Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes
Abstract
1. Introduction
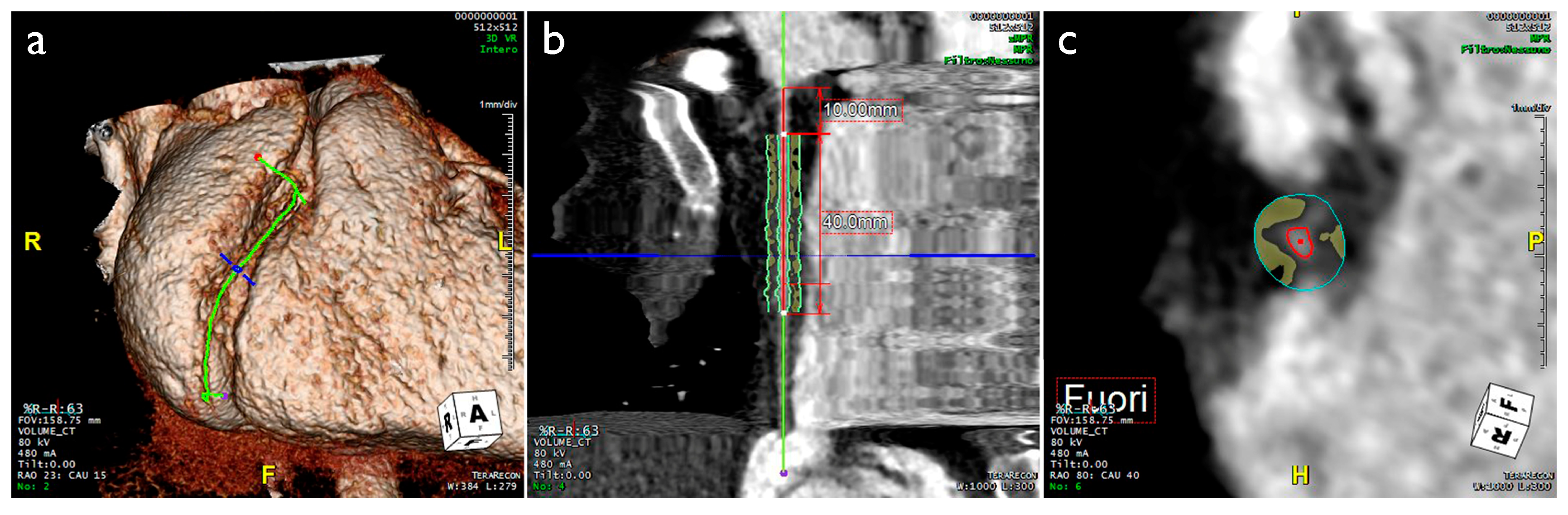
2. Imaging Evaluation of EAT and PCAT
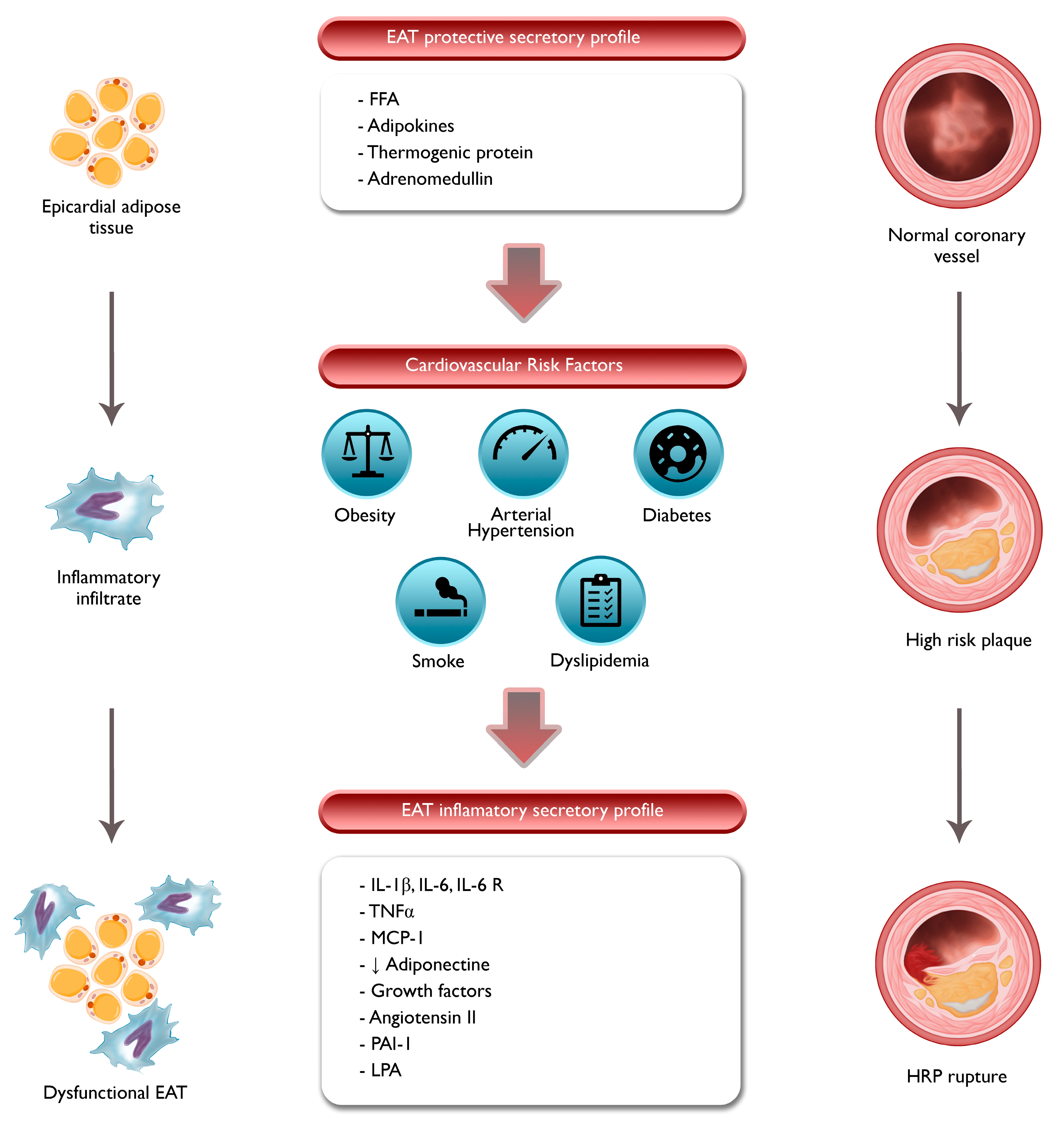
3. Pathophysiologic Role of EAT and PCAT
4. Correlation between EAT, Coronary Inflammation, Coronary Flow Reserve, and Cardiovascular Risk
5. EAT/PCAT Activity Overcomes Systemic Inflammatory Markers in ACS
6. Correlation between PCAT, Plaque Vulnerability, and ACS
Autoptic Evaluation of EAT and Risk of Sudden Cardiac Death
7. Correlation between PCAT and Coronary Arteritis
8. Correlation between PCAT and MINOCA
8.1. Spontaneous Coronary Artery Dissection
8.2. Vasospastic Angina
9. Future Perspectives: Application of Artificial Intelligence
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-FAB | adipocyte fatty-acid-binding protein |
| ACS | acute coronary syndrome |
| AF | atrial fibrillation |
| AI | artificial intelligence |
| AMI | acute myocardial infarction |
| BNP | B-type natriuretic peptide |
| C-TnI | cardiac troponin I |
| CAC | coronary artery calcification |
| CAD | coronary artery disease |
| CCTA | coronary CT angiography |
| CFR | coronary flow reserve |
| CMR | cardiac magnetic resonance |
| CVD | cardiovascular disease |
| CT | computed tomography |
| CT-FFR | CT-derived fractional flow reserve |
| DL | deep learning |
| EAT | epicardial adipose tissue |
| ETT | exercise treadmill testing |
| FFAs | free fatty acids |
| HF | heart failure |
| HRP | high-risk coronary plaques |
| hs-CRP | high-sensitivity C-reactive protein |
| HU | Hounsfield units |
| IL | interleukin |
| KD | Kawasaki disease |
| LAD | left anterior descending |
| LPA | lysophosphatidic acid |
| MACE | major adverse cardiovascular events |
| ML | machine learning |
| MCP-1 | monocyte chemoattractant protein-1 |
| MINOCA | myocardial infarction with non-obstructive coronary arteries |
| MiRNA | microRNA |
| NSTEMI | non-ST-segment elevation myocardial infarction |
| PAAT | periaortic adipose tissue |
| PCAT | pericoronary adipose tissue |
| PET | positron emission tomography |
| pFAI | pericoronary fat attenuation index |
| PVAT | perivascular adipose tissue |
| SCAD | spontaneous coronary artery dissection |
| SCD | sudden cardiac death |
| SMI | silent myocardial infarction |
| T2DM | type 2 diabetes mellitus |
| TAK | Takayasu arteritis |
| TNFα | tumor necrosis factor-α |
| TTE | transthoracic echocardiography |
| VSMCs | vascular smooth muscle cells |
| 18F-NaF | 18F-sodium fluoride |
References
- Bueno, H. Epidemiology of acute coronary syndromes. In The ESC Textbook of Cardiovascular Medicine; James, S., Camm, A.J., Lüscher, T.F., Maurer, G., Serruys, P.W., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 1214–1218. [Google Scholar]
- Yao, H.; Ekou, A.; Brou, I.; Niamkey, T.; Koffi, F.; Tano, S.; Kouamé, I.; N’Guetta, R. Evolution of epidemiology and management of acute coronary syndromes in Abidjan: A cross-sectional study of 1011 patients. Ann. Cardiol. Angeiol. 2022, 71, 130–135. [Google Scholar] [CrossRef]
- Maffei, E.; Seitun, S.; Martini, C.; Palumbo, A.; Tarantini, G.; Berti, E.; Grilli, R.; Tedeschi, C.; Messalli, G.; Guaricci, A.; et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart 2010, 96, 1973–1979. [Google Scholar] [CrossRef]
- Maffei, E.; Seitun, S.; Martini, C.; Aldrovandi, A.; Cervellin, G.; Tedeschi, C.; Guaricci, A.; Messalli, G.; Catalano, O.; Cademartiri, F. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol. Med. 2011, 116, 690–705. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Arcadi, T.; Brunetti, N.D.; Maffei, E.; Montrone, D.; Martini, C.; De Luca, M.; De Rosa, F.; Cocco, D.; Midiri, M.; et al. Carotid intima media thickness and coronary atherosclerosis linkage in symptomatic intermediate risk patients evaluated by coronary computed tomography angiography. Int. J. Cardiol. 2014, 176, 988–993. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Maffei, E.; Brunetti, N.D.; Montrone, D.; Di Biase, L.; Tedeschi, C.; Gentile, G.; Macarini, L.; Midiri, M.; Cademartiri, F.; et al. Heart rate control with oral ivabradine in computed tomography coronary angiography: A randomized comparison of 7.5 mg vs 5 mg regimen. Int. J. Cardiol. 2013, 168, 362–368. [Google Scholar] [CrossRef]
- Narula, J.; Achenbach, S. Napkin-ring necrotic cores: Defining circumferential extent of necrotic cores in unstable plaques. JACC Cardiovasc. Imaging 2009, 2, 1436–1438. [Google Scholar] [CrossRef][Green Version]
- Dodd, J.D.; Rieber, J.; Pomerantsev, E.; Chaithiraphan, V.; Achenbach, S.; Moreiras, J.M.; Abbara, S.; Hoffmann, U.; Brady, T.J.; Cury, R.C. Quantification of nonculprit coronary lesions: Comparison of cardiac 64-MDCT and invasive coronary angiography. AJR Am. J. Roentgenol. 2008, 191, 432–438. [Google Scholar] [CrossRef]
- Si, N.; Shi, K.; Li, N.; Dong, X.; Zhu, C.; Guo, Y.; Hu, J.; Cui, J.; Yang, F.; Zhang, T. Identification of patients with acute myocardial infarction based on coronary CT angiography: The value of pericoronary adipose tissue radiomics. Eur. Radiol. 2022, 32, 6868–6877. [Google Scholar] [CrossRef]
- Pergola, V.; Cabrelle, G.; Mattesi, G.; Cattarin, S.; Furlan, A.; Dellino, C.M.; Continisio, S.; Montonati, C.; Giorgino, A.; Giraudo, C.; et al. Added Value of CCTA-Derived Features to Predict MACEs in Stable Patients Undergoing Coronary Computed Tomography. Diagnostics 2022, 12, 1446. [Google Scholar] [CrossRef]
- Paul, J.F.; Rohnean, A.; Giroussens, H.; Pressat-Laffouilhere, T.; Wong, T. Evaluation of a deep learning model on coronary CT angiography for automatic stenosis detection. Diagn. Interv. Imaging 2022, 103, 316–323. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Bertella, E.; Baggiano, A.; Mushtaq, S.; Loguercio, M.; Segurini, C.; Conte, E.; Beltrama, V.; Annoni, A.; et al. Impact of an intra-cycle motion correction algorithm on overall evaluability and diagnostic accuracy of computed tomography coronary angiography. Eur. Radiol. 2016, 26, 147–156. [Google Scholar] [CrossRef]
- Baggiano, A.; Fusini, L.; Del Torto, A.; Vivona, P.; Guglielmo, M.; Muscogiuri, G.; Soldi, M.; Martini, C.; Fraschini, E.; Rabbat, M.G.; et al. Sequential Strategy Including FFR(CT) Plus Stress-CTP Impacts on Management of Patients with Stable Chest Pain: The Stress-CTP RIPCORD Study. J. Clin. Med. 2020, 9, 2147. [Google Scholar] [CrossRef]
- Esposito, A.; Francone, M.; Andreini, D.; Buffa, V.; Cademartiri, F.; Carbone, I.; Clemente, A.; Guaricci, A.I.; Guglielmo, M.; Indolfi, C.; et al. SIRM-SIC appropriateness criteria for the use of Cardiac Computed Tomography. Part 1: Congenital heart diseases, primary prevention, risk assessment before surgery, suspected CAD in symptomatic patients, plaque and epicardial adipose tissue characterization, and functional assessment of stenosis. Radiol. Med. 2021, 126, 1236–1248. [Google Scholar] [CrossRef]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijić, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2022, 44, 142–158. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Del Torto, A.; Mushtaq, S.; et al. Diagnostic accuracy of simultaneous evaluation of coronary arteries and myocardial perfusion with single stress cardiac computed tomography acquisition compared to invasive coronary angiography plus invasive fractional flow reserve. Int. J. Cardiol. 2018, 273, 263–268. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Fazzari, F.; Berzovini, C.; et al. Quantitative vs. qualitative evaluation of static stress computed tomography perfusion to detect haemodynamically significant coronary artery disease. Eur. Heart. J. Cardiovasc. Imaging 2018, 19, 1244–1252. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Goeller, M.; Rahman Ihdayhid, A.; Cadet, S.; Lin, A.; Adams, D.; Thakur, U.; Yap, G.; Marwan, M.; Achenbach, S.; Dey, D.; et al. Pericoronary adipose tissue and quantitative global non-calcified plaque characteristics from CT angiography do not differ in matched South Asian, East Asian and European-origin Caucasian patients with stable chest pain. Eur. J. Radiol. 2020, 125, 108874. [Google Scholar] [CrossRef]
- Cosson, E.; Nguyen, M.T.; Rezgani, I.; Tatulashvili, S.; Sal, M.; Berkane, N.; Allard, L.; Brillet, P.-Y.; Bihan, H. Epicardial adipose tissue volume and coronary calcification among people living with diabetes: A cross-sectional study. Cardiovasc. Diabetol. 2021, 20, 35. [Google Scholar] [CrossRef]
- Yerramasu, A.; Dey, D.; Venuraju, S.; Anand, D.V.; Atwal, S.; Corder, R.; Berman, D.S.; Lahiri, A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012, 220, 223–230. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Danial, J.S.H.; Henein, M.Y. Coronary Artery Microcalcification: Imaging and Clinical Implications. Diagnostics 2019, 9, 125. [Google Scholar] [CrossRef]
- Galli, E.; Muratore, F.; Boiardi, L.; Restuccia, G.; Cavazza, A.; Catanoso, M.; Macchioni, P.; Spaggiari, L.; Casali, M.; Pipitone, N.; et al. Significance of inflammation restricted to adventitial/periadventitial tissue on temporal artery biopsy. Semin. Arthritis Rheum. 2020, 50, 1064–1072. [Google Scholar] [CrossRef]
- Wall, C.; Huang, Y.; Le, E.P.V.; Ćorović, A.; Uy, C.P.; Gopalan, D.; Ma, C.; Manavaki, R.; Fryer, T.D.; Aloj, L.; et al. Pericoronary and periaortic adipose tissue density are associated with inflammatory disease activity in Takayasu arteritis and atherosclerosis. Eur. Heart. J. Open 2021, 1, oeab019. [Google Scholar] [CrossRef]
- Marwan, M.; Hell, M.; Schuhbäck, A.; Gauss, S.; Bittner, D.; Pflederer, T.; Achenbach, S. CT Attenuation of Pericoronary Adipose Tissue in Normal Versus Atherosclerotic Coronary Segments as Defined by Intravascular Ultrasound. J. Comput. Assist. Tomogr. 2017, 41, 762–767. [Google Scholar] [CrossRef]
- Nakajima, A.; Sugiyama, T.; Araki, M.; Seegers, L.M.; Dey, D.; McNulty, I.; Lee, H.; Yonetsu, T.; Yasui, Y.; Teng, Y.; et al. Plaque Rupture, Compared with Plaque Erosion, Is Associated with a Higher Level of Pancoronary Inflammation. JACC Cardiovasc. Imaging 2022, 15, 828–839. [Google Scholar] [CrossRef]
- Iacobellis, G.; Willens, H.J. Echocardiographic Epicardial Fat: A Review of Research and Clinical Applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319. [Google Scholar] [CrossRef]
- Natale, F.; Tedesco, M.A.; Mocerino, R.; de Simone, V.; Di Marco, G.M.; Aronne, L.; Credendino, M.; Siniscalchi, C.; Calabrò, P.; Cotrufo, M.; et al. Visceral adiposity and arterial stiffness: Echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur. J. Echocardiogr. 2009, 10, 549–555. [Google Scholar] [CrossRef]
- Guglielmo, M.; Lin, A.; Dey, D.; Baggiano, A.; Fusini, L.; Muscogiuri, G.; Pontone, G. Epicardial fat and coronary artery disease: Role of cardiac imaging. Atherosclerosis 2021, 321, 30–38. [Google Scholar] [CrossRef]
- Hell, M.M.; Achenbach, S.; Schuhbaeck, A.; Klinghammer, L.; May, M.S.; Marwan, M. CT-based analysis of pericoronary adipose tissue density: Relation to cardiovascular risk factors and epicardial adipose tissue volume. J. Cardiovasc. Comput. Tomogr. 2016, 10, 52–60. [Google Scholar] [CrossRef]
- Ma, R.; Fari, R.; van der Harst, P.; De Cecco, C.N.; Stillman, A.E.; Vliegenthart, R.; van Assen, M. Evaluation of pericoronary adipose tissue attenuation on CT. Br. J. Radiol. 2023, 96, 20220885. [Google Scholar] [CrossRef]
- Duncker, H.; Achenbach, S.; Moshage, M.; Dey, D.; Bittner, D.O.; Ammon, F.; Marwan, M.; Goeller, M. Computed Tomography-derived Characterization of Pericoronary, Epicardial, and Paracardial Adipose Tissue and Its Association with Myocardial Ischemia as Assessed by Computed Fractional Flow Reserve. J. Thorac. Imaging 2023, 38, 46–53. [Google Scholar] [CrossRef]
- Yuvaraj, J.; Cheng, K.; Lin, A.; Psaltis, P.J.; Nicholls, S.J.; Wong, D.T.L. The Emerging Role of CT-Based Imaging in Adipose Tissue and Coronary Inflammation. Cells 2021, 10, 1196. [Google Scholar] [CrossRef]
- Toemen, L.; Santos, S.; Roest, A.A.; Jelic, G.; van der Lugt, A.; Felix, J.F.; Helbing, W.A.; Gaillard, R.; Jaddoe, V.W.V. Body Fat Distribution, Overweight, and Cardiac Structures in School-Age Children: A Population-Based Cardiac Magnetic Resonance Imaging Study. J. Am. Heart Assoc. 2020, 9, e014933. [Google Scholar] [CrossRef]
- Marciniak, M.; van Deutekom, A.W.; Toemen, L.; Lewandowski, A.J.; Gaillard, R.; Young, A.A.; Jaddoe, V.W.V.; Lamata, P. A three-dimensional atlas of child’s cardiac anatomy and the unique morphological alterations associated with obesity. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1645–1653. [Google Scholar] [CrossRef]
- Wong, C.; Marwick, T.H. Obesity cardiomyopathy: Pathogenesis and pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 436–443. [Google Scholar] [CrossRef]
- Corradi, D.; Maestri, R.; Callegari, S.; Pastori, P.; Goldoni, M.; Luong, T.V.; Bordi, C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004, 13, 313–316. [Google Scholar] [CrossRef]
- Iacobellis, G.; Ribaudo, M.C.; Zappaterreno, A.; Iannucci, C.V.; Leonetti, F. Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol. 2004, 94, 1084–1087. [Google Scholar] [CrossRef]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp. Biochem. Physiol. B 1989, 94, 225–232. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Silaghi, A.; Achard, V.; Paulmyer-Lacroix, O.; Scridon, T.; Tassistro, V.; Duncea, I.; Clément, K.; Dutour, A.; Grino, M. Expression of adrenomedullin in human epicardial adipose tissue: Role of coronary status. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1443–E1450. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Chen, Y.-C.; Chen, J.-H.; Chen, S.-A.; Chen, Y.-J. Adipocytes modulate the electrophysiology of atrial myocytes: Implications in obesity-induced atrial fibrillation. Basic Res. Cardiol. 2012, 107, 293. [Google Scholar] [CrossRef]
- Parisi, V.; Rengo, G.; Perrone-Filardi, P.; Pagano, G.; Femminella, G.D.; Paolillo, S.; Petraglia, L.; Gambino, G.; Caruso, A.; Grimaldi, M.G.; et al. Increased Epicardial Adipose Tissue Volume Correlates with Cardiac Sympathetic Denervation in Patients with Heart Failure. Circ. Res. 2016, 118, 1244–1253. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; di Gioia, C.R. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef]
- McAninch, E.A.; Fonseca, T.L.; Poggioli, R.; Panos, A.L.; Salerno, T.A.; Deng, Y.; Li, Y.; Bianco, A.C.; Iacobellis, G. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity 2015, 23, 1267–1278. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mahabadi, A.A. Is epicardial fat attenuation a novel marker of coronary inflammation? Atherosclerosis 2019, 284, 212–213. [Google Scholar] [CrossRef]
- Christensen, R.H.; von Scholten, B.J.; Hansen, C.S.; Jensen, M.T.; Vilsbøll, T.; Rossing, P.; Jørgensen, P.G. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2019, 18, 114. [Google Scholar] [CrossRef]
- Yang, X.; Feng, C.; Feng, J. Epicardial Adipose Tissue and Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231151820. [Google Scholar] [CrossRef]
- Maffei, E.; Seitun, S.; Nieman, K.; Martini, C.; Guaricci, A.I.; Tedeschi, C.; Weustink, A.C.; Mollet, N.R.; Berti, E.; Grilli, R.; et al. Assessment of coronary artery disease and calcified coronary plaque burden by computed tomography in patients with and without diabetes mellitus. Eur. Radiol. 2011, 21, 944–953. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Lorenzoni, V.; Guglielmo, M.; Mushtaq, S.; Muscogiuri, G.; Cademartiri, F.; Rabbat, M.; Andreini, D.; Serviddio, G.; Gaibazzi, N.; et al. Prognostic relevance of subclinical coronary and carotid atherosclerosis in a diabetic and nondiabetic asymptomatic population. Clin. Cardiol. 2018, 41, 769–777. [Google Scholar] [CrossRef]
- Basile, P.; Guaricci, A.I.; Piazzolla, G.; Volpe, S.; Vozza, A.; Benedetto, M.; Carella, M.C.; Santoro, D.; Monitillo, F.; Baggiano, A.; et al. Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study. J. Clin. Med. 2023, 12, 1586. [Google Scholar] [CrossRef]
- Nomura, C.H.; Assuncao-Jr, A.N.; Guimarães, P.O.; Liberato, G.; Morais, T.C.; Fahel, M.G.; Giorgi, M.C.P.; Meneghetti, J.C.; Parga, J.R.; Dantas-Jr, R.N.; et al. Association between perivascular inflammation and downstream myocardial perfusion in patients with suspected coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 599–605. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: A systematic review and meta-analysis. Eur. Heart J.—Cardiovasc. Imaging 2017, 19, 490–497. [Google Scholar] [CrossRef]
- Rajani, R.; Shmilovich, H.; Nakazato, R.; Nakanishi, R.; Otaki, Y.; Cheng, V.Y.; Hayes, S.W.; Thomson, L.E.; Friedman, J.D.; Slomka, P.J.; et al. Relationship of epicardial fat volume to coronary plaque, severe coronary stenosis, and high-risk coronary plaque features assessed by coronary CT angiography. J. Cardiovasc. Comput. Tomogr. 2013, 7, 125–132. [Google Scholar] [CrossRef]
- Bo, X.; Ma, L.; Fan, J.; Jiang, Z.; Zhou, Y.; Zhang, L.; Li, W. Epicardial fat volume is correlated with coronary lesion and its severity. Int. J. Clin. Exp. Med. 2015, 8, 4328–4334. [Google Scholar]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Pontone, G.; Fusini, L.; De Luca, M.; Cafarelli, F.P.; Guglielmo, M.; Baggiano, A.; Beltrama, V.; Muscogiuri, G.; Mushtaq, S.; et al. Additional value of inflammatory biomarkers and carotid artery disease in prediction of significant coronary artery disease as assessed by coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1049–1056. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef]
- Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; Nelson, C.P.; et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef]
- Roongsritong, C.; Warraich, I.; Bradley, C. Common causes of troponin elevations in the absence of acute myocardial infarction: Incidence and clinical significance. Chest 2004, 125, 1877–1884. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Masci, P.G.; Muscogiuri, G.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Lorenzoni, V.; Martini, C.; Andreini, D.; Pavon, A.G.; et al. CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Ischaemic dilated CardioMyopathy: An international Registry. Europace 2021, 23, 1072–1083. [Google Scholar] [CrossRef]
- Morita, E.; Yasue, H.; Yoshimura, M.; Ogawa, H.; Jougasaki, M.; Matsumura, T.; Mukoyama, M.; Nakao, K. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993, 88, 82–91. [Google Scholar] [CrossRef]
- Wong, Y.-K.; Tse, H.-F. Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients with Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 713191. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Santoro, F.; Paoletti Perini, A.; Ioffredo, L.; Trivedi, C.; Pontone, G.; Di Biase, M.; Brunetti, N.D. Correlations between NT-proBNP, outcome and haemodynamics in patients with septic shock. Acta Cardiol. 2015, 70, 545–552. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Bulzis, G.; Pontone, G.; Scicchitano, P.; Carbonara, R.; Rabbat, M.; De Santis, D.; Ciccone, M.M. Current interpretation of myocardial stunning. Trends Cardiovasc. Med. 2018, 28, 263–271. [Google Scholar] [CrossRef]
- Hsu, B.G.; Chen, Y.C.; Lee, R.P.; Lee, C.C.; Lee, C.J.; Wang, J.H. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ. J. 2010, 74, 327–331. [Google Scholar] [CrossRef]
- Bao, Y.; Lu, Z.; Zhou, M.; Li, H.; Wang, Y.; Gao, M.; Wei, M.; Jia, W. Serum levels of adipocyte fatty acid-binding protein are associated with the severity of coronary artery disease in Chinese women. PLoS ONE 2011, 6, e19115. [Google Scholar] [CrossRef]
- Zografos, T.; Haliassos, A.; Korovesis, S.; Giazitzoglou, E.; Voridis, E.; Katritsis, D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am. J. Cardiol. 2009, 104, 917–920. [Google Scholar] [CrossRef]
- Elkhidir, A.E.; Eltaher, H.B.; Mohamed, A.O. Association of lipocalin-2 level, glycemic status and obesity in type 2 diabetes mellitus. BMC Res. Notes 2017, 10, 285. [Google Scholar] [CrossRef]
- Caselli, C.; Rovai, D.; Lorenzoni, V.; Carpeggiani, C.; Teresinska, A.; Aguade, S.; Todiere, G.; Gimelli, A.; Schroeder, S.; Casolo, G.; et al. A New Integrated Clinical-Biohumoral Model to Predict Functionally Significant Coronary Artery Disease in Patients with Chronic Chest Pain. Can. J. Cardiol. 2015, 31, 709–716. [Google Scholar] [CrossRef]
- Caselli, C.; De Graaf, M.A.; Lorenzoni, V.; Rovai, D.; Marinelli, M.; Del Ry, S.; Giannessi, D.; Bax, J.J.; Neglia, D.; Scholte, A.J. HDL cholesterol, leptin and interleukin-6 predict high risk coronary anatomy assessed by CT angiography in patients with stable chest pain. Atherosclerosis 2015, 241, 55–61. [Google Scholar] [CrossRef]
- Pepe, M.; Napoli, G.; Biondi-Zoccai, G.; Giordano, A. Anti-Inflammatory Therapy for Acute Coronary Syndromes: Is It Time for a Shift in the Treatment Paradigm? J. Cardiovasc. Pharmacol. 2022, 80, 633–635. [Google Scholar] [CrossRef]
- Held, C.; White, H.D.; Stewart, R.A.H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 6, e005077. [Google Scholar] [CrossRef]
- Zebrack, J.S.; Anderson, J.L.; Maycock, C.A.; Horne, B.D.; Bair, T.L.; Muhlestein, J.B. Usefulness of high-sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am. J. Cardiol. 2002, 89, 145–149. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Yuvaraj, J.; Fernandes, K.; Jiang, C.; Nicholls, S.J.; Dey, D.; Wong, D.T.L. Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: A cross-sectional study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 298–306. [Google Scholar] [CrossRef]
- Araki, M.; Sugiyama, T.; Nakajima, A.; Yonetsu, T.; Seegers, L.M.; Dey, D.; Lee, H.; McNulty, I.; Yasui, Y.; Teng, Y.; et al. Level of Vascular Inflammation Is Higher in Acute Coronary Syndromes Compared with Chronic Coronary Disease. Circ. Cardiovasc. Imaging 2022, 15, e014191. [Google Scholar] [CrossRef]
- Tzolos, E.; Williams, M.C.; McElhinney, P.; Lin, A.; Grodecki, K.; Flores Tomasino, G.; Cadet, S.; Kwiecinski, J.; Doris, M.; Adamson, P.D.; et al. Pericoronary Adipose Tissue Attenuation, Low-Attenuation Plaque Burden, and 5-Year Risk of Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1078–1088. [Google Scholar] [CrossRef]
- Kubo, T.; Imanishi, T.; Kashiwagi, M.; Ikejima, H.; Tsujioka, H.; Kuroi, A.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; Ino, Y.; et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am. J. Cardiol. 2010, 105, 318–322. [Google Scholar] [CrossRef]
- Dawson, L.P.; Layland, J. High-Risk Coronary Plaque Features: A Narrative Review. Cardiol. Ther. 2022, 11, 319–335. [Google Scholar] [CrossRef]
- Nerlekar, N.; Ha, F.J.; Cheshire, C.; Rashid, H.; Cameron, J.D.; Wong, D.T.; Seneviratne, S.; Brown, A.J. Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2018, 11, e006973. [Google Scholar] [CrossRef]
- Narula, J.; Nakano, M.; Virmani, R.; Kolodgie, F.D.; Petersen, R.; Newcomb, R.; Malik, S.; Fuster, V.; Finn, A.V. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J. Am. Coll. Cardiol. 2013, 61, 1041–1051. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared with Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Balcer, B.; Dykun, I.; Forsting, M.; Schlosser, T.; Heusch, G.; Rassaf, T. Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction. PLoS ONE 2017, 12, e0183514. [Google Scholar] [CrossRef]
- Barandier, C.; Montani, J.-P.; Yang, Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: Effects of aging and obesity. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H1807–H1813. [Google Scholar] [CrossRef]
- Gennero, I.; Xuereb, J.M.; Simon, M.F.; Girolami, J.P.; Bascands, J.L.; Chap, H.; Boneu, B.; Sié, P. Effects of lysophosphatidic acid on proliferation and cytosolic Ca++ of human adult vascular smooth muscle cells in culture. Thromb. Res. 1999, 94, 317–326. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M.; Verheule, S.; Recalde, A.; Sanna, F.; Herdman, L.; Psarros, C.; Nasrallah, H.; Coutinho, P.; Akoumianakis, I.; et al. Mutual Regulation of Epicardial Adipose Tissue and Myocardial Redox State by PPAR-γ/Adiponectin Signalling. Circ. Res. 2016, 118, 842–855. [Google Scholar] [CrossRef]
- Verhagen, S.N.; Vink, A.; van der Graaf, Y.; Visseren, F.L. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis 2012, 225, 99–104. [Google Scholar] [CrossRef]
- Sequeira, D.I.; Ebert, L.C.; Flach, P.M.; Ruder, T.D.; Thali, M.J.; Ampanozi, G. The correlation of epicardial adipose tissue on postmortem CT with coronary artery stenosis as determined by autopsy. Forensic Sci. Med. Pathol. 2015, 11, 186–192. [Google Scholar] [CrossRef]
- Kelly, K.L.; Lin, P.T.; Basso, C.; Bois, M.; Buja, L.M.; Cohle, S.D.; d’Amati, G.; Duncanson, E.; Fallon, J.T.; Firchau, D.; et al. Sudden cardiac death in the young: A consensus statement on recommended practices for cardiac examination by pathologists from the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2023, 63, 107497. [Google Scholar] [CrossRef]
- Hogea, T.; Noemi, N.; Suciu, B.A.; Brinzaniuc, K.; Chinezu, L.; Arbănași, E.M.; Kaller, R.; Carașca, C.; Arbănași, E.M.; Vunvulea, V.; et al. Increased Epicardial Adipose Tissue and Heart Characteristics Are Correlated with BMI and Predict Silent Myocardial Infarction in Sudden Cardiac Death Subjects: An Autopsy Study. Diagnostics 2023, 13, 2157. [Google Scholar] [CrossRef]
- Hogea, T.; Suciu, B.A.; Ivănescu, A.D.; Carașca, C.; Chinezu, L.; Arbănași, E.M.; Russu, E.; Kaller, R.; Arbănași, E.M.; Mureșan, A.V.; et al. Increased Epicardial Adipose Tissue (EAT), Left Coronary Artery Plaque Morphology, and Valvular Atherosclerosis as Risks Factors for Sudden Cardiac Death from a Forensic Perspective. Diagnostics 2023, 13, 142. [Google Scholar] [CrossRef]
- Harnden, A.; Takahashi, M.; Burgner, D. Kawasaki disease. BMJ 2009, 338, b1514. [Google Scholar] [CrossRef]
- Shi, H.; Wu, H.; Winkler, M.A.; Belin de Chantemèle, E.J.; Lee, R.; Kim, H.W.; Weintraub, N.L. Perivascular adipose tissue in autoimmune rheumatic diseases. Pharmacol. Res. 2022, 182, 106354. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, Q.; Wu, T.; Zhu, B.; Liu, S.; Liu, S.; Aierken, X.; Ahmat, A.; Li, N. Association of circulating resistin and adiponectin levels with Kawasaki disease: A meta-analysis. Exp. Ther. Med. 2020, 19, 1033–1041. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2016, 38, 143–153. [Google Scholar] [CrossRef]
- Occhipinti, G.; Bucciarelli-Ducci, C.; Capodanno, D. Diagnostic pathways in myocardial infarction with non-obstructive coronary artery disease (MINOCA). Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 813–822. [Google Scholar] [CrossRef]
- Pergola, V.; Previtero, M.; Cecere, A.; Storer, V.; Castiello, T.; Baritussio, A.; Cabrelle, G.; Mele, D.; Motta, R.; Caforio, A.P.; et al. Clinical Value and Time Course of Pericoronary Fat Inflammation in Patients with Angiographically Nonobstructive Coronaries: A Preliminary Report. J. Clin. Med. 2021, 10, 1786. [Google Scholar] [CrossRef]
- Robinowitz, M.; Virmani, R.; McAllister, H.A.J. Spontaneous coronary artery dissection and eosinophilic inflammation: A cause and effect relationship? Am. J. Med. 1982, 72, 923–928. [Google Scholar] [CrossRef]
- Pitliya, A.; Datta, S.; Kalayci, A.; Kahe, F.; Sharfaei, S.; Jafarizade, M.; Goudarzi, S.; Chi, G. Eosinophilic inflammation in spontaneous coronary artery dissection: A potential therapeutic target? Med. Hypotheses 2018, 121, 91–94. [Google Scholar] [CrossRef]
- Margaritis, M.; Sheppard, M.; Parsons, S.; Robertus, J.L.; Vink, A.; Samani, N.; Adlam, D. Abstract 15829: Periadvential Inflammation in Spontaneous Coronary Artery Dissection: Causal Role or Response to Injury? Circulation 2018, 138, A15829. [Google Scholar]
- Hedgire, S.; Baliyan, V.; Zucker, E.J.; Bittner, D.O.; Staziaki, P.V.; Takx, R.A.P.; Scholtz, J.E.; Meyersohn, N.; Hoffmann, U.; Ghoshhajra, B. Perivascular Epicardial Fat Stranding at Coronary CT Angiography: A Marker of Acute Plaque Rupture and Spontaneous Coronary Artery Dissection. Radiology 2018, 287, 808–815. [Google Scholar] [CrossRef]
- Yuvaraj, J.; Lin, A.; Nerlekar, N.; Rashid, H.; Cameron, J.D.; Seneviratne, S.; Nicholls, S.; Psaltis, P.J.; Wong, D.T.L. Is spontaneous coronary artery dissection (SCAD) related to vascular inflammation and epicardial fat? -insights from computed tomography coronary angiography. Cardiovasc. Diagn. Ther. 2020, 10, 239–241. [Google Scholar] [CrossRef]
- Tweet, M.S.; Akhtar, N.J.; Hayes, S.N.; Best, P.J.; Gulati, R.; Araoz, P.A. Spontaneous coronary artery dissection: Acute findings on coronary computed tomography angiography. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 467–475. [Google Scholar] [CrossRef]
- Pergola, V.; Continisio, S.; Mantovani, F.; Motta, R.; Mattesi, G.; Marrazzo, G.; Dellino, C.M.; Montonati, C.; De Conti, G.; Galzerano, D.; et al. Spontaneous coronary artery dissection: The emerging role of coronary computed tomography. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 839–850. [Google Scholar] [CrossRef]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of coronary vasomotion abnormalities—From bench to bedside. Eur. Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef]
- Forman, M.B.; Oates, J.A.; Robertson, D.; Robertson, R.M.; Roberts, L.J., 2nd; Virmani, R. Increased adventitial mast cells in a patient with coronary spasm. N. Engl. J. Med. 1985, 313, 1138–1141. [Google Scholar] [CrossRef]
- Lange, R.A.; Cigarroa, R.G.; Yancy, C.W., Jr.; Willard, J.E.; Popma, J.J.; Sills, M.N.; McBride, W.; Kim, A.S.; Hillis, L.D. Cocaine-induced coronary-artery vasoconstriction. N. Engl. J. Med. 1989, 321, 1557–1562. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Nishimiya, K.; Hao, K.; Tsuburaya, R.; Ota, H.; Amamizu, H.; Uzuka, H.; Takahashi, J.; Ito, K.; et al. Increased Coronary Perivascular Adipose Tissue Volume in Patients with Vasospastic Angina. Circ. J. 2016, 80, 1653–1656. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Amamizu, H.; Uzuka, H.; Nishimiya, K.; Morosawa, S.; Hirano, M.; Watabe, H.; Funaki, Y.; Miyata, S.; et al. Association of Coronary Perivascular Adipose Tissue Inflammation and Drug-Eluting Stent-Induced Coronary Hyperconstricting Responses in Pigs: (18)F-Fluorodeoxyglucose Positron Emission Tomography Imaging Study. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1757–1764. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients with Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Owen, M.K.; Witzmann, F.A.; McKenney, M.L.; Lai, X.; Berwick, Z.C.; Moberly, S.P.; Alloosh, M.; Sturek, M.; Tune, J.D. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation 2013, 128, 9–18. [Google Scholar] [CrossRef]
- Lin, A.; Kolossváry, M.; Motwani, M.; Išgum, I.; Maurovich-Horvat, P.; Slomka, P.J.; Dey, D. Artificial Intelligence in Cardiovascular Imaging for Risk Stratification in Coronary Artery Disease. Radiol. Cardiothorac. Imaging 2021, 3, e200512. [Google Scholar] [CrossRef]
- Al’Aref, S.J.; Singh, G.; Choi, J.W.; Xu, Z.; Maliakal, G.; van Rosendael, A.R.; Lee, B.C.; Fatima, Z.; Andreini, D.; Bax, J.J.; et al. A Boosted Ensemble Algorithm for Determination of Plaque Stability in High-Risk Patients on Coronary CTA. JACC Cardiovasc. Imaging 2020, 13, 2162–2173. [Google Scholar] [CrossRef]
- Dey, D.; Gaur, S.; Ovrehus, K.A.; Slomka, P.J.; Betancur, J.; Goeller, M.; Hell, M.M.; Gransar, H.; Berman, D.S.; Achenbach, S.; et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: A multicentre study. Eur. Radiol. 2018, 28, 2655–2664. [Google Scholar] [CrossRef]
- Driessen, R.S.; Danad, I.; Stuijfzand, W.J.; Raijmakers, P.G.; Schumacher, S.P.; van Diemen, P.A.; Leipsic, J.A.; Knuuti, J.; Underwood, S.R.; van de Ven, P.M.; et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J. Am. Coll. Cardiol. 2019, 73, 161–173. [Google Scholar] [CrossRef]
- Commandeur, F.; Goeller, M.; Razipour, A.; Cadet, S.; Hell, M.M.; Kwiecinski, J.; Chen, X.; Chang, H.-J.; Marwan, M.; Achenbach, S.; et al. Fully Automated CT Quantification of Epicardial Adipose Tissue by Deep Learning: A Multicenter Study. Radiol. Artif. Intell. 2019, 1, e190045. [Google Scholar] [CrossRef]
- Otaki, Y.; Hell, M.; Slomka, P.J.; Schuhbaeck, A.; Gransar, H.; Huber, B.; Nakazato, R.; Germano, G.; Hayes, S.W.; Thomson, L.E.; et al. Relationship of epicardial fat volume from noncontrast CT with impaired myocardial flow reserve by positron emission tomography. J. Cardiovasc. Comput. Tomogr. 2015, 9, 303–309. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chiesa, M.; Baggiano, A.; Spadafora, P.; De Santis, R.; Guglielmo, M.; Scafuri, S.; Fusini, L.; Mushtaq, S.; Conte, E.; et al. Diagnostic performance of deep learning algorithm for analysis of computed tomography myocardial perfusion. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3119–3128. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Williams, M.C.; Kotanidis, C.P.; Desai, M.Y.; Marwan, M.; Antonopoulos, A.S.; Thomas, K.E.; Thomas, S.; Akoumianakis, I.; Fan, L.M.; et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur. Heart J. 2019, 40, 3529–3543. [Google Scholar] [CrossRef]
- Lin, A.; Kolossváry, M.; Yuvaraj, J.; Cadet, S.; McElhinney, P.A.; Jiang, C.; Nerlekar, N.; Nicholls, S.J.; Slomka, P.J.; Maurovich-Horvat, P.; et al. Myocardial Infarction Associates with a Distinct Pericoronary Adipose Tissue Radiomic Phenotype: A Prospective Case-Control Study. JACC Cardiovasc. Imaging 2020, 13, 2371–2383. [Google Scholar] [CrossRef]

| TTE | CCTA | CMR | |
|---|---|---|---|
| Availability |    |    |    |
| Cost |    |    |    |
| Lack of iodine contrast use |  |  |  |
| Lack of ionizing radiation exposure |  |  |  |
| Reproducibility |    |    |    |
| Spatial resolution |    |    |    |
| 3D volume data |  |  |  |
| AT thickness |  |  |  |
| AT area |  |  |  |
| AT volume |  |  |  |
| AT attenuation |  |  |  |
| AT radiomic profile |  |  |  |


 , high;
, high; 

 , medium;
, medium; 

 , low;
, low;  , condition satisfied/measure allowed;
, condition satisfied/measure allowed;  , N/A.
, N/A.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoli, G.; Pergola, V.; Basile, P.; De Feo, D.; Bertrandino, F.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Carrabba, N.; et al. Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes. J. Clin. Med. 2023, 12, 7212. https://doi.org/10.3390/jcm12237212
Napoli G, Pergola V, Basile P, De Feo D, Bertrandino F, Baggiano A, Mushtaq S, Fusini L, Fazzari F, Carrabba N, et al. Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes. Journal of Clinical Medicine. 2023; 12(23):7212. https://doi.org/10.3390/jcm12237212
Chicago/Turabian StyleNapoli, Gianluigi, Valeria Pergola, Paolo Basile, Daniele De Feo, Fulvio Bertrandino, Andrea Baggiano, Saima Mushtaq, Laura Fusini, Fabio Fazzari, Nazario Carrabba, and et al. 2023. "Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes" Journal of Clinical Medicine 12, no. 23: 7212. https://doi.org/10.3390/jcm12237212
APA StyleNapoli, G., Pergola, V., Basile, P., De Feo, D., Bertrandino, F., Baggiano, A., Mushtaq, S., Fusini, L., Fazzari, F., Carrabba, N., Rabbat, M. G., Motta, R., Ciccone, M. M., Pontone, G., & Guaricci, A. I. (2023). Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes. Journal of Clinical Medicine, 12(23), 7212. https://doi.org/10.3390/jcm12237212














