Abstract
Introduction: Insufficient nutrient intake is a strong independent predictor of mortality in elderly patients with heart failure. However, it is unclear to what extent energy intake affects their prognosis. This study investigated the association between patient outcomes and actual measured energy intake in elderly patients (≥65 years) with heart failure. Methods: This study enrolled 139 elderly patients who were hospitalized with worsening heart failure at Shingu Municipal Medical Center, Shingu, Japan, between May 2017 and April 2018. Energy intake was evaluated for three days (from three days prior to the day of discharge until the day of discharge). Based on basal energy expenditure calculated using the Harris–Benedict equation, the patients were classified into a low-energy group (n = 38) and a high-energy group (n = 101). We assessed the prognosis in terms of both all-cause mortality and readmission due to worsening heart failure as a primary outcome. Results: Compared to the patients in the high-energy group, the patients in the low-energy group were predominantly female, less frequently had smoking habits and ischemic heart diseases, and had a higher left ventricular ejection fraction. The low-energy group had higher mortality than the high-energy group (p = 0.028), although the two groups showed equivalent event rates of the primary outcome (p = 0.569). Conclusion: Calculations based on the Harris–Benedict equation revealed no significant difference in the primary outcome between the two groups, with a secondary outcome that showed worse mortality in the low-energy group. Given this result, energy requirement-based assessments using the Harris–Benedict equation might help in the management of elderly heart failure patients in terms of improved life outcomes.
1. Introduction
Insufficient nutrient intake is a strong independent predictor of mortality in patients with heart failure (HF) [1,2]. The Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score [3] is a widely used tool for predicting mortality after the discharge of patients with HF [4,5]. It is calculated using a number of individual risk factors, such as serum sodium, sex, and preserved or reduced left ventricular ejection fraction (LVEF). However, nutritional factors are not included in the MAGGIC risk score. Meanwhile, although there are a number of malnutrition scores, including the Geriatric Nutritional Risk Index (GNRI) [6] and the Controlling Nutritional Status (CONUT) Index [7], the typical presentation of malnutrition is a loss of appetite, which represents an independent factor of hospital mortality [8]. Energy deficits are associated with an increased proportion of infections in patients in intensive care units [9]. However, it is unclear to what extent energy intake affects the prognosis of HF.
The Harris–Benedict equation (HBE) [10] is considered to be the best equation to predict basal energy expenditure (BEE) [11]; however, in elderly patients (≥65 years), it has been reported to underestimate energy requirements [12]. Conversely, the HBE has been shown to overestimate BEE in patients with HF [13]. At present, the best indicators of the appropriate energy needs of elderly patients with HF remain unclear.
We hypothesized that the HBE would be reliable for elderly patients with HF and that elderly patients with inadequate energy intake with HF would have a much worse prognosis than those with adequate energy intake. This study investigated the association between the outcomes and actual measured energy intake of elderly patients with HF.
2. Materials and Methods
2.1. Subjects
We included 196 consecutive elderly HF patients (aged 65 years or older) without severe valvular disease, congenital disease, complete atrioventricular block, pericardial disease, primary pulmonary hypertension, or acute coronary syndrome who were admitted to Shingu Municipal Medical Center, Shingu, Japan, due to worsening HF between May 2017 and April 2018 and discharged home. HF was defined as follows: HF symptoms according to the Framingham criteria and increased plasma concentration of brain natriuretic peptide (BNP) (>100 pg/mL) at admission. We excluded patients who died during index hospitalization (n = 10), patients already enrolled in the study who were admitted to the hospital with an HF exacerbation during the follow-up period (1 year) (to avoid double counting) (n = 45), and those who could not be followed up (n = 2). Finally, 139 patients were enrolled in the present study (Figure 1).
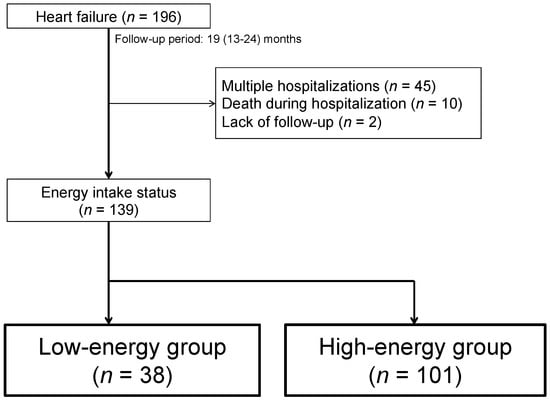
Figure 1.
Flowchart of the study population and design.
This study was approved by the Shingu Municipal Medical Center Ethics Committee (Number: 65) and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients in accordance with the committee guidelines.
2.2. Patient Characteristics
Clinical and laboratory data were retrospectively collected from the patients’ records. BNP was measured with a commercially available kit (BNP-JP 471680R03, Abbott Japan, Tokyo, Japan). The LVEF was quantitated via echocardiogram at admission. Medical histories and findings in the physical examination were also collected from the records. Ischemic heart disease was defined as any medical history of acute coronary syndrome, percutaneous coronary intervention, or coronary artery bypass surgery or a diagnosis of myocardial ischemia based on invasive/noninvasive diagnostic tests.
2.3. Dietary Assessment
Meals with a set total energy content were provided, and their intake was directly observed. Nurses in our cardiovascular section visually estimated dietary intake after each meal (main dishes and staple meals were estimated separately). This is standard practice in most hospitals in Japan [14], and nurses are trained in standardized visual dietary intake assessment based on protocols implemented throughout the Shingu Municipal Medical Center. The consumption of each dietary item is recorded as a percentage (expressed in ×10%) of the total amount. From the data recorded in these medical charts, the overall average percentage intake of main and side dishes was calculated for each meal. With reference to a previous study [15], the average energy intake was estimated and calculated under the supervision of a dietitian from the average percentage intake of the three meals (breakfast, lunch, and dinner) obtained from the medical record review and the total energy of the meals provided during the three days before discharge. BEE was calculated using the following HBE formulas [10]: 66.5 + 13.76 × weight (kg) + 5.003 × height (cm) − 6.755 × age (years) for men and 655 + 9.563 × weight (kg) + 1.850 × height (cm) − 4.676 × age (years) for women. We defined patients with HF above BEE as the high-energy group and patients with HF below BEE as the low-energy group.
2.4. Malnutrition Screening Tools
Both the GNRI score [6] and the CONUT score [7] are nutrition screening tools. The GNRI was calculated using the following formula: 1.489 × serum albumin (g/L) + 41.7 × (body weight in kilograms/ideal body weight). The ideal body weight was calculated using the following formula: 22 × square of height in meters. A score > 98 is considered normal; scores of 92 to 98, 82 to 91, and <82 reflect mild, moderate, and severe malnutrition, respectively. The CONUT score takes into account serum albumin levels, cholesterol levels, and total lymphocyte counts. A score of 0 to 1 is considered normal, while scores of 2 to 4, 5 to 8, and 9 to 12 reflect mild, moderate, and severe malnutrition, respectively.
2.5. MAGGIC Risk Score
The MAGGIC risk score is a well-validated risk score for predicting the 1-year mortality of outpatients with HF [5]. It is calculated for each patient based on 13 variables (age, sex, current smoker, systolic blood pressure, body mass index, serum creatinine, diabetes, chronic obstructive pulmonary disease, New York Heart Association functional class, time since diagnosis of HF, LVEF, nonprescription of beta-blockers, and nonprescription of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers).
2.6. Outcomes and Follow-Up
Patients were followed until 31 March 2020. The median follow-up period in this study was 19 (13–24) months. The primary outcome was the composite outcome of all-cause death or readmission due to worsening HF. Decisions regarding the need for readmission due to HF were made according to the directions of the treating physicians and according to standard practice. The exploratory secondary outcomes comprised each outcome taken separately.
2.7. Statistical Analyses
Statistical analysis was performed using JMP Pro version 16 (SAS Institute, Cary, NC, USA) and R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables are presented as numbers (%) and were compared using the χ2 test. Continuous variables are presented as the mean ± SD or median [25th percentile, 75th percentile] and were compared using Student’s t-test. The Wilcoxon test was applied for nonparametric comparisons. To identify determinants of low energy intake, we selected variables showing p < 0.05 in the univariate analysis and performed multivariable logistic regression analysis. OR indicates the unit odds ratio, and HR indicates the unit hazard ratio. The p value was calculated with a Wald test. The log-rank test was used for event-free survival curves. In all analyses, statistical significance was defined by the criterion of p < 0.05.
3. Results
3.1. General Observations
Based on the BEE calculation, 38 patients with HF were classified into the low-energy group, and 101 patients with HF were classified into the high-energy group. The patient characteristics are summarized in Table 1. Compared to the patients in the high-energy group, the patients in the low-energy group were predominantly female (low-energy group vs. high-energy group, 92% vs. 24%, p < 0.0001), were less likely to smoke (current and past smoker) (current/past/never, 5%/16%/79% vs. 10%/42%/49%, p = 0.005), had a lower rate of ischemic heart diseases (8% vs. 33%, p = 0.002) and had a higher LVEF (44.6 ± 16.4% vs. 38.3 ± 15.6%, p = 0.041). Vital, physical, laboratory, echocardiographic, and nutritional data at both admission and at discharge are shown in Table 1. At admission, although patients in the low-energy group showed a higher heart rate (102.5 ± 29.1/min vs. 90.9 ± 23.8/min, p = 0.017), there were no significant differences in other parameters, including BNP (651.7 [interquartile range (IQR): 447.0, 893.8] pg/mL vs. 756.0 [IQR: 504.9, 1203.4] pg/mL, p = 0.525) or nutritional items, such as the GNRI (p = 0.572) and CONUT (p = 0.737). At discharge, there was no significant difference in the BNP (261.0 [IQR: 124.7, 460.0] pg/mL vs. 259.7 [138.0, 520.0] pg/mL, p = 0.677) or MAGGIC risk score (p = 0.139), which is a prognostic index. Furthermore, no significant differences in the qualitative nutritional status ratios or CONUT and GNRI scores were noted between the two groups (Table 2). There was no significant difference in medications at discharge (Table 3) between the two groups.

Table 1.
Baseline clinical characteristics and clinical data at both admission and discharge.

Table 2.
Nutritional assessment data.

Table 3.
Medication at discharge.
3.2. Multivariate Analysis of the Determinants of Low Energy Intake
The results of the multivariate analysis are summarized in Table 4. Being female (p < 0.001) was an independent predictor of low energy intake.

Table 4.
Multivariate analysis: determinants of low energy intake.
3.3. Prognosis of Patients with Low Energy Intake
A total of 26 fatal cases, including 21 cardiovascular disease (CV) deaths and 5 non-CV deaths, were observed. Among the fatal CV cases, 19 were due to HF, and 2 were due to stroke. Among the non-CV fatal cases, two were due to cancer, one to senility, one was a postoperative complication of gastroenterological disease, and one case had an unknown cause. In the low-energy group, 10 out of 11 deaths (90%) were CV deaths (9 HF and 1 stroke), while 11 out of 15 deaths (73%) in the high-energy group were CV deaths (10 HF and 1 stroke), indicating a similar ratio of CV deaths between the two groups (p = 0.356).
Rehospitalization for worsening HF was observed in 11 (29%) patients in the low-energy group and 39 (39%) patients in the high-energy group, with similar levels of rehospitalization for worsening HF between the two groups (p = 0.290).
The Kaplan–Meier (KM) curve of the primary outcome showed that the two groups had equivalent event rates (p = 0.569) (Figure 2A). On the other hand, the KM curve of all-cause death demonstrated a worse prognosis in the low-energy group than in the high-energy group (p = 0.028, Figure 2B), although the two groups showed equivalent readmission rates (p = 0.403, Figure 2C).
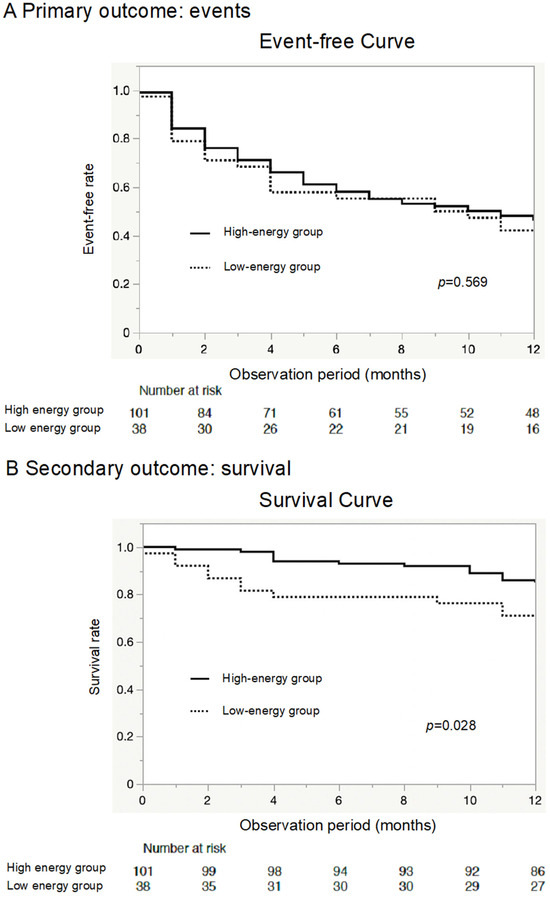

Figure 2.
Primary outcome, all-cause mortality, and readmission due to worsening heart failure. The Kaplan–Meier (KM) curve shows that the rate of the primary outcome (all-cause mortality or heart failure readmission) was similar between the two groups (A). The KM curve shows that elderly heart failure patients in the low-energy group were associated with relatively poor outcomes for mortality compared to the high-energy group (B). However, the rehospitalization rates for heart failure were similar between the two groups (C).
4. Discussion
There were three major findings of this study. First, there was no significant difference in the BNP levels between patients with adequate and inadequate energy intake, as classified according to the energy requirements determined using the Harris–Benedict equation, at admission and at discharge. Second, the nutritional scores and the prognostic index for patients with HF were similar between the two groups. Third, the low-energy group showed similar rates of the primary endpoint (composite outcome of all-cause death or readmission due to worsening HF) to the high-energy group.
Poor nutritional status in patients with HF is generally recognized to increase the risk of future mortality. In particular, the GNRI is thought to have the greatest incremental value in predicting the risk of death among HF patients [16]. The GNRI is associated with the duration of hospital stay, congestion, cardiovascular events, and long-term prognosis among patients with HF [17]. However, in this study, regardless of the similar GNRI scores and status between the two groups, the low-energy group showed worse outcomes. The CONUT score is a nutritional index derived from albumin levels, total cholesterol levels, and lymphocyte counts [7]. On the other hand, albumin levels are influenced by diuretics, and total cholesterol levels are influenced by statins. Therefore, it may not be appropriate to use the CONUT score as a nutritional indicator in HF patients who frequently use these drugs. Because of these factors, the CONUT scores probably did not differ significantly between the two groups. Various studies have reported that patients with HF and high BNP levels have a poor prognosis [18,19,20]. Many studies on HF related to poor nutrition have reported significantly lower BNP levels in malnutrition groups, with a poor prognosis [5,14,21,22,23]. BNP is a biochemical marker that sensitively reflects the degree of ventricular overload, so it is possible that the undernourished groups in these studies may have had worse control of HF than the control groups. However, we found no significant differences in BNP levels between the groups. Furthermore, the medical treatment was similar between the groups. Patients with HF and malnutrition are generally associated with a low body mass index (BMI). Patients with HF and a low BMI have a poor prognosis [24,25,26] because cases of low BMI include those with cardiac cachexia caused by chronic inflammation or physiologic abnormalities, and cardiac cachexia has a serious negative impact on HF [27]. The patients in this study had appropriate BMIs, and it was thought that there would be no prognostic impact of an abnormal BMI. In the present study, the proportion of women was higher in the low-energy group than in the high-energy group, while women generally tended to have a lower risk of mortality. If the sex ratio is equal, there might be a significant difference in events, especially mortality. From the above results, this study showed that a low energy intake is associated with higher future rates of mortality, even in the context of similar BNP levels, body weights, and nutritional status scores.
Energy deprivation induces alterations in intestinal permeability and digestive ability [28,29,30]. Genton et al. demonstrated that the gut microbiota’s composition and function were altered in nutritionally depleted states [29]. An altered microbial composition, which is caused by the irreversible loss of gut microbes, is referred to as dysbiosis [31] and is considered to play a role in cardiovascular diseases [32]. Indeed, patients with HF in the low-energy group may have experienced dysbiosis. Additionally, energy deprivation influences cytokine levels (e.g., increased serum levels of tumor necrosis factor-alpha (TNF-a) and decreased levels of interleukin-2 [33]) and immune-related cells (e.g., diminished phagocytic activity of macrophages [34] and decreased natural killer cell activity and T-cell proliferation [35]). However, whether these conditions are related to a deficient intake of energy, along with whether they are aggravated by components other than nutritional intake, such as chronic inflammation, remains unclear.
To the best of our knowledge, no previous studies have evaluated the association between energy intake and the mid-term prognosis of elderly patients with HF. Ill patients, including those with HF, often have energy needs that are greater than expected based on their body composition [36]. The Harris–Benedict equation (HBE) [10] is a classical predictor of basal energy expenditure [11]. Although the HBE is thought to underestimate energy requirements in elderly patients [12], the HBE may have just the right balance for assessing the energy requirements of elderly patients with HF.
Several previous observational surveys have shown that insufficient food intake is associated with adverse outcomes. Yoshida et al. found that inadequate food intake in relatively young patients with acute cardiac insufficiency increased mortality and HF rehospitalization rates. Hersberger et al. demonstrated that nutritional support for patients with HF reduced the risk of short-term (30-day) mortality [37]. The results of these studies support the conclusion that inadequate energy intake at discharge is associated with a poor prognosis and are in agreement with our findings among patients with HF.
There were no significant differences in readmission rates due to worsening HF between the two groups (the low-energy group and the high-energy group). The background of the patients enrolled in this study was approximately the same. Although there were no significant differences in age or NYHA classification/BNP/Hb levels/renal function at discharge between the two groups, both groups were very old, had low LVEF and poor renal function, and were hospitalized approximately two times on average for HF exacerbations. The patients in this study were considered to have late stage C to stage D HF and were more likely to have acute exacerbations of chronic HF due to factors other than energy intake, which would have resulted in a higher rehospitalization rate [38]. Therefore, we consider that the rehospitalization rate was not significantly different between the two groups.
This study has several limitations. It was an observational study, so some unmeasured variables might have influenced the outcomes. Additionally, it had a relatively small sample size and was conducted at a single center. The medications in this study did not include some new types of drugs, such as sodium glucose cotransporter 2 inhibitors (SGLT2i) or angiotensin receptor–neprilysin inhibitors (ARNi). This study is based on patient data obtained from 2018, so not all patients were taking these new types of drugs, and their impact does not need to be considered. Finally, we investigated energy intake only in the last three days before discharge, and changes in energy intake during hospitalization may have different implications.
5. Conclusions
In elderly patients with HF, the composite endpoint (all-cause mortality within one year and rehospitalization for worsening HF) did not differ significantly between the two groups diagnosed using the Harris–Benedict formula with regard to energy intake. On the other hand, inadequate energy intake diagnosed with the formula was associated with a higher incidence of all-cause mortality within one year in elderly HF patients. The monitoring of energy intake may therefore be useful in predicting mortality risk in elderly patients with HF, and the basal energy requirement derived from the Harris–Benedict equation may serve as an indicator for such monitoring. However, in the present study, no intervention using the Harris–Benedict formula for basal energy expenditure as a reference was performed, and further studies with interventions for energy intake are needed to verify whether it is a useful indicator for elderly patients with HF.
Author Contributions
Conceptualization, methodology, formal analysis, Writing—Original draft, project administration, A.T. (Akira Taruya); conceptualization, formal analysis, investigation, T.N.; formal analysis, investigation, S.O., M.T., M.K. and Y.S.; statistical analysis, K.W.; investigation, supervision, Y.I.; writing—review and editing, supervision, A.T. (Atsushi Tanaka). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 19K17612 (Taruya). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Shingu Municipal Medical Center (protocol code 65 on 3 February 2020).
Informed Consent Statement
Patient consent was waived by the Ethics Committee of Shingu Municipal Medical Center considering the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
We acknowledge the proofreading and editing by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Honda, Y.; Nagai, T.; Iwakami, N.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Usefulness of Geriatric Nutritional Risk Index for Assessing Nutritional Status and Its Prognostic Impact in Patients Aged >/=65 Years With Acute Heart Failure. Am. J. Cardiol. 2016, 118, 550–555. [Google Scholar] [CrossRef]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Kober, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Sawano, M.; Shiraishi, Y.; Kohsaka, S.; Nagai, T.; Goda, A.; Mizuno, A.; Sujino, Y.; Nagatomo, Y.; Kohno, T.; Anzai, T.; et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 2018, 5, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Khanam, S.S.; Choi, E.; Son, J.W.; Lee, J.W.; Youn, Y.J.; Yoon, J.; Lee, S.H.; Kim, J.Y.; Ahn, S.G.; Ahn, M.S.; et al. Validation of the MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) heart failure risk score and the effect of adding natriuretic peptide for predicting mortality after discharge in hospitalized patients with heart failure. PLoS ONE 2018, 13, e0206380. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Hiesmayr, M.; Schindler, K.; Pernicka, E.; Schuh, C.; Schoeniger-Hekele, A.; Bauer, P.; Laviano, A.; Lovell, A.D.; Mouhieddine, M.; Schuetz, T.; et al. Decreased food intake is a risk factor for mortality in hospitalised patients: The NutritionDay survey 2006. Clin. Nutr. 2009, 28, 484–491. [Google Scholar] [CrossRef]
- Villet, S.; Chiolero, R.L.; Bollmann, M.D.; Revelly, J.P.; Cayeux, R.N.M.; Delarue, J.; Berger, M.M. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Jesus, P.; Achamrah, N.; Grigioni, S.; Charles, J.; Rimbert, A.; Folope, V.; Petit, A.; Dechelotte, P.; Coeffier, M. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a Nutrition Unit. Clin. Nutr. 2015, 34, 529–535. [Google Scholar] [CrossRef]
- Compher, C.; Cato, R.; Bader, J.; Kinosian, B. Harris-Benedict equations do not adequately predict energy requirements in elderly hospitalized African Americans. J. Natl. Med. Assoc. 2004, 96, 209–214. [Google Scholar]
- Anderson, T.; Cascino, T.M.; Koelling, T.M.; Perry, D.; Grafton, G.; Houston, D.K.; Upadhya, B.; Kitzman, D.W.; Hummel, S.L. Measured Versus Estimated Resting Metabolic Rate in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e007962. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Shoji, S.; Shiraishi, Y.; Kawana, M.; Kohno, T.; Inoue, K.; Fukuda, K.; Heidenreich, P.A.; Kohsaka, S. Hospital meal intake in acute heart failure patients and its association with long-term outcomes. Open Heart 2020, 7, e001248. [Google Scholar] [CrossRef]
- Breslow, R.A.; Sorkin, J.D. Comparison of one-day and three-day calorie counts in hospitalized patients: A pilot study. J. Am. Geriatr. Soc. 1993, 41, 923–927. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.F.; Wong, K.; Clark, A.L. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure: A Comparison With Body Mass Index. JACC. Heart Fail. 2018, 6, 476–486. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Zhang, J.; Clark, A.L. Malnutrition, congestion and mortality in ambulatory patients with heart failure. Heart 2019, 105, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Tsutamoto, T.; Wada, A.; Maeda, K.; Hisanaga, T.; Maeda, Y.; Fukai, D.; Ohnishi, M.; Sugimoto, Y.; Kinoshita, M. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: Prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997, 96, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Price, J.F.; Thomas, A.K.; Grenier, M.; Eidem, B.W.; O’Brian Smith, E.; Denfield, S.W.; Towbin, J.A.; Dreyer, W.J. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation 2006, 114, 1063–1069. [Google Scholar] [CrossRef]
- Nishii, M.; Inomata, T.; Takehana, H.; Naruke, T.; Yanagisawa, T.; Moriguchi, M.; Takeda, S.; Izumi, T. Prognostic utility of B-type natriuretic peptide assessment in stable low-risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J. Am. Coll. Cardiol. 2008, 51, 2329–2335. [Google Scholar] [CrossRef]
- Nakamura, T.; Haraguchi, Y.; Matsumoto, M.; Ishida, T.; Momomura, S.I. Prognostic impact of malnutrition in elderly patients with acute myocardial infarction. Heart Vessel. 2022, 37, 385–391. [Google Scholar] [CrossRef]
- Kinugasa, Y.; Kato, M.; Sugihara, S.; Hirai, M.; Yamada, K.; Yanagihara, K.; Yamamoto, K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, K.; Abe, H.; Iida, Y.; Kato, T.; Nakamura, M.; Toriyama, C.; Nishida, H.; Idemoto, A.; Shinouchi, K.; Mishima, T.; et al. Prognostic impact of nutritional status and physical capacity in elderly patients with acute decompensated heart failure. ESC Heart Fail. 2020, 7, 1801–1808. [Google Scholar] [CrossRef]
- Horwich, T.B.; Fonarow, G.C.; Hamilton, M.A.; MacLellan, W.R.; Woo, M.A.; Tillisch, J.H. The relationship between obesity and mortality in patients with heart failure. J. Am. Coll. Cardiol. 2001, 38, 789–795. [Google Scholar] [CrossRef]
- Anker, S.D.; Negassa, A.; Coats, A.J.; Afzal, R.; Poole-Wilson, P.A.; Cohn, J.N.; Yusuf, S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: An observational study. Lancet 2003, 361, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Pocock, S.J.; Wang, D.; Finn, P.V.; Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; Yusuf, S.; Swedberg, K.; Michelson, E.L.; et al. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 116, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef]
- Hodin, C.M.; Lenaerts, K.; Grootjans, J.; de Haan, J.J.; Hadfoune, M.; Verheyen, F.K.; Kiyama, H.; Heineman, E.; Buurman, W.A. Starvation compromises Paneth cells. Am. J. Pathol. 2011, 179, 2885–2893. [Google Scholar] [CrossRef]
- Genton, L.; Cani, P.D.; Schrenzel, J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin. Nutr. 2015, 34, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Behrens, R.; Northrop, C.; Wraight, P.; Neale, G. Evaluation of mannitol, lactulose and 51Cr-labelled ethylenediaminetetra-acetate as markers of intestinal permeability in man. Clin. Sci. 1987, 73, 197–204. [Google Scholar] [CrossRef]
- Kane, A.V.; Dinh, D.M.; Ward, H.D. Childhood malnutrition and the intestinal microbiome. Pediatr. Res. 2015, 77, 256–262. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- McMurray, D.N.; Mintzer, C.L.; Bartow, R.A.; Parr, R.L. Dietary protein deficiency and Mycobacterium bovis BCG affect interleukin-2 activity in experimental pulmonary tuberculosis. Infect. Immun. 1989, 57, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Novotny Nunez, I.; de Moreno de LeBlanc, A.; Carmuega, E.; Weill, R.; Perdigon, G. Impact of a probiotic fermented milk in the gut ecosystem and in the systemic immunity using a non-severe protein-energy-malnutrition model in mice. BMC Gastroenterol. 2011, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Wang, J.T.; Wei, S.C.; Ni, Y.H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Kim, J.N.; Bader, J.G. Nutritional requirements of an aging population with emphasis on subacute care patients. AACN Clin. Issues 1998, 9, 441–450. [Google Scholar] [CrossRef]
- Hersberger, L.; Dietz, A.; Burgler, H.; Bargetzi, A.; Bargetzi, L.; Kagi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.A.; Jacobsen, S.J.; Mahoney, D.W.; Kors, J.A.; Redfield, M.M.; Burnett, J.C., Jr.; Rodeheffer, R.J. Prevalence and prognostic significance of heart failure stages: Application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007, 115, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).