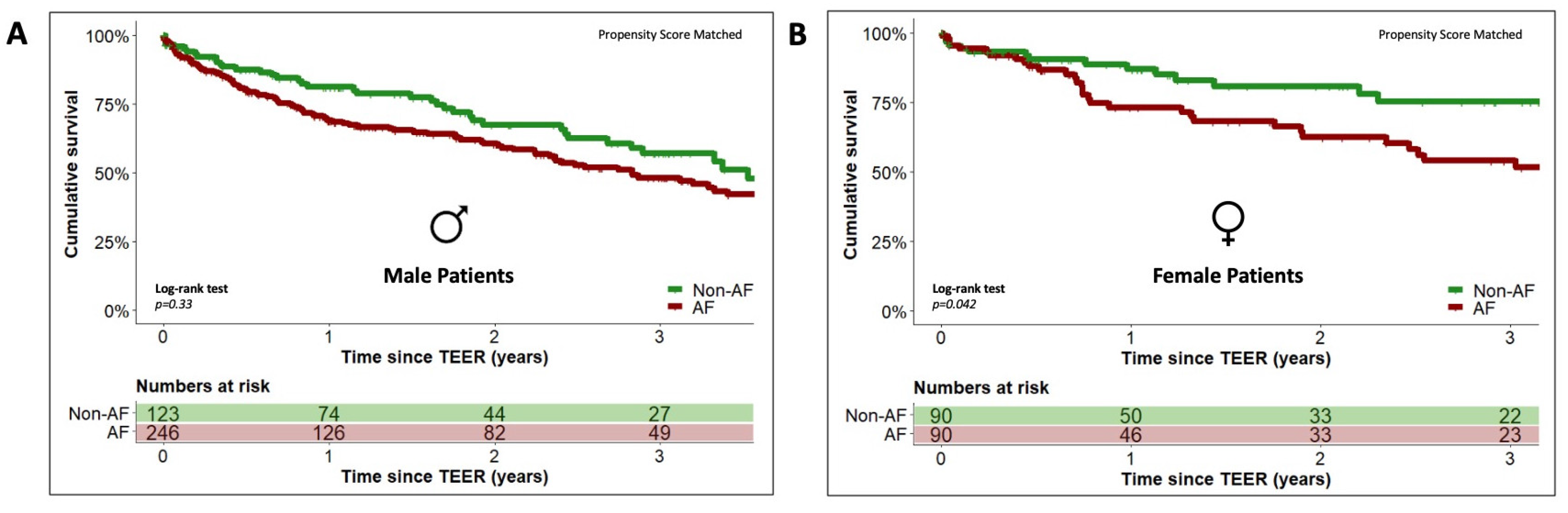
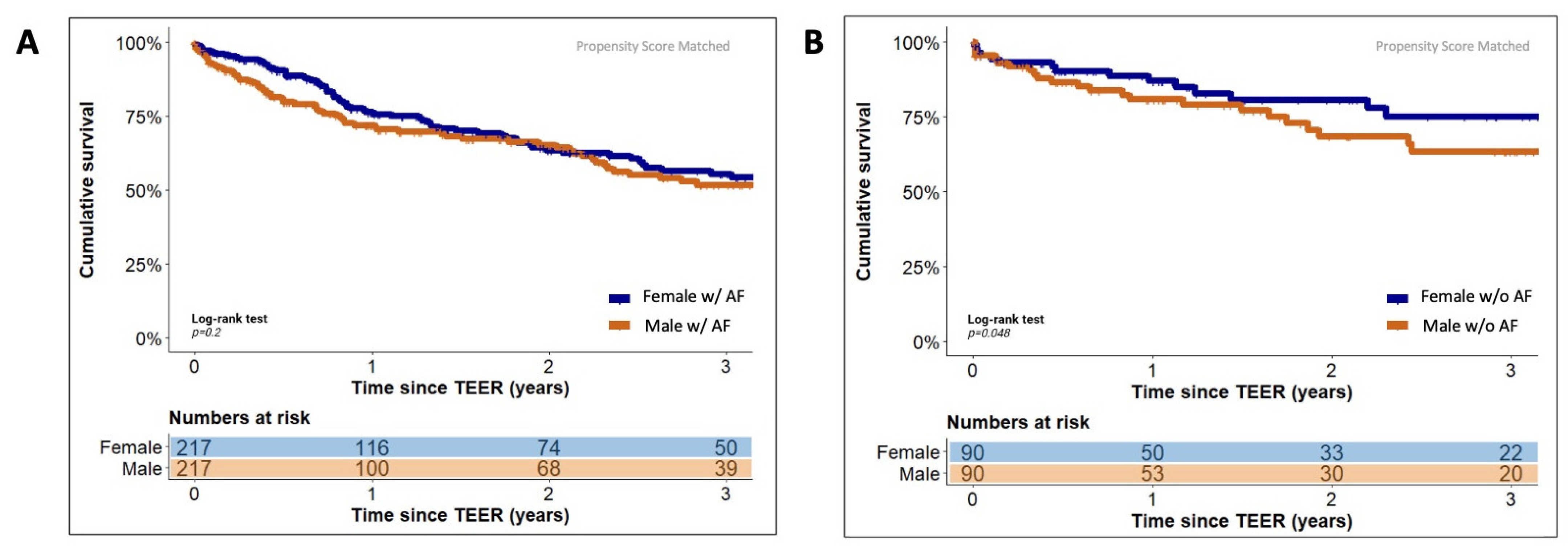
3.4. Sex-Specific Effects of Concomitant Atrial Fibrillation on Long-Term Outcome
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global Epidemiology of Valvular Heart Disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- DesJardin, J.T.; Chikwe, J.; Hahn, R.T.; Hung, J.W.; Delling, F.N. Sex Differences and Similarities in Valvular Heart Disease. Circ. Res. 2022, 130, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of Valvular Heart Diseases: A Population-Based Study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of Valvular Heart Disease in a Swedish Nationwide Hospital-Based Register Study. Heart 2017, 103, 1696. [Google Scholar] [CrossRef]
- d’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-Scale Community Echocardiographic Screening Reveals a Major Burden of Undiagnosed Valvular Heart Disease in Older People: The OxVALVE Population Cohort Study. Eur. Hear. J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of Heart Failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Tersalvi, G.; Gaiero, L.; Capriolo, M.; Cristoforetti, Y.; Salizzoni, S.; Senatore, G.; Pedrazzini, G.; Biasco, L. Sex Differences in Epidemiology, Morphology, Mechanisms, and Treatment of Mitral Valve Regurgitation. Medicina 2023, 59, 1017. [Google Scholar] [CrossRef]
- Vassileva, C.M.; McNeely, C.; Mishkel, G.; Boley, T.; Markwell, S.; Hazelrigg, S. Gender Differences in Long-Term Survival of Medicare Beneficiaries Undergoing Mitral Valve Operations. Ann. Thorac. Surg. 2013, 96, 1367–1373. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Candolfi, P.; Vahanian, A.; Chan, V.; Burwash, I.G.; Philippon, J.; Toussaint, J.; Verta, P.; Feldman, T.E.; Iung, B.; et al. Dismal Outcomes and High Societal Burden of Mitral Valve Regurgitation in France in the Recent Era: A Nationwide Perspective. J. Am. Hear. Assoc. 2020, 9, e016086. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Candolfi, P.; Enriquez-Sarano, M.; Burwash, I.G.; Chan, V.; Philippon, J.-F.; Toussaint, J.-M.; Verta, P.; Feldman, T.E.; Iung, B.; et al. Presentation and Outcomes of Mitral Valve Surgery in France in the Recent Era: A Nationwide Perspective. Open Hear. 2020, 7, e001339. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; Bonis, M.D.; Paulis, R.D.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart DiseaseDeveloped by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, ehab395. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Glower, D.G.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Waechter, C.; Ausbuettel, F.; Chatzis, G.; Fischer, D.; Nef, H.; Barth, S.; Halbfaß, P.; Deneke, T.; Kerber, S.; Divchev, D.; et al. Analysis of Atrial Fibrillation Treatment Regimes in a Multicenter Cohort of Transcatheter Edge-to-Edge Mitral Valve Repair Patients. J. Interv. Cardiol. 2020, 2020, 6542028. [Google Scholar] [CrossRef]
- Waechter, C.; Ausbuettel, F.; Chatzis, G.; Cheko, J.; Fischer, D.; Nef, H.; Barth, S.; Halbfass, P.; Deneke, T.; Kerber, S.; et al. Impact of Rhythm vs. Rate Control in Atrial Fibrillation on the Long-Term Outcome of Patients Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. J. Clin. Med. 2021, 10, 5044. [Google Scholar] [CrossRef]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.-H.; Leon, M.B.; Piazza, N.; et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint DefinitionsA Consensus Document from the Mitral Valve Academic Research Consortium. Eur. Hear. J. 2015, 36, 1878–1891. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet Women and Cardiovascular Disease Commission: Reducing the Global Burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Sun, F.; Liu, H.; Zhang, Q.; Zhou, J.; Zhan, H.; Lu, F. Sex-Specific Difference in Outcomes after Transcatheter Mitral Valve Repair with MitraClip Implantation: A Systematic Review and Meta-Analysis. J. Interv. Cardiol. 2022, 2022, 5488654. [Google Scholar] [CrossRef]
- Ya’Qoub, L.; Gad, M.; Faza, N.N.; Kunkel, K.J.; Ya’acoub, R.; Villablanca, P.; Bagur, R.; Alasnag, M.; Eng, M.; Elgendy, I.Y. Sex Differences in Outcomes of Transcatheter Edge-to-edge Repair with MitraClip: A Meta-analysis. Cathet. Cardiovasc. Intervent. 2022, 99, 1819–1828. [Google Scholar] [CrossRef]
- Villablanca, P.A.; Vemulapalli, S.; Stebbins, A.; Dai, D.; So, C.; Eng, M.H.; Wang, D.D.; Frisoli, T.M.; Lee, J.C.; Kang, G.; et al. Sex-Based Differences in Outcomes with Percutaneous Transcatheter Repair of Mitral Regurgitation with the MitraClip System: Transcatheter Valve Therapy Registry From 2011 to 2017. Circ. Cardiovasc. Interv. 2021, 14, e009374. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Zahid, S.; Khan, M.U.; Khan, S.U.; Munir, M.B.; Balla, S. Gender Disparities in Percutaneous Mitral Valve Repair (from the National Inpatient Sample). Am. J. Cardiol. 2020, 132, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Tigges, E.; Kalbacher, D.; Thomas, C.; Appelbaum, S.; Deuschl, F.; Schofer, N.; Schlüter, M.; Conradi, L.; Schirmer, J.; Treede, H.; et al. Transcatheter Mitral Valve Repair in Surgical High-Risk Patients: Gender-Specific Acute and Long-Term Outcomes. Biomed. Res. Int. 2016, 2016, 3934842. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Puls, M.; Baldus, S.; Lubos, E.; Bekeredjian, R.; Sievert, H.; Schofer, J.; Kuck, K.; Möllmann, H.; Hehrlein, C.; et al. Gender-related Differences in Patients Undergoing Transcatheter Mitral Valve Interventions in Clinical Practice: 1-year Results from the German TRAMI Registry. Catheter. Cardiovasc. Interv. 2020, 95, 819–829. [Google Scholar] [CrossRef]
- Park, S.-D.; Orban, M.; Karam, N.; Lubos, E.; Kalbacher, D.; Braun, D.; Stolz, L.; Neuss, M.; Butter, C.; Praz, F.; et al. Sex-Related Clinical Characteristics and Outcomes of Patients Undergoing Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. Jacc Cardiovasc. Interv. 2021, 14, 819–827. [Google Scholar] [CrossRef]
- Kosmidou, I.; Lindenfeld, J.; Abraham, W.T.; Rinaldi, M.J.; Kapadia, S.R.; Rajagopal, V.; Sarembock, I.J.; Brieke, A.; Gaba, P.; Rogers, J.H.; et al. Sex-Specific Outcomes of Transcatheter Mitral-Valve Repair and Medical Therapy for Mitral Regurgitation in Heart Failure. Jacc Hear Fail 2021, 9, 674–683. [Google Scholar] [CrossRef]
- Namazi, F.; Bijl, P.; Vo, N.M.; Wijngaarden, S.E.; Marsan, N.A.; Delgado, V.; Bax, J.J. Sex Differences in Prognosis of Significant Secondary Mitral Regurgitation. Esc. Hear Fail 2021, 8, 3539–3546. [Google Scholar] [CrossRef]
- Gafoor, S.; Sievert, H.; Maisano, F.; Baldus, S.; Schaefer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Geist, V.; Widder, J.D.; et al. Gender in the ACCESS-EU Registry: A Prospective, Multicentre, Non-Randomised Post-Market Approval Study of MitraClip® Therapy in Europe. EuroIntervention 2016, 12, e257–e264. [Google Scholar] [CrossRef]
- EL-Andari, R.; Bozso, S.J.; Fialka, N.M.; Kang, J.J.H.; Nagendran, J. Does Sex Impact Outcomes after Mitral Valve Surgery? A Systematic Review and Meta-Analysis. Scand. J. Surg. 2022, 111, 99–109. [Google Scholar] [CrossRef]
- Doshi, R.; Shlofmitz, E.; Vadher, A.; Shah, J.; Meraj, P. Impact of Sex on Short Term In-Hospital Outcomes with Transcatheter Edge-to-Edge Mitral Valve Repair. Cardiovasc. Revascularization Med. 2018, 19, 182–185. [Google Scholar] [CrossRef]
- Elbadawi, A.; Elzeneini, M.; Thakker, R.; Mahmoud, K.; Elgendy, I.Y.; Megaly, M.; Hamed, M.; Omer, M.A.; Chowdhury, M.; Ogunbayo, G.; et al. Sex Differences in In-Hospital Outcomes of Transcatheter Mitral Valve Repair (from a National Database). Am. J. Cardiol. 2020, 125, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Hioki, H.; Watanabe, Y.; Kataoka, A.; Kozuma, K.; Shirai, S.; Naganuma, T.; Yamawaki, M.; Enta, Y.; Mizuno, S.; Ueno, H.; et al. Impact of Gender on Mortality after Transcatheter Edge-to-Edge Repair for Functional Mitral Regurgitation. Am. J. Cardiol. 2023, 205, 12–19. [Google Scholar] [CrossRef]
- Attizzani, G.F.; Ohno, Y.; Capodanno, D.; Cannata, S.; Dipasqua, F.; Immé, S.; Mangiafico, S.; Barbanti, M.; Ministeri, M.; Cageggi, A.; et al. Gender-related Clinical and Echocardiographic Outcomes at 30-day and 12-month Follow up after MitraClip Implantation in the GRASP Registry. Catheter. Cardiovasc. Interv. 2015, 85, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Pleger, S.T.; Schulz-Schönhagen, M.; Geis, N.; Mereles, D.; Chorianopoulos, E.; Antaredja, M.; Lewening, M.; Katus, H.A.; Bekeredjian, R. One Year Clinical Efficacy and Reverse Cardiac Remodelling in Patients with Severe Mitral Regurgitation and Reduced Ejection Fraction after MitraClip© Implantation. Eur. J. Heart Fail. 2013, 15, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Godino, C.; Giannini, C.; Scotti, A.; Liga, R.; Curello, S.; Fiorina, C.; Chiari, E.; Chizzola, G.; Abbenante, A.; et al. Left Ventricular Reverse Remodelling Predicts Long-term Outcomes in Patients with Functional Mitral Regurgitation Undergoing MitraClip Therapy: Results from a Multicentre Registry. Eur. J. Heart Fail. 2019, 21, 196–204. [Google Scholar] [CrossRef]
- Velu, J.F.; Kortlandt, F.A.; Hendriks, T.; Schurer, R.A.J.; van Boven, A.J.; Koch, K.T.; Vis, M.M.; Henriques, J.P.; Piek, J.J.; den Branden, B.J.L.V.; et al. Comparison of Outcome after Percutaneous Mitral Valve Repair with the MitraClip in Patients with Versus without Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 2035–2040. [Google Scholar] [CrossRef]
- Keßler, M.; Pott, A.; Mammadova, E.; Seeger, J.; Wöhrle, J.; Rottbauer, W.; Markovic, S. Atrial Fibrillation Predicts Long-Term Outcome after Transcatheter Edge-to-Edge Mitral Valve Repair by MitraClip Implantation. Biomol 2018, 8, 152. [Google Scholar] [CrossRef]
- Arora, S.; Vemulapalli, S.; Stebbins, A.; Ramm, C.J.; Kosinski, A.S.; Sorajja, P.; Piccini, J.P.; Cavender, M.A.; Vavalle, J.P. The Prevalence and Impact of Atrial Fibrillation on 1-Year Outcomes in Patients Undergoing Transcatheter Mitral Valve Repair Results From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Jacc Cardiovasc. Interv. 2019, 12, 569–578. [Google Scholar] [CrossRef]
- Subahi, A.; Munir, A.; Abubakar, H.; Akintoye, E.; Yassin, A.S.; Adegbala, O.; Alraies, M.C.; Elder, M.; Mohamad, T.; Kaki, A.; et al. The Impact of Atrial Fibrillation on Transcatheter Mitral Valve Repair Outcomes: A Propensity-matched Analysis. J. Interv. Cardiol. 2018, 31, 925–931. [Google Scholar] [CrossRef]
- Shah, S.; Raj, V.; Abdelghany, M.; Mena-Hurtado, C.; Riaz, S.; Patel, S.; Wiener, H.; Chaudhuri, D. Impact of Atrial Fibrillation on the Outcomes of Transcatheter Mitral Valve Repair Using MitraClip: A Systematic Review and Meta-Analysis. Heart Fail Rev. 2020, 26, 531–543. [Google Scholar] [CrossRef]
- Emdin, C.A.; Wong, C.X.; Hsiao, A.J.; Altman, D.G.; Peters, S.A.; Woodward, M.; Odutayo, A.A. Atrial Fibrillation as Risk Factor for Cardiovascular Disease and Death in Women Compared with Men: Systematic Review and Meta-Analysis of Cohort Studies. BMJ 2016, 352, h7013. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M.; Kannankeril, P.; Darbar, D. On the Relationship among QT Interval, Atrial Fibrillation, and Torsade de Pointes. EP Eur. 2007, 9, iv1–iv3. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, G.S.; Almodaimegh, H.; McMurtry, M.S.; Wu, C. Do Women Bleed More than Men When Prescribed Novel Oral Anticoagulants for Venous Thromboembolism? A Sex-Based Meta-Analysis. Thromb. Res. 2013, 132, 185–189. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall (n = 821) | Before Propensity-Score-Matching | After Propensity-Score-Matching | ||||
|---|---|---|---|---|---|---|---|
| Female (n = 307) | Male (n = 514) | p-Value | Female (n = 307) | Male (n = 307) | p-Value | ||
| Age (years) | 78.3 ± 8 | 80.4 ± 6.7 | 77 ± 8.5 | 0.03 | 80.4 ± 6.6 | 79.8 ± 6.5 | 0.2 |
| euroSCORE II * | 16.0% (20) | 13.1 (20.1) | 16.5 (20.2) | 0.054 | 13.1 (20.1) | 14.9 (18) | 0.9 |
| STS Risk Score * | 6.6% (8) | 6.1 (7.5) | 7 (8.6) | 0.2 | 6.1 (7.5) | 6.4 (7.1) | 0.8 |
| NYHA class I NYHA class II NYHA class III NYHA class IV | 0.1% (1) 3.2% (26) 75.6% (621) 21.7% (173) | 0.3% (1) 2.9% (9) 76.9% (236) 19.9% (61) | 0% (0) 3.3% (17) 74.9% (385) 21.8% (112) | 0.6 | 0.3% (1) 2.9% (9) 76.9% (236) 19.9% (61) | 0% (0) 2.6% (8) 76.2% (234) 21.2% (65) | 0.9 |
| COPD | 17.8% (146) | 15.3% (47) | 19.3% (99) | 0.2 | 15.3% (47) | 15.6% (48) | 1 |
| CAD | 62.2% (511) | 53.1% (163) | 67.7% (348) | <0.0001 | 53.1% (163) | 60.3% (185) | 0.1 |
| Prior CAB-OP | 27.8% (228) | 18.2% (56) | 33.5% (172) | <0.0001 | 18.2% (56) | 22.1% (68) | 0.3 |
| Prior PCI | 54.0% (443) | 50.5% (155) | 56.0% (288) | 0.1 | 50.5% (155) | 54.1% (166) | 0.4 |
| Pre-existing ICD | 22.3% (183) | 9.4% (29) | 30.0% (154) | <0.0001 | 9.4% (29) | 11.1% (34) | 0.6 |
| Pre-existing CRT | 14.4% (118) | 7.2% (22) | 18.7% (96) | <0.0001 | 7.2% (22) | 10.1% (31) | 0.3 |
| Diabetes mellitus | 29.8% (245) | 28.7% (88) | 30.5% (157) | 0.6 | 28.7% (88) | 27.7% (85) | 0.9 |
| Arterial hypertension | 81% (665) | 79.2% (243) | 82.1% (422) | 0.3 | 79.2% (243) | 81.8% (251) | 0.5 |
| Prior Stroke | 9.7% (80) | 9.8% (30) | 9.7% (50) | 1 | 9.8% (30) | 8.8% (27) | 0.8 |
| Atrial Fibrillation Paroxysmal Non-paroxysmal | 74.1% (608) 21.2% (174) 52.9% (434) | 70.7% (217) 21.8% (67) 48.9% (150) | 76.1% (391) 20.8% (107) 55.3% (284) | 0.1 0.2 0.2 | 70.7% (217) 21.8% (67) 48.9% (150) | 73.9% (227) 20.8% (64) 53.1% (163) | 0.4 0.2 0.3 |
| LVEF ≥ 50% LVEF 41–49% LVEF ≤ 40% | 39.2% (322) 11.6% (95) 49.2% (404) | 50.5% (155) 13.4% (41) 36.2% (111) | 32.5% (167) 10.5% (54) 57.0% (293) | <0.0001 | 50.5% (155) 13.4% (41) 36.2% (111) | 46.9% (144) 13.4% (41) 39.7% (122) | 0.6 |
| GFR (mL/Min) | 50 ± 25.5 | 48.5 ± 21.3 | 51.2 ± 27.7 | 0.1 | 48.5 ± 21 | 51.5 ± 31 | 0.2 |
| NT-proBNP (ng/L) * | 2262 (4936) | 1919 (4328) | 2463 (5288) | 0.1 | 1919 (4328) | 2465 (5028) | 0.2 |
| TR grade III | 18.6% (153) | 19.9% (61) | 17.9% (92) | 0.5 | 19.9% (61) | 19.9% (61) | 1 |
| Degenerative MR etiology Functional MR etiology Mixed MR etiology | 35.7% (293) 52.6% (432) 11.7% (96) | 38.8% (119) 47.9% (147) 13.4% (41) | 33.9% (174) 55.4% (285) 10.7% (55) | 0.1 | 38.8% (119) 47.9% (147) 13.4% (41) | 37.5% (115) 49.8% (153) 12.7% (39) | 0.9 |
| Heart Failure Therapy | |||||||
| ACE-/AT1 Inhibitors | 72.2% (593) | 76.5% (235) | 69.6% (358) | 0.04 | 76.5% (235) | 74.3% (228) | 0.6 |
| ARN Inhibitor | 13.5% (111) | 8.1% (25) | 16.7% (86) | 0.004 | 8.1% (25) | 11.1% (34) | 0.3 |
| Beta Blockers | 88.8% (729) | 89.9% (276) | 88.1% (453) | 0.5 | 89.9% (276) | 85.7% (263) | 0.1 |
| Loop diuretics | 90.6% (744) | 92.2% (283) | 89.7% (461) | 0.3 | 92.2% (283) | 89.6% (275) | 0.3 |
| Thiazid diuretics | 17.4% (143) | 15.3% (47) | 18.7% (96) | 0.3 | 15.3% (47) | 19.2% (59) | 0.2 |
| Aldosteron antagonists | 48.2% (396) | 45% (138) | 50.2% (258) | 0.1 | 45% (138) | 45.3% (139) | 1 |
| Ivabradin | 49.5% (10) | 0.7% (2) | 1.6% (8) | 0.3 | 0.7% (2) | 1% (3) | 1 |
| Digitalis | 6.8% (56) | 5.9% (18) | 7.4% (38) | 0.5 | 5.9% (18) | 5.5% (17) | 1 |
| SGLT-II-Inhibitors | 4.8% (39) | 2.6% (8) | 6.0% (31) | 0.02 | 2.6% (8) | 5.5% (17) | 0.1 |
| Vericiguat | 0.1% (1) | 0.0% (0) | 0.2% (1) | 1 | 0.0% (0) | 0.0% (0) | 1 |
| Variable | Overall | Female | Male | p-Value |
|---|---|---|---|---|
| (n = 821) | (n = 307) | (n = 514) | ||
| Procedural duration (min) * | 80 (60) | 75 (54) | 83 (63) | 0.003 |
| Number of clips implanted * | 1 (1) | 1 (1) | 2 (1) | <0.001 |
| Periprocedual MR reduction # | Δ2.04 ± 0.6 | Δ2 ± 0.6 | Δ2.1 ± 0.6 | 0.1 |
| Postprocedural MR grade # | 0.7 | |||
| ≤mild-to-moderate | 88.6% (727) | 88.9% (273) | 88.3% (454) | |
| moderate | 11.4% (94) | 11.1% (34) | 11.7% (60) | |
| Length of hospital stay (days) * | 6 (5) | 7 (4) | 6 (5) | 0.2 |
| Variable | Overall (n = 821) | Female (n = 307) | Male (n = 514) | p-Value |
|---|---|---|---|---|
| Stroke | 0.6% (5) | 0.3% (1) | 0.8% (4) | 0.7 |
| Myocardial infarction | 0% (0) | 0% (0) | 0% (0) | 1 |
| Bleeding complications MVARC I MVARC II MVARC III MVARC IV MVARC V | 3.2% (26) 2.1% (17) 0.6% (5) 0.1% (1) 0.4% (3) 0% (0) | 3.3% (10) 1.6% (5) 1.3% (4) 0.3% (1) 0% (0) 0% (0) | 3.1% (16) 2.3% (12) 0.2% (1) 0% (0) 0.6% (3) 0% (0) | 1 0.08 |
| Cardiac conduction system disturbances | 0% (0) | 0% (0) | 0% (0) | 1 |
| In-hospital mortality Cardiac cause Non-cardiac cause | 3.5% (29) 2.2% (18) 1.3% (11) | 2.6% (8) 2% (6) 0.6% (2) | 4.1% (21) 2.3% (12) 1.8% (9) | 0.3 0.8 0.4 |
| Male Sex | Female Sex | |||||
|---|---|---|---|---|---|---|
| Variable | No AF (n = 123) | AF (n = 391) | p-Value | No AF (n = 90) | AF (n = 217) | p-Value |
| Age (years) | 75.0 ± 9.0 | 77.6 ± 8.3 | 0.01 | 79.0 ± 8 | 81.0 ± 6.0 | 0.1 |
| euroSCORE II * | 16.7 (18.5) | 16.5 (21.4) | 0.7 | 15.0 (17) | 13.0 (21) | 0.8 |
| STS Risk Score * | 6.9 (8.8) | 7.0 (8.5) | 0.4 | 5.5 (5.5) | 6.7 (8.6) | 0.008 |
| NYHA class I NYHA class II NYHA class III NYHA class IV | 0% (0) 2.4% (3) 78.9% (97) 18.7% (23) | 0% (0) 3.6% (14) 73.7% (288) 22.8% (89) | 0.6 | 0% (0) 6.7% (6) 75.6% (68) 17.8% (16) | 0.5% (1) 1.4% (3) 77.4% (168) 20.7% (45) | 0.08 |
| COPD | 21.1% (26) | 18.7% (73) | 0.6 | 18.9% (17) | 13.8% (30) | 0.3 |
| CAD | 73.2% (90) | 66% (258) | 0.2 | 58.9% (53) | 50.7% (110) | 0.2 |
| Prior CAB-OP | 42.3% (52) | 30.7% (120) | 0.02 | 22.2% (20) | 16.6% (36) | 0.3 |
| Prior PCI | 58.5% (72) | 55.2% (216) | 0.5 | 56.7% (51) | 47.9% (104) | 0.2 |
| Pre-existing ICD | 29.3% (36) | 30.2% (118) | 0.9 | 7.8% (7) | 10.4% (22) | 0.7 |
| Pre-existing CRT | 18.7% (23) | 18.7% (73) | 1 | 3.3% (3) | 8.8% (19) | 0.1 |
| Diabetes mellitus | 31.7% (39) | 30.2% (118) | 0.7 | 30% (27) | 28.1% (61) | 0.8 |
| Arterial hypertension | 81.3% (100) | 82.4% (322) | 0.8 | 68.9% (62) | 83.4% (181) | 0.005 |
| Prior Stroke | 6.5% (8) | 10.7% (42) | 0.2 | 8.9% (8) | 10.1% (22) | 0.8 |
| LVEF ≥ 50% LVEF 41–49% LVEF ≤ 40% | 25.2% (31) 8.9% (11) 65.9% (81) | 34.8% (136) 11% (43) 54.2% (212) | 0.08 | 45.6% (41) 10% (9) 44.4% (40) | 52.5% (114) 14.7% (32) 32.7% (71) | 0.2 |
| GFR (mL/Min) | 54 ± 26 | 51 ± 28 | 0.3 | 55 ± 23 | 46 ± 20 | 0.03 |
| NT-proBNP (ng/L) * | 2069 (6005) | 2473 (5049) | 0.6 | 2080 (4437) | 1888 (3857) | 0.6 |
| TR grade III | 11.4% (14) | 19.9% (78) | 0.03 | 10% (9) | 24% (52) | 0.004 |
| Degenerative MR etiology Functional MR etiology Mixed MR etiology | 35.8% (44) 57.7% (71) 6.5% (8) | 33.2% (130) 54.7% (214) 12% (47) | 0.2 | 35.6% (32) 52.2% (47) 12.2% (11) | 40.1% (87) 46.1% (100) 13.8% (30) | 0.6 |
| Procedural duration (min) * | 85 (62) | 83 (63) | 0.9 | 82 (39) | 86 (49) | 1 |
| Number of clips implanted * | 2 (1) | 2 (1) | 1 | 1 (1) | 1 (1) | 0.4 |
| Periprocedual MR reduction # | Δ2.0 ± 0.6 | Δ2.1 ± 0.6 | 0.3 | Δ1.9 ± 0.6 | Δ2.0 ± 0.5 | 0.2 |
| Length of hospital stay (days) * | 6 (5) | 6 (5) | 0.6 | 6.5 (4) | 7 (5) | 0.1 |
| Overall-MACCE Cerebral/systemic thromboembolic event Bleeding requiring intervention In-hospital death from cardiovasc. cause | 6.5% (8) 0.8% (1) 3.3% (4) 2.4% (3) | 5.4% (21) 0.8% (3) 3.1% (12) 2.3% (9) | 0.7 1 1 1 | 6.7% (6) 0% (0) 4.4% (4) 2.2% (2) | 4.1% (9) 0.5% (1) 2.8% (6) 1.8% (4) | 0.4 1 0.5 1 |
| In-hospital death from any cause | 4.9% (6) | 3.8% (15) | 0.6 | 3.3% (3) | 2.3% (5) | 0.7 |
| Heart Failure Therapy | ||||||
| ACE/AT1 Inhibitors | 66.7% (82) | 70.6% (276) | 0.4 | 80% (72) | 75.1% (163) | 0.4 |
| ARN Inhibitor | 17.1% (21) | 16.6% (65) | 0.9 | 7.8% (7) | 8.3% (18) | 1 |
| Beta Blockers | 87.8% (108) | 88.2% (345) | 0.9 | 87.8% (79) | 90.8% (197) | 0.4 |
| Loop diuretics | 86.2% (106) | 90.8% (355) | 0.2 | 90% (81) | 93.1% (202) | 0.4 |
| Thiazid diuretics | 18.7% (23) | 18.7% (73) | 1 | 13.3% (12) | 16.1% (35) | 0.6 |
| Aldosteron antagonists | 50.4% (62) | 50.1% (196) | 1 | 41.1% (37) | 46.5% (101) | 0.4 |
| Ivabradin | 4.1% (5) | 0.8% (3) | 0.02 | 1.1% (1) | 0.5% (1) | 0.5 |
| Digitalis | 0.8% (1) | 9.5% (37) | <0.001 | 0% (0) | 8.3% (18) | 0.002 |
| SGLT-II-Inhibitors | 8.1% (10) | 5.4% (21) | 0.3 | 3.3% (3) | 2.3% (5) | 0.7 |
| Vericiguat | 0% (0) | 0.3% (1) | 1 | 0% (0) | 0% (0) | 1 |
| Oral anticoagulants | 92.3% (361) | - | - | 94% (204) | - | |
| VKA | - | 45% (176) | - | - | 44.7% (97) | - |
| NOAC | - | 47.3% (185) | - | - | 49.3% (107) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ausbuettel, F.; Barth, S.; Chatzis, G.; Sassani, K.; Fischer, D.; Weyand, S.; Mueller, J.; Schuett, H.; Schieffer, B.; Luesebrink, U.; et al. Sex-Specific Disparities in Outcomes of Transcatheter Edge-to-Edge Repair for Mitral Regurgitation: A Multicenter “Real-World” Analysis. J. Clin. Med. 2023, 12, 7231. https://doi.org/10.3390/jcm12237231
Ausbuettel F, Barth S, Chatzis G, Sassani K, Fischer D, Weyand S, Mueller J, Schuett H, Schieffer B, Luesebrink U, et al. Sex-Specific Disparities in Outcomes of Transcatheter Edge-to-Edge Repair for Mitral Regurgitation: A Multicenter “Real-World” Analysis. Journal of Clinical Medicine. 2023; 12(23):7231. https://doi.org/10.3390/jcm12237231
Chicago/Turabian StyleAusbuettel, Felix, Sebastian Barth, Georgios Chatzis, Kiarash Sassani, Dieter Fischer, Sebastian Weyand, Julian Mueller, Harald Schuett, Bernhard Schieffer, Ulrich Luesebrink, and et al. 2023. "Sex-Specific Disparities in Outcomes of Transcatheter Edge-to-Edge Repair for Mitral Regurgitation: A Multicenter “Real-World” Analysis" Journal of Clinical Medicine 12, no. 23: 7231. https://doi.org/10.3390/jcm12237231
APA StyleAusbuettel, F., Barth, S., Chatzis, G., Sassani, K., Fischer, D., Weyand, S., Mueller, J., Schuett, H., Schieffer, B., Luesebrink, U., & Waechter, C. (2023). Sex-Specific Disparities in Outcomes of Transcatheter Edge-to-Edge Repair for Mitral Regurgitation: A Multicenter “Real-World” Analysis. Journal of Clinical Medicine, 12(23), 7231. https://doi.org/10.3390/jcm12237231






