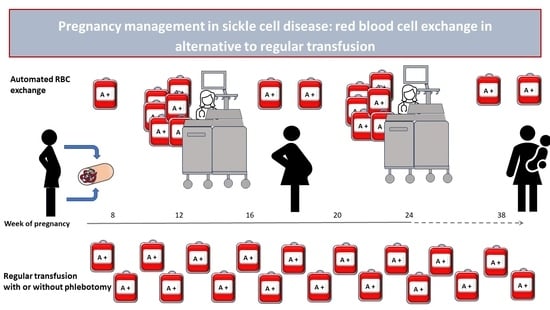
Red Blood Cell Exchange as a Valid Therapeutic Approach for Pregnancy Management in Sickle Cell Disease: Three Explicative Cases and Systematic Review of Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Treatment and Procedures
2.2. Systematic Review
3. Results
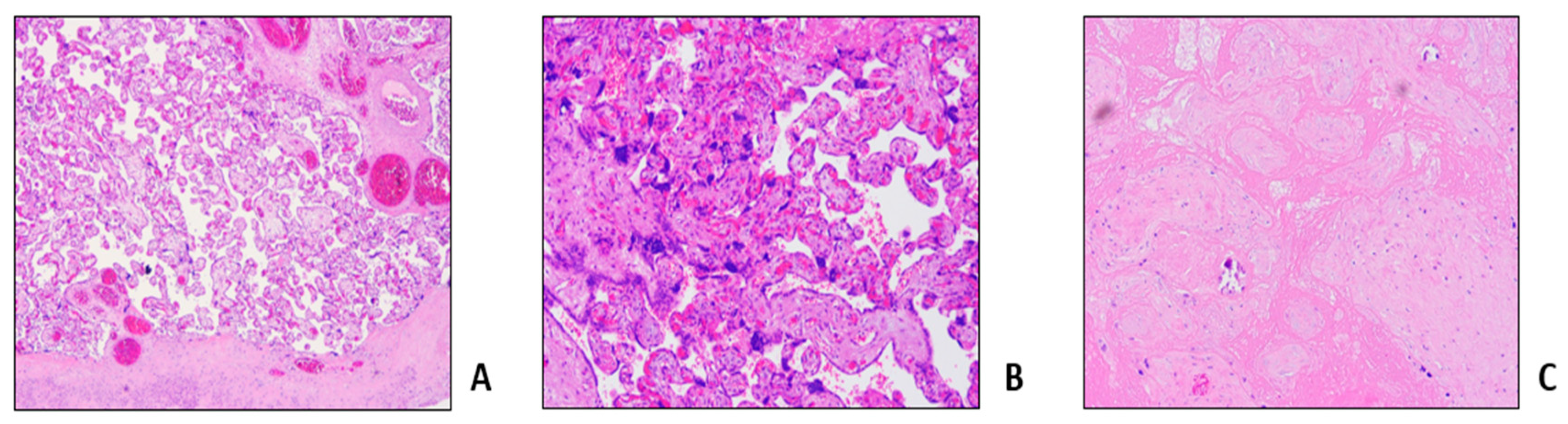
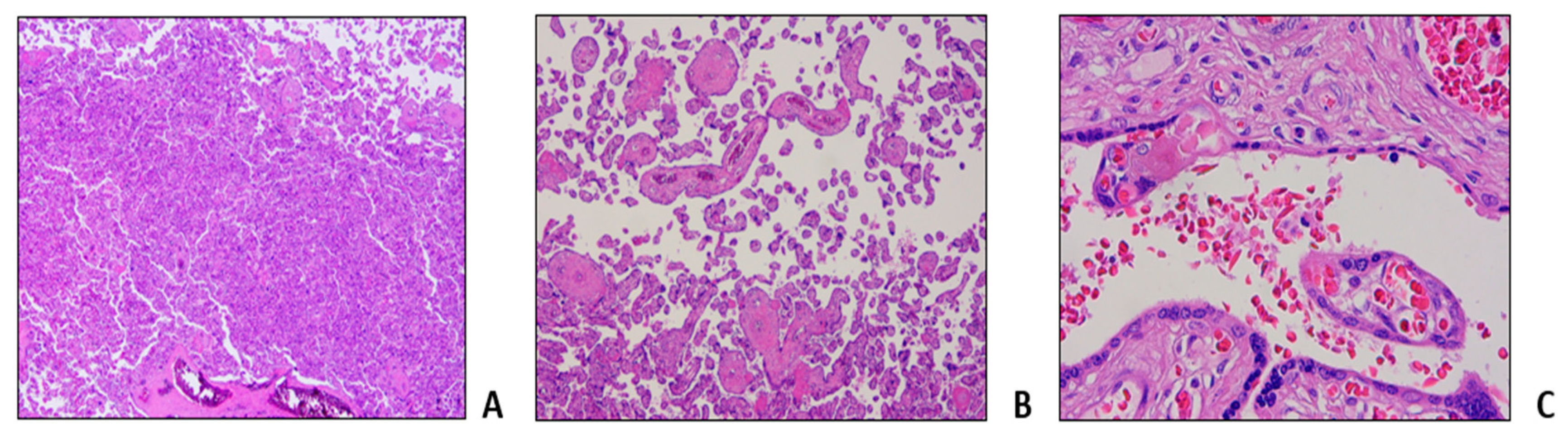
3.1. Clinical Reports
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.2. Literature Revision
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Serjeant, G.R. Sickle-cell disease. Lancet 1997, 350, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.J.; Nagel, R.L. Sickle-cell disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.; Hofrichter, J. Hemoglobin S Gelation and Sickle Cell Disease. Blood 1987, 70, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, V.; Ally, M.; Isa, H.; Anie, K.A.; Mgaya, J.; Nkanyemka, M.; Nembaware, V.; Oppong-Mensah, Y.G.; Ndobho, F.; Chirande, L.; et al. Development of multi-level standards of care recommendations for sickle cell disease: Experience from SickleInAfrica. Front Genet. 2023, 13, 1052179. [Google Scholar] [CrossRef]
- Manwani, D.; Frenette, P.S. Vaso-occlusion in sickle cell disease: Pathophysiology and novel targeted therapies. Blood 2013, 122, 3892–3898. [Google Scholar] [CrossRef]
- Early, M.L.; Eke, A.C.; Gemmill, A.; Lanzkron, S.; Pecker, L.H. Comparisons of Severe Maternal Morbidity and Other Adverse Pregnancy Outcomes in Pregnant People with Sickle Cell Disease vs Anemia. JAMA Netw. Open 2023, 6, e2254545. [Google Scholar] [CrossRef]
- Wilson, S.; Ellsworth, P.; Key, N.S. Pregnancy in sickle cell trait: What we do and don’t know. Br. J. Haematol. 2020, 190, 328–335. [Google Scholar] [CrossRef]
- Ha, T.K.; Boulet, S.L.; Cotsonis, G.; Geary, F.; Jamieson, D.J.; Lindsay, M. Association of Sickle Cell Disease with Severe Maternal Morbidity. Obstet. Gynecol. 2023, 141, 163–169. [Google Scholar] [CrossRef]
- Chou, S.T.; Alsawas, M.; Fasano, R.M.; Field, J.J.; Hendrickson, J.E.; Howard, J.; Kameka, M.; Kwiatkowski, J.L.; Pirenne, F.; Shi, P.A.; et al. American Society of Hematology 2020 guidelines for sickle cell disease: Transfusion support. Blood Adv. 2020, 4, 327–355. [Google Scholar] [CrossRef]
- Oteng-Ntim, E.; Pavord, S.; Howard, R.; Robinson, S.; Oakley, L.; Mackillop, L.; Pancham, S.; Howard, J.; British Society for Haematology Guideline. Management of sickle cell disease in pregnancy. A British Society for Haematology Guideline. Br. J. Haematol. 2021, 194, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.A.; Allard, S.; Qureshi, A.; Porter, J.B.; Pancham, S.; Win, N.; Cho, G.; Ryan, K.; British Society for Haematology. Guidelines on red cell transfusion in sickle cell disease Part II: Indications for transfusion. Br. J. Haematol. 2017, 176, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Putzulu, R.; Piccirillo, N.; Orlando, N.; Massini, G.; Maresca, M.; Scavone, F.; Ricerca, B.M.; Zini, G. The role of molecular typing and perfect match transfusion in sickle cell disease and thalassaemia: An innovative transfusion strategy. Transfus. Apher. Sci. 2017, 56, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Stussi, G.; Buser, A.; Holbro, A. Red Blood Cells: Exchange, Transfuse, or Deplete. Transfus. Med. Hemother. 2019, 46, 407–416. [Google Scholar] [CrossRef]
- Biller, E.; Zhao, Y.; Berg, M.; Boggio, L.; Capocelli, K.E.; Fang, D.C.; Koepsell, S.; Music-Aplenc, L.; Pham, H.P.; Treml, A.; et al. Red blood cell exchange in patients with sickle cell disease-indications and management: A review and consensus report by the therapeutic apheresis subsection of the AABB. Transfusion 2018, 58, 1965–1972. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Rees, D.C.; Brousse, V.A.M.; Brewin, J.N. Determinants of severity in sickle cell disease. Blood Rev. 2022, 56, 100983. [Google Scholar] [CrossRef]
- Key, T.C.; Horger, E.O.; Walker, E.M.; Mitchum, E.N. Automated erythrocytopheresis for sickle cell anemia during pregnancy. Am. J. Obstet. Gynecol. 1980, 138, 731–737. [Google Scholar] [CrossRef]
- Lee, W.; Werch, J.; Rokey, R.; Pivarnik, J.; Miller, J. Physiologic observations of pregnant women undergoing prophylactic erythrocytapheresis for sickle cell disease. Transfusion 1991, 31, 59–62. [Google Scholar] [CrossRef]
- Morrison, J.C.; Morrison, F.S.; Floyd, R.C.; Roberts, W.E.; Hess, L.W.; Wiser, W.L. Use of continuous flow erythrocytapheresis in pregnant patients with sickle cell disease. J. Clin. Apher. 1991, 6, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Gilli, S.C.; De Paula, E.V.; Biscaro, F.P.; Marques, J.F.; Costa, F.F.; Saad, S.T. Third-trimester erythrocytapheresis in pregnant patients with sickle cell disease. Int. J. Gynaecol. Obstet. 2007, 96, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.; Kozanoglu, I.; Tarım, E.; Sarıturk, C.; Gereklioglu, C.; Akdeniz, A.; Kasar, M.; Turgut, N.H.; Yeral, M.; Kandemir, F.; et al. Prophylactic red blood cell exchange may be beneficial in the management of sickle cell disease in pregnancy. Transfusion 2015, 55, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Vencato, E.; Cantini, M.; Zanconato, G.; Manfrin, E.; Zamo, A.; Zorzi, F.; Mazzi, F.; Martinelli, N.; Cavaliere, E.; et al. Improvement of maternal and fetal outcomes in women with sickle cell disease treated with early prophylactic erythrocytapheresis. Transfusion 2018, 58, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Baran, Ş.; Kozanoğlu, İ.; Korur, A.; Doğan Durdağ, G.; Kalaycı, H.; Alemdaroğlu, S.; Asma, S.; Kılıçdağ, E.B.; Boğa, C. Role of prophylactic and therapeutic red blood cell exchange in pregnancy with sickle cell disease: Maternal and perinatal outcomes. J. Clin. Apher. 2021, 36, 283–290. [Google Scholar] [CrossRef]
- Davis, B.A.; Allard, S.; Qureshi, A.; Porter, J.B.; Pancham, S.; Win, N.; Cho, G.; Ryan, K.; British Committee for Standards in Haematology. Guidelines on red cell transfusion in sickle cell disease. Part I: Principles and laboratory aspects. Br. J. Haematol. 2017, 176, 179–191. [Google Scholar] [CrossRef]
- Connelly-Smith, L.; Alquist, C.R.; Aqui, N.A.; Hofmann, J.C.; Klingel, R.; Onwuemene, O.A.; Patriquin, C.J.; Pham, H.P.; Sanchez, A.P.; Schneiderman, J.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J. Clin. Apher. 2023, 38, 77–278. [Google Scholar] [CrossRef]
- Motta, S.; Perseghin, P.; Consonni, S.; Regalia, A.L.; Masera, N. A case report of a successful monochorionic diamniotic twin pregnancy in a patient affected by sickle cell disease treated with erythrocytapheresis. Ther. Apher. Dial. 2010, 14, 112–115. [Google Scholar] [CrossRef]
- Froehly, S.; Diemunsch, P.; Waller, C.; Renaud, R.; Gros, H. Utilisation de l’échange érythrocytaire chez une femme enceinte atteinte de drépanocytose [Use of erythrocytapheresis in a pregnant woman with sickle cell anemia]. Ann. Fr. Anesth. Reanim. 1989, 8, 67–69. (In French) [Google Scholar] [CrossRef]
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Temperley, W.H.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013, 381, 142–151. [Google Scholar] [CrossRef]
- Ally, M.; Balandya, E. Current challenges and new approaches to implementing optimal management of sickle cell disease in sub-Saharan Africa. Semin. Hematol. 2023, 60, 192–199. [Google Scholar] [CrossRef]
- Chamba, C.; Iddy, H.; Tebuka, E.; Tluway, F.; Osati, E.; Budodi, N.; Meda, C.; Yonazi, M.; Schuh, A.; Luzzatto, L.; et al. Limited Exchange Transfusion Can Be Very Beneficial in Sickle Cell Anemia with Acute Chest Syndrome: A Case Report from Tanzania. Case Rep. Hematol. 2018, 2018, 5253625. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, A.K.; Dziegielewski, C.; Keating, S.; Parks, T.; Kingdom, J.; Shehata, N.; Rizov, E.; D’Souza, R. Placental histopathology in sickle cell disease: A descriptive and hypothesis-generating study. Placenta 2020, 95, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.C.; Costa, M.L.; Surita, F.G.; Rocha, C.S.; Lopes-Cendes, I.; Souza, B.B.; Costa, F.F.; Melo, M.B. Placental transcriptome profile of women with sickle cell disease reveals differentially expressed genes involved in migration, trophoblast differentiation and inflammation. Blood Cells Mol. Dis. 2020, 84, 102458. [Google Scholar] [CrossRef]
- Gil, G.P.; Ananina, G.; Maschietto, M.; Lima, S.C.S.; da Silva Costa, S.M.; Baptista, L.C.; Ito, M.T.; Costa, F.F.; Costa, M.L.; de Melo, M.B. Epigenetic analysis in placentas from sickle cell disease patients reveals a hypermethylation profile. PLoS ONE 2022, 17, e0274762. [Google Scholar] [CrossRef]
- Malinowski, A.K.; Shehata, N.; D’Souza, R.; Kuo, K.H.; Ward, R.; Shah, P.S.; Murphy, K. Prophylactic transfusion for pregnant women with sickle cell disease: A systematic review and meta-analysis. Blood 2015, 126, 2424–2435. [Google Scholar] [CrossRef]
- Mokrzycki, M.H.; Balogun, R.A. Therapeutic apheresis: A review of complications and recommendations for prevention and management. J. Clin. Apher. 2011, 26, 243–248. [Google Scholar] [CrossRef]
- Kundrapu, S.; Datla, S.; Griffin, V.; Maitta, R.W. Adverse events during apheresis: A 10-year experience at a tertiary academic medical center. J. Clin. Apher. 2019, 34, 528–536. [Google Scholar] [CrossRef]
- Oakley, L.L.; Awogbade, M.; Brien, S.; Briley, A.; Chorozoglou, M.; Drasar, E.; Johns, J.; Rhodes, E.; Robinson, V.; Seed, P.; et al. Serial prophylactic exchange blood transfusion in pregnant women with sickle cell disease (TAPS-2): Study protocol for a randomized controlled feasibility trial. Trials 2020, 21, 347. [Google Scholar] [CrossRef]
| Case 1 | Case 2 | Case 3 | |||||
|---|---|---|---|---|---|---|---|
| Genotype | HbS c.20A>T + trait alfa-thalassemia (del alfa 3.7) | HbS c.20A>T | HbS c.20A>T | ||||
| Blood group and red cell phenotype | A POS ccee kk | AB pos ccee kk Fya-b- | 0 pos ccee kk Fya+b- | ||||
| Fya-b- Jka+b- Kpa-b+ | Jka+b- Kpa-b+ | Jka+b- Kpa-b+ | |||||
| Jsa-b+ Ns Lua-b+ Dia-b+ | Jsa-b+ MNs Lua-b+ Dia-b+ | Jsa-b+ MNs Lua-b+ Dia-b+ | |||||
| Doa+b+ Coa+b- Sc 1,2 | Doa-b+ Coa+b- Sc 1,2 | Doa+b+ Coa+b- Sc 1,2 | |||||
Obstetric history (n)
| |||||||
| 4 | 4 | 4 | |||||
| 1 | 2 | 2 | |||||
| 3 (10, 14, 15) | 2 (8, 8) | 2 (11, 10) | |||||
| At term pregnancy | First | First | Second | First | Second | ||
| Antithrombotic prophylaxis | Cardioaspirin 100 mg/die + Enoxaparin 4000 U/die from 14 weeks to delivery | None | Enoxaparin 4000 U/die from 12 weeks to delivery | Enoxaparin 4000 U/die from 25 weeks to delivery | Enoxaparin 4000 U/die from the beginning to delivery | ||
| Median transfusion requirement pre-apheresis (RBC units/month) | 1 | 1 | 1 | 1 | 0 | ||
| Procedure 1A | Procedure 2A | Procedure 2B | Procedure 2C | Procedure 3A | Procedure 3B | Procedure 3C | |
| aRBCX indication | Vaso-occlusive crisis | Vaso-occlusive crisis | Prophylactic | Prophylactic | Prophylactic | Prophylactic | Prophylactic |
| Gestational age at procedure (weeks) | 38 + 2 | 19 + 5 | 16 + 3 | 28 + 1 | 24 + 0 | 7 + 5 | 34 + 0 |
| Weight (kg) | 80 | 68 | 68 | 75 | 51 | 49 | 50 |
| Pre-treatment HbS (%) | 65 | 64.7 | 46 | 40 | 45.4 | 87 | 47 |
| Vascular access | Peripheral | Temporary CVC | Peripheral | Peripheral | Temporary CVC | Peripheral | Peripheral |
| Post-treatment HbS (%) | 29 | 7.8 | 19 | 25 | 27 | 21 | 25 |
| Blood volume substituted (mL) | 4164 | 4796 | 4930 | 3276 | 2769 | 4497 | 2844 |
| Target FCR (%) | 26 | 28 | 38 | 49 | 39 | 19 | 39 |
Obstetric outcome:
| C-section at week 39 + 0 | C-section at week 35 + 1 | C-section at week 36 + 4 | C-section at week 37 + 3 | C-section at week 39 + 2 | ||
| Reference | Study Type | Population/Pregnancies (n) | Apheresis Sessions Schedule (n = Pregnancies) | Total Procedures (n) | Apheresis System | Technical Details | Vascular Access | Outcome | Level of Evidence (Quality Assessment [17]) |
|---|---|---|---|---|---|---|---|---|---|
| Key et al., 1980 [19] | Single-center, retrospective | 8/8 | First prophylactic RBCX at variable gestational age (range 17–30 weeks); 2 patients underwent a second procedure for HbA < 25% | 10 | IBM COBE 2997 Blood Cell Separator | Not reported | Peripheral | All pregnancies were carried to term. No fetal or neonatal morbidity. | VERY LOW (⨁) |
| Lee et al., 1990 [20] | Single-center, prospective | 5/5 | Prophylactic RBCX during second or early third trimester when Hct < 25%, and HbS > 65% | 5 | IBM COBE 2997 Blood Cell Separator | Not reported | Not reported | Significant increases in the Hct and % HbA. Negligible changes in maternal hemodynamic and metabolic function. | NA |
| Morrison et al., 1990 [21] | Single-center, retrospective | 131/131 | Prophylactic RBCX (n = 103): first procedure as early in pregnancy as possible, then if HbA < 20% or severe crisis or morbidity. Control group (n = 28): simple transfusion support. | Not reported | IBM COBE 2997 Blood Cell Separator | HbA target > 50% | Not reported | Lower maternal morbidity rates and hospitalization days. Decreased number of preterm deliveries, decreased prevalence of low birthweight infants and perinatal death rate. | VERY LOW (⨁) |
| Gilli et al., 2007 [22] | Single-center, retrospective | 31/31 | Prophylactic RBCX from the 28th week onwards (n = 14). Control group (n = 17): simple prophylactic transfusions. | Not stated | Not reported | Not reported | Not reported | Lower risk of intrauterine growth restriction and oligohydramnios. | VERY LOW (⨁) |
| Asma et al., 2015 [23] | Single-center, retrospective, cross-sectional | 37/37 | Prophylactic RBCX (n = 24): 1–3 at variable pregnancy time points. Control group (n = 13): simple transfusion support. | 43 | Cobe Spectra 7.0, Spectra Optia 7.0 | Hbs target < 30% FCR: 60–70% | Not reported | Higher rates of maternal mortality, maternal complications, incidence of VOC crises and fetal complications in control group. | VERY LOW (⨁) |
| Vianello et al., 2018 [24] | Double-center retrospective cross-sectional study | 18/46 | Every 3–4 weeks during pregnancy until delivery, starting at variable pregnancy time points (range from 22 to 28 weeks). | 160 | COM.TEC, Fresenius Kabi | Hbs target <30% | 157 peripheral (98.1%), 3 temporary CVC (1.9%) | No severe VOCs, sepsis, severe infection. Normal umbilical artery impedance during pregnancy. Improvement in new-born birthweight compared to mean values of SCD pregnancies reported in literature. | VERY LOW (⨁) |
| Baran et al., 2021 [25] | Single-center, retrospective, cross-sectional | 37/46 | Prophylactic RBCX (n = 27): 1 or more sessions at variable pregnancy time points (25–30 weeks). Therapeutic RBCX (n = 7): severe VOCs. Control group (n = 19): simple transfusion support | 43 | Spectra Optia 7.0 | Hbs target < 30% FCR: 60–70% | 20 peripheral (60.6%), 13 temporary CVC (39.4%) | Higher rate of painful crises, preeclampsia and preterm birth in control vs. prophylactic RBCX group. | VERY LOW (⨁) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, C.G.; Pellegrino, C.; Ceglie, S.; Arena, V.; Di Landro, F.; Chiusolo, P.; Teofili, L. Red Blood Cell Exchange as a Valid Therapeutic Approach for Pregnancy Management in Sickle Cell Disease: Three Explicative Cases and Systematic Review of Literature. J. Clin. Med. 2023, 12, 7123. https://doi.org/10.3390/jcm12227123
Valentini CG, Pellegrino C, Ceglie S, Arena V, Di Landro F, Chiusolo P, Teofili L. Red Blood Cell Exchange as a Valid Therapeutic Approach for Pregnancy Management in Sickle Cell Disease: Three Explicative Cases and Systematic Review of Literature. Journal of Clinical Medicine. 2023; 12(22):7123. https://doi.org/10.3390/jcm12227123
Chicago/Turabian StyleValentini, Caterina Giovanna, Claudio Pellegrino, Sara Ceglie, Vincenzo Arena, Francesca Di Landro, Patrizia Chiusolo, and Luciana Teofili. 2023. "Red Blood Cell Exchange as a Valid Therapeutic Approach for Pregnancy Management in Sickle Cell Disease: Three Explicative Cases and Systematic Review of Literature" Journal of Clinical Medicine 12, no. 22: 7123. https://doi.org/10.3390/jcm12227123
APA StyleValentini, C. G., Pellegrino, C., Ceglie, S., Arena, V., Di Landro, F., Chiusolo, P., & Teofili, L. (2023). Red Blood Cell Exchange as a Valid Therapeutic Approach for Pregnancy Management in Sickle Cell Disease: Three Explicative Cases and Systematic Review of Literature. Journal of Clinical Medicine, 12(22), 7123. https://doi.org/10.3390/jcm12227123