The Antineoplastic Effect of Heparin on Colorectal Cancer: A Review of the Literature
Abstract
1. Introduction
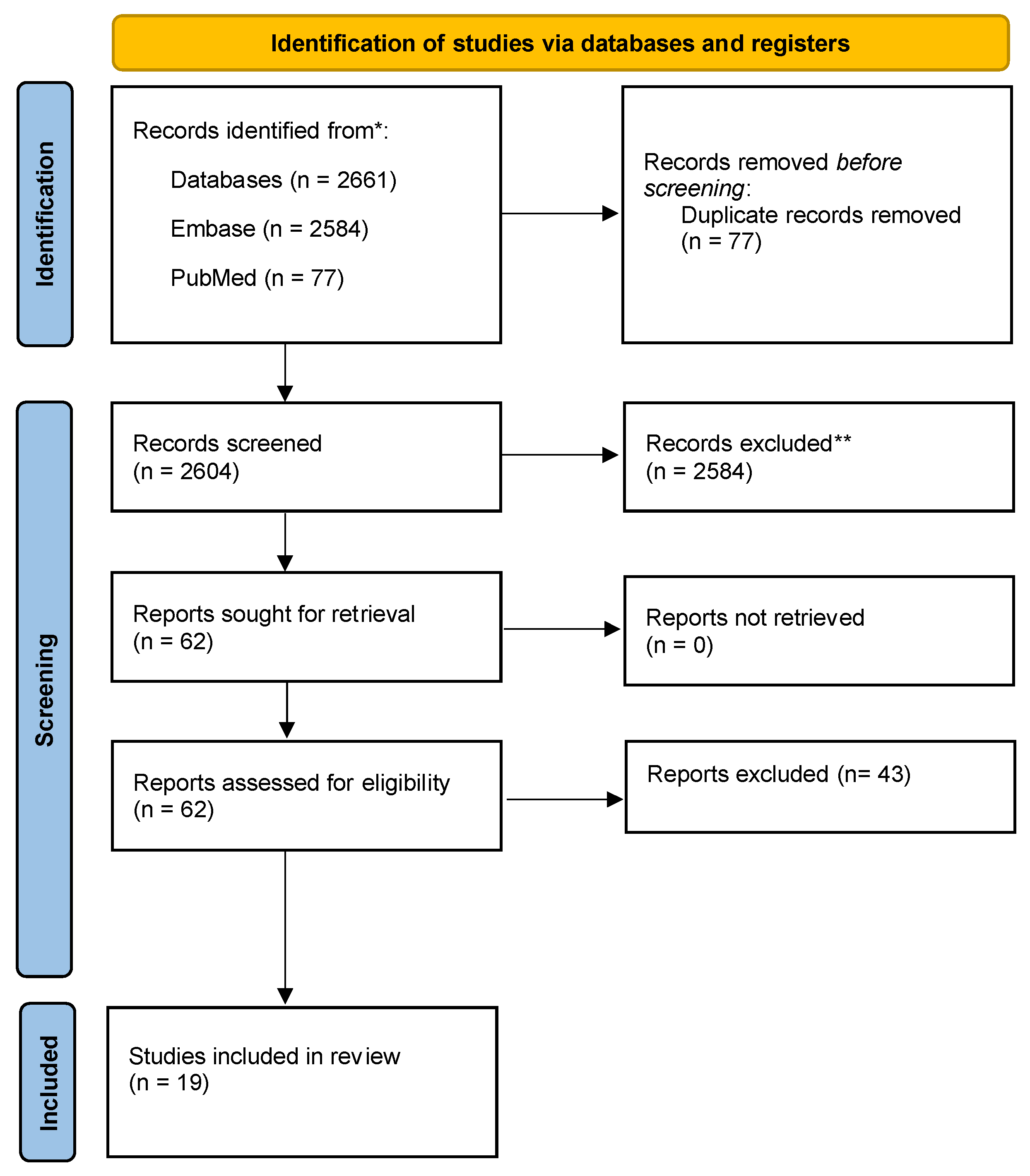
2. Methods
2.1. Literature Search
2.2. Search Strategy
2.3. Citation Management and Data Extraction
2.4. Inclusion Criteria
2.5. Exclusion Criteria
3. Results
3.1. In Vitro Cell Studies
3.2. In Vivo Rat Studies
3.3. In Vivo Human Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antachopoulos, C.T.; Iliopoulos, D.C.; Agapitos, M.V.; Karayannacos, P.E.; Roboli, S.K.; Skalkeas, G.D. In Vitro Effects of Heparin on SW480 Tumor Cell-Matrix Interaction. Anticancer Res. 1995, 15, 1411–1416. [Google Scholar]
- Shahbazi, B.; Mafakher, L.; Arab, S.S.; Teimoori-Toolabi, L. Kallistatin as an inhibitory protein against colorectal cancer cells through binding to LRP6. J. Biomol. Struct. Dyn. 2023, 1–17. [Google Scholar] [CrossRef]
- Liu, J.-J. Heparin/heparan sulfate interacting protein plays a role in apoptosis induced by anticancer drugs. Carcinogenesis 2004, 25, 873–879. [Google Scholar] [CrossRef]
- Yao, Y.; Li, L.; Huang, X.; Gu, X.; Xu, Z.; Zhang, Y.; Huang, L.; Li, S.; Dai, Z.; Li, C.; et al. SERPINA3K induces apoptosis in human colorectal cancer cells via activating the FAS/FASL/caspase-8 signaling pathway. FEBS J. 2013, 280, 3244–3255. [Google Scholar] [CrossRef]
- Takei, Y.; Kadomatsu, K.; Matsuo, S.; Itoh, H.; Nakazawa, K.; Kubota, S.; Muramatsu, T. Antisense oligodeoxynucleotide targeted to midkine, a heparin-binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res. 2001, 61, 8486–8491. [Google Scholar]
- Smorenburg, S.M.; Vink, R.; Lintelo, M.T.; Tigchelaar, W.; Maas, A.; Büller, H.R.; van Noorden, C.J. In vivo treatment of rats with unfractionated heparin (UFH) or low molecular weight heparin (LMWH) does not affect experimentally induced colon carcinoma metastasis. Clin. Exp. Metastasis 1999, 17, 451–456. [Google Scholar] [CrossRef]
- Ma, S.-N.; Mao, Z.-X.; Wu, Y.; Liang, M.-X.; Wang, D.-D.; Chen, X.; Chang, P.-A.; Zhang, W.; Tang, J.-H. The anti-cancer properties of heparin and its derivatives: A review and Prospect. Cell Adh. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-M.; Mi, Y.-S.; Yu, F.-D.; Han, Y.; Liu, X.-S.; Lu, S.; Zhang, Y.; Zhao, S.-L.; Ye, L.; Liu, T.-T.; et al. SERPINA4 is a novel independent prognostic indicator and a potential therapeutic target for colorectal cancer. Am. J. Cancer Res. 2016, 6, 1636–1649. [Google Scholar]
- De Robertis, M.; Greco, M.R.; Cardone, R.A.; Mazza, T.; Marzano, F.; Mehterov, N.; Kazakova, M.; Belev, N.; Tullo, A.; Pesole, G.; et al. Upregulation of YKL-40 promotes metastatic phenotype and correlates with poor prognosis and therapy response in patients with colorectal cancer. Cells 2022, 11, 3568. [Google Scholar] [CrossRef]
- Ma, L.; Qiao, H.; He, C.; Yang, Q.; Cheung, C.H.A.; Kanwar, J.R.; Sun, X. Modulating the interaction of CXCR4 and CXCL12 by low-molecular-weight heparin inhibits hepatic metastasis of colon cancer. Investig. New Drugs 2010, 30, 508–517. [Google Scholar] [CrossRef]
- Boothello, R.S.; Patel, N.J.; Sharon, C.; Abdelfadiel, E.I.; Morla, S.; Brophy, D.F.; Lippman, H.R.; Desai, U.R.; Patel, B.B. A unique nonsaccharide mimetic of heparin hexasaccharide inhibits colon cancer stem cells via p38 MAP kinase activation. Mol. Cancer Ther. 2019, 18, 51–61. [Google Scholar] [CrossRef]
- Spijkers-Shaw, S.; Campbell, K.; Shields, N.J.; Miller, J.H.; Rendle, P.M.; Jiao, W.; Young, S.L.; Zubkova, O.V. Synthesis of novel glycolipid mimetics of heparan sulfate and their application in colorectal cancer treatment in a mouse model. Chem. Asian J. 2022, 17, e202200228. [Google Scholar] [CrossRef]
- Diao, Y.; Ma, J.; Xiao, W.-D.; Luo, J.; Li, X.-Y.; Chu, K.-W.; Fung, P.W.; Habib, N.; Farzaneh, F.; Xu, R.-A. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by Kallistatin. World J. Gastroenterol. 2007, 13, 4615–4619. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Alam, F.; Chung, S.W.; Park, J.; Jeon, O.C.; Kim, S.Y.; Son, W.C.; Byun, Y. Combinational chemoprevention effect of celecoxib and an oral antiangiogenic LHD4 on colorectal carcinogenesis in mice. Anti-Cancer Drugs 2014, 25, 1061–1071. [Google Scholar] [CrossRef]
- Debergh, I.; Pattyn, P.; Ceelen, W. Microvascular effects of the low molecular weight heparins in a colorectal xenograft model: An intravital microscopy study. J. Surg. Res. 2015, 194, 488–495. [Google Scholar] [CrossRef]
- Djaafar, S.; Dunand-Sautier, I.; Gonelle-Gispert, C.; Lacotte, S.; Agostini, A.D.E.; Petro, M.; Rubbia-Brandt, L.; Morel, P.; Toso, C.; Mentha, G.; et al. Enoxaparin Attenuates Mouse Colon Cancer Liver Metastases by Inhibiting Heparanase and Interferon-Œ≥-inducible Chemokines. Anticancer Res. 2016, 36, 4019–4032. [Google Scholar]
- Mitsis, M.; Koliou, P.; Bali, C.; Ntounousi, E.; Tatsis, V.; Nousias, V.; Lianos, G.D.; Vartholomatos, G.; Nastos, D. In surgical colon cancer patients extended-duration thromboprophylaxis (30 days) with the highest dose of tinzaparin (4500 IU S.C./Q.D.) normalizes the postoperative VEGF levels. J. Cancer 2017, 8, 2899–2906. [Google Scholar] [CrossRef][Green Version]
- Yamaoka, Y.; Ikeda, M.; Ikenaga, M.; Murakami, H.; Haraguchi, N.; Miyake, M.; Yamamoto, K.; Asaoka, T.; Nishikawa, K.; Miyamoto, A.; et al. Anti-metastatic effect of short-term postoperative anticoagulation for patients undergoing curative resection of colorectal cancer. Anticancer Res. 2016, 36, 5425–5430. [Google Scholar] [CrossRef]
- Klerk, C.P.W.; Smorenburg, S.M.; Otten, H.-M.; Lensing, A.W.; Prins, M.H.; Piovella, F.; Prandoni, P.; Bos, M.M.; Richel, D.J.; van Tienhoven, G.; et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 2005, 23, 2130–2135. [Google Scholar] [CrossRef]
- Auer, R.C.; Ott, M.; Karanicolas, P.; Brackstone, M.R.; Ashamalla, S.; Weaver, J.; Tagalakis, V.; Boutros, M.; Stotland, P.; Marulanda, A.C.; et al. Efficacy and safety of extended duration to perioperative thromboprophylaxis with low molecular weight heparin on disease-free survival after surgical resection of colorectal cancer (PERIOP-01): Multicentre, open label, Randomised Controlled Trial. BMJ 2022, 378, e071375. [Google Scholar] [CrossRef]
- Petersen, L.; Mousa, S. Anti-cancer properties of low-molecular-weight heparin: Preclinical evidence. Thromb. Haemost. 2009, 102, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bokas, A.; Papakotoulas, P.; Sarantis, P.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Mechanisms of the antitumor activity of low molecular weight heparins in pancreatic adenocarcinomas. Cancers 2020, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Lazo-langner, A.; Goss, G.D.; Spaans, J.N.; Rodger, M.A. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J. Thromb. Haemost. 2007, 5, 729–737. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The Prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ge, P.; Reyila, A.; Li, X.; Liu, S.; Jiang, Y.; Yang, Y.; Li, X.; Bian, Y. Efficacy and safety of AFLIBERCEPT plus chemotherapy in metastatic colorectal cancer: A systematic review and prisma-compliant single-Arm meta-analysis of noncomparative clinical studies and randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 798–808. [Google Scholar] [CrossRef]
- Su, J.-Y.; Wang, Y.; Wu, S.-S.; Li, W.-K.; Wang, C.-Y.; Ma, J.-Y.; Qiu, Y.-T.; Zhou, M.-S.; Wang, Z.; Li, P.; et al. Association between new plasma inflammatory markers and risk of colorectal neoplasms in individuals over 50 years old. Carcinogenesis 2023, bgad064. [Google Scholar] [CrossRef]
- Gou, M.; Men, K.; Zhang, J.; Li, Y.; Song, J.; Luo, S.; Shi, H.; Wen, Y.; Guo, G.; Huang, M.; et al. Efficient inhibition of C-26 colon carcinoma by VSVMP gene delivered by biodegradable cationic Nanogel derived from polyethyleneimine. ACS Nano 2010, 4, 5573–5584. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, X.; Men, K.; Wang, B.; Zhang, S.; Qiu, J.; Huang, M.; Gou, M.; Huang, N.; Qian, Z.; et al. Gene therapy for C-26 colon cancer using heparin-polyethyleneimine nanoparticle-mediated Survivin T34A. Int. J. Nanomed. 2011, 6, 2419–2427. [Google Scholar] [CrossRef]
- Mulita, F.; Liolis, E.; Akinosoglou, K.; Tchabashvili, L.; Maroulis, I.; Kaplanis, C.; Vailas, M.; Panos, G. Postoperative sepsis after colorectal surgery: A prospective single-center observational study and review of the literature. Gastroenterol. Rev. 2021, 17, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Milanchi, S.; Nasserl, Y.; Westhout, F.; Murrell, Z.A.; Fleshner, P.R. Does prophylactic subcutaneous heparin increase the risk of wound infection after colorectal surgery? Am. Surg. 2010, 76, 1412–1415. [Google Scholar] [CrossRef]
- Bousis, D.; Verras, G.-I.; Bouchagier, K.; Antzoulas, A.; Panagiotopoulos, I.; Katinioti, A.; Kehagias, D.; Kaplanis, C.; Kotis, K.; Anagnostopoulos, C.-N.; et al. The role of Deep Learning in diagnosing colorectal cancer. Gastroenterol. Rev. 2023, 18, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, L.; Zhai, Q.; Zhao, R.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. In vitro fermentation of heparin by the human gut microbiota: Changes in the microbiota community and metabolic functions. Food Chem. 2023, 406, 135010. [Google Scholar] [CrossRef] [PubMed]
| Study Number | Author | Primary Study Centre | Study Design | Study Population | Study Groups | Type of Heparin Administered | Main Finding |
|---|---|---|---|---|---|---|---|
| 1 | Antachopoulos 1995 [1] | Greece | In vitro, lab study | Human colon adenocarcinoma cells (SW480) | Heparin groups with different concentrations and control | Heparin sodium salt | Chemically modified heparins probably play a role in the in vivo inhibition of tumor cell metastasis |
| 2 | Shahbazi 2023 [2] | Iran | In vitro, lab study | Human CRC cells (SW480 HCT116) | Kallistatin and control | Kallistatin–LRP6 complex | Kallistatin has anti-tumor effects |
| 3 | Liu 2004 [3] | Singapore and USA | In vitro, lab study | Human CC Cell Lines (HT-29 and HCT-116) | Sodium butyrate, adherent and floating cell lines | Heparin sulfate-interacting protein (HIP) | HIP is an anti-apoptotic peptide and is involved in regulating apoptosis induced by anticancer drugs |
| 4 | Yao 2013 [4] | China | In vitro, lab study | Human CRC cells (SW480 and HT-29) | SERPINA3K and control | SERPINA3K (kallikrein-binding protein) | SERPINA3K exerted anti-tumor activity by suppressing the rate of proliferation and inducing CRC apoptosis |
| 5 | Takei 2001 [5] | Japan | In vitro and in vivo, lab study | Mice rectal carcinoma cells (CMT-93), athymic nude mice from SLC (Tokyo, Japan) | NR | Midkine (heparin-binding growth factor) | Inhibitory effect of midkine antisense oligodeoxynucleotide on rectal carcinoma |
| 6 | Sun 2016 [8] | China | In vitro and in vivo, lab study |
| CRC mucosa matched with healthy colonic mucosa Cell cultures Mouse xenografts | SERPINA4 (kallistatin) | SERPINA4 is significantly correlated with aggressive phenotype and poor clinical outcomes in CRC. SERPINA4 suppresses cancer progression and serves as a potential therapeutic target for CRC |
| 7 | DeRobertis 2022 [9] | Italy and Bulgaria | In vitro and in vivo, lab study | Human CRC cell lines (9HCT116 and Caco2), BALB/c female mice, and 41 paired CRC mucosa and healthy mucosa of human patients | NR | YKL-40 (heparin-binding glycoprotein) | YKL-40 appeared to promote the metastatic phenotype during CRC carcinogenesis |
| 8 | Ma 2012 [10] | China | In vitro and in vivo, lab study | Male nude BALB/c mice and human colon cancer cell lines (HT29, LS-174 T, HCT-199) | Control, CXCL12, CXCL12 and LMWH, CXCL12 and CXCR4 Ab, Placebo, LMWH | LMWH (enoxaparin) | LMWH may help prevent the seeding and subsequent growth of hepatic metastasis of colon cancer cells |
| 9 | Boothello 2019 [11] | USA | In vitro and in vivo, lab study | Human CRC cells (HT29 and HCT116) and pancreatic (Pan = 1), female NCr nude mice | NR | G2.2 (mimetic of heparin hexasaccharide) | G2.2 is a promising therapeutic agent for cancer |
| 10 | Spijkers-Shaw 2022 [12] | New Zealand | In vitro and in vivo, lab study | MC38 murine model of colon adenocarcinoma were implanted in flanks of syngeneic C57BL/6 mice, HT29 human colorectal adenocarcinoma cells | Treatment, control | Tet-29 (heparin sulfate mimetic developed in the lab) | Novel glycolipid (HS mimetic) delayed tumor growth and improved overall survival |
| 11 | Diao 2007 [13] | China | In vivo, lab study | Male BALB/c mice | rAVV-GFP, rAVV-KAL | Kallistatin (KAL) | KAL suppressed angiogenesis and resulted in growth retardation of colon tumors |
| 12 | Smorenburg 1999 [6] | Netherlands | In vivo, lab study | WAG-Rjj Rats | LMWH, UFH, control | LMWH (reviparin), UFH | Heparins do not affect colon carcinoma metastasis in liver |
| 13 | Kim 2014 [14] | South Korea | In vivo, lab study | Male ICR mice | Control, AOM and DS, celecoxib, LHD4, celecoxib and LHD4 | LHD4 (derived from fraxiparine, 4500 Da) | The combined use of celecoxib and LHD4 could significantly enhance chemoprevention of CRC in terms of polyp formation and malignancy development |
| 14 | Debergh 2015 [15] | Belgium | In vivo, lab study | Male athymic mice with human CC cell line (HT29) | Group I—0.1 mL saline Group II—200 IU aXa nadroparin Group III—200 IU aXa dalteparin | Nadroparin, dalteparin | Nadroparin and enoxaparin inhibit tumor-associated angiogenesis and normalize microvessel structure in this mouse xenograft |
| 15 | Djaafar 2016 [16] | Switzerland | In vivo, lab study | Mice wild type C57BL/6 with mouse CC cells (MCA38) and mouse melanoma cells (B16-F10 BL6) | Enoxaparin and placebo (phosphate buffered saline) | Enoxaparin | Enoxaparin significantly reduced CC metastatic tumors in the mouse liver at early stages of development. |
| 16 | Mitsis 2017 [17] | Greece | In vivo, prospective cohort study | Human patients with endoscopy-biopsy-proven CC undergoing colectomy with curative intent | Group I: 3500 I.U. for 10 days, Group II: 3500 I.U. for 30 days, Group III: 4500 I.U. for 10 days, Group IV: 4500 I.U. for 30 days | LMWH (tinzaparin) | Post-op VEGF levels may contribute to future progress of disease. The use of high-dose tinzaparin for a long period may help better control VEGF fluctuations. |
| 17 | Yamaoka 2016 [18] | Japan | In vivo, retrospective cohort study | Human with primary CRC and pathologically diagnosed lymph-node metastasis who underwent curative (R0) resection | Fondaparinux (FPX) and intermittent pneumatic compression (IPC) vs. intermittent pneumatic compression (IPC) only | Fondaparinux (FPX) | Short-term postoperative use of FPX as VTE prophylaxis does not prevent CRC recurrence after curative resection. |
| 18 | Klerk 2005 [19] | Netherlands | In vivo, randomized control trial | Human patients with uncurable advanced solid organ malignancy | Nadroparin and placebo | Nadroparin | Six week course of LMWH in patients with advanced solid malignancy reduces mortality at 12 and 24 months by 12% and 10% and prolongs median survival from 6.6 months to 8.0 months |
| 19 | Auer 2022 [20] | Canada | In vivo, randomized control trial | Patients with CRC, no evidence of metastatic disease, and scheduled to undergo surgical resection | Treatment and control | LMWH (tinzaparin) | Extended duration to perioperative thrombophylaxis with tinzaparin (given before surgery and for 56 days after surgery) does not increase disease-free survival at 3 years. Rates of clinically detected VTE were low and extended-duration thrombophylaxis was not associated with a reduction in VTE. |
| Molecular Pathway | Mechanism |
|---|---|
| p38 MAPK | Activation of p38 MAPK to inhibit CRC [9,11] |
| Wnt signaling pathway | Inhibited [2] |
| β-catenin | Reduced expression in heparin treated cells and rats [2] |
| Cyclin D1 | Reduced expression in heparin treated cells and rats [2] |
| c-Myc | Reduced expression in heparin treated cells and rats [2] |
| Caspase 3 | Activation [3] |
| FasL/caspase-8 | Activation [4] |
| PI3K/AKT signaling pathway | Activation [9] |
| CXCR4-CXCL12 | Downregulates [10] |
| VEGF and bFGF | Reduce VEGF and bFGF binding activity [13,15,17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannas, E.; Kontovounisios, C. The Antineoplastic Effect of Heparin on Colorectal Cancer: A Review of the Literature. J. Clin. Med. 2023, 12, 7173. https://doi.org/10.3390/jcm12227173
Giannas E, Kontovounisios C. The Antineoplastic Effect of Heparin on Colorectal Cancer: A Review of the Literature. Journal of Clinical Medicine. 2023; 12(22):7173. https://doi.org/10.3390/jcm12227173
Chicago/Turabian StyleGiannas, Emmanuel, and Christos Kontovounisios. 2023. "The Antineoplastic Effect of Heparin on Colorectal Cancer: A Review of the Literature" Journal of Clinical Medicine 12, no. 22: 7173. https://doi.org/10.3390/jcm12227173
APA StyleGiannas, E., & Kontovounisios, C. (2023). The Antineoplastic Effect of Heparin on Colorectal Cancer: A Review of the Literature. Journal of Clinical Medicine, 12(22), 7173. https://doi.org/10.3390/jcm12227173






