Contemporary Echocardiographic Evaluation of Mitral Regurgitation and Guidance for Percutaneous Mitral Valve Repair
Abstract
1. Contemporary Echocardiographic Evaluation of Mitral Valve Regurgitation
1.1. MR Etiology Evaluation
1.2. MR Severity Quantification
1.2.1. Qualitative Assessment
- Type I: normal leaflet motion, which may be seen in primary MR due to endocarditis, perforation, or clefts and in primary or secondary MR due to isolated annular dilation.
- Type II: excessive leaflet motion, which is most seen with MV prolapse or flail leaflet. Leaflet prolapse occurs when the leaflet body moves above the saddle-shaped annulus in systole, whereas leaflet flail occurs when a focal portion of the leaflet edge moves above the annulus and zone of coaptation. With flail leaflets, torn chords are usually visible.
- Type III: restricted leaflet motion, which is subclassified into restriction during both systole and diastole (IIIA) present in rheumatic MV disease, radiation- or drug-induced injury, or other inflammatory conditions. Restricted leaflet motion during systole only (IIIB) is present in functional MR.
1.2.2. Semi-Quantitative and Quantitative Assessment
1.3. Treatment Considerations
1.4. Heart Remodeling Evaluation
1.5. Stress Echocardiography and Functional MR
1.6. Artificial Intelligence and Machine Learning
| Mild | Moderate | Severe | ||
|---|---|---|---|---|
| Qualitative | ||||
| Valve morphology | Mild leaflets alteration | Moderately abnormal leaflets | Severe prolapse, flail leaflet, ruptured papillary muscle, large perforation, severe retraction | |
| Color flow jet | Small left atrium penetration | Moderate LA penetration/large late systolic | Deep LA penetration, Coanda effect | |
| Flow convergence zone | Not visible/transient/small | Intermediate size and duration | Large throughout systole/flattened, hardly measurable in strongly eccentric jets | |
| CW signal | Faint/partial, parabolic | Dense but partial (e.g., tele-systolic jet), parabolic and light density | Holosystolic, dense, triangular signal | |
| MR duration (Color, M-mode) | Protosystolic or tele-systolic | Moderate and holosystolic or late systolic | Dense, holosystolic signal | |
| Semi-quantitative | ||||
| Vena contracta width (mm) | <3 | 4–6 | ≥7 | |
| Pulmonary vein flow | Systolic dominance (diastolic dominance in AF and young patients) | Systolic blunting | Systolic flow reversal or Normal with low LA pressure | |
| Mitral inflow | A-wave dominance | Variable | E-wave dominance (>1.5 cm/s) | |
| Mitral TVI/Aortic TVI | <1.0 | 1.0–1.4 | >1.4 | |
| Quantitative | Mild/ moderate | Moderate/ severe | ||
| EROA (mm2) | <20 | 20–29 | 30–39 | ≥40 (≥30 in functional MR if elliptical orifice) |
| Regurgitant volume (mL) | <30 | 30–44 | 45–59 | ≥60 |
| Regurgitant fraction (%) | <30 | 30–49 | >50 | |
| Collateral findings | ||||
| LV and LA size (chronic severe MR) | Normal/mild LV dilation in secondary MR | Normal/mild dilation | Severe dilation | |
| PA systolic pressure (mmHg) | Normal | Normal | Usually elevated | |
2. Echocardiographic Guidance for Percutaneous Mitral Valve Repair: A Practical Overview
- -
- Clinical decision-making regarding the appropriateness and feasibility of procedures;
- -
- Pre-procedural planning;
- -
- Intraprocedural guidance;
- -
- Post-procedural follow-up.
2.1. Two-Dimensional TEE—Pre-Procedural Planning
- -
- FOUR-CHAMBER view (0°): A2-P2;
- -
- DUAL-CHAMBER view (90°): P1-A2-P3;
- -
- BI-COMMISSURAL view (110°): A counterclockwise rotation of the probe displays A1-P1 (and the anterolateral commissure), while a clockwise rotation of the probe displays A3-P3 (and the posteromedial commissure);
- -
- LVOT view (120°): A2-P2;
- -
- TRANSGASTRIC view (0°): view of all the scallops; planimetric area of the MV.
- -
- Mitral valve area (≥4 cm2);
- -
- Distance Fossa–Coaptation (≥4 cm);
- -
- Flap length (≥10 mm), flap thickness (≤5 mm);
- -
- Absence of calcification in the grasping area.
- -
- Flail gap < 10 mm;
- -
- Flail length < 15 mm.
- -
- Coaptation length ≥ 2 mm;
- -
- Coaptation depth < 11 mm.
- -
- Large coaptation gap (≥15 mm);
- -
- Large flail gap (≥10 mm);
- -
- Jets outside A2-P2;
- -
- Small mitral valve area (MVA);
- -
- Calcified landing zone;
- -
- Minimal tissue of the flaps.
2.2. Selection of TEER System—Pre-Procedural Planning
2.3. TEE 3D—Pre-Procedural Planning
- -
- Analyze the extent of commissural fusion in rheumatic MR or commissural prolapse in degenerative disease;
- -
- Identify leaflets and scallops involved in degenerative myxomatous disease and diagnose the associated presence of chordal rupture or prolapse of several scallops;
- -
- Establish the differential diagnosis between “indentation” and “cleft”;
- -
- Reliably measure the mitral valve annulus, valve area, and tenting area
3. Procedural Phase
3.1. Trans-Septal Puncture
- -
- The BICAVAL view (110°) for superior-inferior axis;
- -
- The SHORT-AXIS view (45°) of the aortic valve for antero-posterior axis;
- -
- The FOUR-CHAMBER view for puncture height.
- BICAVAL view: The puncture needle within the sheath is advanced from the right femoral vein down to the superior vena cava (SVC). Under TEE and fluoroscopic guidance, the puncture needle is retracted along the intra-atrial septum, down the SVC to the junction of the muscular and membranous septum.
- SHORT AXIS view: The trans-septal sheath and needle assembly should be rotated clockwise until the tenting is visible in the posterior part of the membranous septum. Because the mitral valve coaptation line is in the posterior LA, a posterior puncture allows the system to maneuver almost only laterally and medially, without excessive antero-posterior movement, as the system is advanced or retracted. If the puncture is too anterior, the guidewire may be too close to the aorta, resulting in an “aortic hugger” scenario where the system runs nearly parallel to the aorta in the LVOT view.
- FOUR-CHAMBER view: In this view, it is possible to measure the ideal distance between the puncture point in the septal wall and the mitral annular plane. An excessively high puncture could create too much distance to the MV coaptation line; the height of the system could be too short to reach and grip the flaps. If punctured too low, there could be inadequate space to orient the system and to grasp the leaflets, as the catheter tip may be just above and too close to the plane of the mitral annulus.
- The guide catheter is then inserted into the left atrium. The disappearance of the tenting on the inter-atrial septum is an indicator of complete crossing; the dilator can be removed when the guide catheter crosses the inter-atrial septum by at least 2 cm. The tip of the sheath and dilator should be clearly visualized on TEE, which allows for safe advancement.
- -
- The catheter and fossa ovalis are displayed simultaneously on the fluoroscopic screen and the transesophageal echocardiogram, allowing operators to understand the spatial position of the catheter in superior-inferior and anterior-posterior directions.
- -
- The downward pull of the catheter in the direction of the inter-atrial septum can be monitored using the 2D bicaval view superimposed on the left anterior oblique (LAO) view, in which the lateral profile of the septum is well visualized.
3.2. Clip Implantation
- -
- BI-COMMISSURAL view (60°) for information on the medial/lateral orientation;
- -
- LVOT view (120°) for anterior/posterior orientation of the clip within the mitral orifice;
- -
- THREE-DIMENSIONAL EN-FACE-view of the mitral valve from the atrial side, with or without color Doppler, to understand the origin and direction of regurgitant jet/jets.
- Following atrial septal puncture, the delivery system is advanced into the LA.
- The device tip should be advanced under echocardiographic guidance to avoid contact with the LA wall. Since the greatest distance from the septal puncture to the lateral wall of the LA in many patients is in the direction of the left superior pulmonary vein, the clip should be advanced in this direction, parallel to the mitral valvular plan.
- The clip should be placed in the LA, over the center of the mitral valve, pointing toward the apex of the LV in the BI-COMMISSURAL view and above the coaptation line in the LVOT view. A 120–140° X-Plane view allows simultaneous projection. The trajectory must ensure perpendicularity to the plane of the mitral valve (MV). Once proper axial alignment is achieved on the target lesion, the device arms are unfolded and oriented perpendicular to the coaptation line. Off-axis grasping of the leaflets can lead to valve distortion which can lead to worsening mitral regurgitation or leaflet injury. The LVOT view should show both clip arms of the same length if the clip arm orientation is perpendicular to the coaptation line. The BI-COMMISSURAL view should show the clip as a bar, as this plane is oriented 90 degrees to the LVOT view and perpendicular to the clip arms.
- The clip should be oriented perpendicularly to the coaptation line; a 3D EN-FACE view helps to orientate when viewed from the LA. Clockwise rotation of the system causes clockwise rotation of the clip arms; conversely, when viewed from the LV, counterclockwise rotation of the system results in clockwise rotation of the clip arms.
- The system is then advanced through the MV with the clip arms partially open. Once below the MV plane, the arms are fully opened.
- The clip should be oriented to achieve perpendicularity to the coaptation line of the flaps using the 3D EN-FACE view.
- Leaflet grasping: After partial closure of the clip, the delivery catheter is retracted to grab the leaflets. The entire clip is then closed to about 60 degrees.
- Correct flap insertion and clip location at the origin of the MR jet should be ensured. The LVOT/4CH view can confirm that mitral leaflets are adequately captured, whilst the BICOMMUSSUAL view is used to ensure that the flap on each side bisects the clip. Finally, an EN-FACE 3-D view shows the presence of a stable double orifice and the reduction of the MR jet.
- After adequate insertion of the flaps at 60 degrees of clip closure has been confirmed, the clip should be slowly closed further until the flaps are covered. The degree of MR reduction should be evaluated with color Doppler in all TEE views. Once the result is satisfactory, the device is detached from the delivery system Figure 6.
3.3. Post-Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MR | mitral regurgitation |
| TTE | transthoracic echocardiography |
| TEE | transesophageal echocardiography |
| AI | artificial intelligence |
| TEER | transcatheter edge-to-edge mitral valve repair |
| PWD | pulsed-wave doppler |
| EROA | effective regurgitant orifice area |
| LVEF | left ventricle ejection fraction |
| LA | left atrium |
| 2DE | two-dimensional echo |
| 3DE | three-dimensional echo |
| GLS | left ventricular longitudinal strain |
| PALS | peak left atrial longitudinal strain |
| PISA | proximal isovelocity surface area |
| ESC | European Society of Cardiology |
| SHD | Structural Heart Disease |
| AHA/ACC | American Heart Association/American College of Cardiology |
References
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Grayburn, P.A. Contrasting Effects of Pharmacological, Procedural, and Surgical Interventions on Proportionate and Disproportionate Functional Mitral Regurgitation in Chronic Heart Failure. Circulation 2019, 140, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Lee, A.P.W. Strain and mitral regurgitation: Is atrial functional mitral regurgitation a ventricular disease? Heart 2023, 109, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Dal-Bianco, J.P.; Levine, R.A.; Hung, J.W. Secondary Mitral Regurgitation: Cardiac Remodeling, Diagnosis, and Management. Struct. Heart 2023, 7, 100129. [Google Scholar] [CrossRef] [PubMed]
- Mihăilă, S.; Muraru, D.; Piasentini, E.; Miglioranza, M.H.; Peluso, D.; Cucchini, U.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Quantitative Analysis of Mitral Annular Geometry and Function in Healthy Volunteers Using Transthoracic Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 846–857. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Igata, S.; Cotter, B.R.; Hang, C.T.; Morikawa, N.; Strachan, M.; Raisinghani, A.; Blanchard, D.G.; DeMaria, A.N. Optimal Quantification of Functional Mitral Regurgitation: Comparison of Volumetric and Proximal Isovelocity Surface Area Methods to Predict Outcome. J. Am. Heart Assoc. 2021, 10, e018553. [Google Scholar] [CrossRef] [PubMed]
- Nishino, S.; Watanabe, N.; Kimura, T.; Kuriyama, N.; Shibata, Y. Acute Versus Chronic Ischemic Mitral Regurgitation: An Echocardiographic Study of Anatomy and Physiology. Circ. Cardiovasc. Imaging 2018, 11, e007028. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, V.; Barr, B.; Srivastava, M. Acute Valvular Heart Disease. Cardiol. Clin. 2018, 36, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Meyer, T.E. Left Ventricular Response to Mitral Regurgitation: Implications for Management. Circulation 2008, 118, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Hagendorff, A.; Knebel, F.; Helfen, A.; Stöbe, S.; Haghi, D.; Ruf, T.; Lavall, D.; Knierim, J.; Altiok, E.; Brandt, R.; et al. Echocardiographic assessment of mitral regurgitation: Discussion of practical and methodologic aspects of severity quantification to improve diagnostic conclusiveness. Clin. Res. Cardiol. 2021, 110, 1704–1733. [Google Scholar] [CrossRef] [PubMed]
- Hien, M.D.; Großgasteiger, M.; Weymann, A.; Rauch, H.; Rosendal, C. Reproducibility in Echocardiographic Two- and Three-Dimensional Mitral Valve Assessment. Echocardiography 2014, 31, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Jungels, V.M.; Heidrich, F.M.; Pfluecke, C.; Linke, A.; Sveric, K.M. Benefit of 3D Vena Contracta Area over 2D-Based Echocardiographic Methods in Quantification of Functional Mitral Valve Regurgitation. Diagnostics 2023, 13, 1176. [Google Scholar] [CrossRef]
- Lozano-Edo, S.; Jover-Pastor, P.; Osa-Saez, A.; Buendia-Fuentes, F.; Rodriguez-Serrano, M.; Arnau-Vives, M.A.; Rueda-Soriano, J.; Calvillo-Batlles, P.; Fonfria-Esparcia, C.; Martinez-Dolz, L.; et al. Spatiotemporal Complexity of Vena Contracta and Mitral Regurgitation Grading Using Three-Dimensional Echocardiographic Analysis. J. Am. Soc. Echocardiogr. 2023, 36, 77–86.e7. [Google Scholar] [CrossRef]
- Cherry, S.V.; Jain, P.; Rodriguez-Blanco, Y.F.; Fabbro, M., 2nd. Noninvasive Evaluation of Native Valvular Regurgitation: A Review of the 2017 American Society of Echocardiography Guidelines for the Perioperative Echocardiographer. J. Cardi-Othorac. Vasc. Anesth. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Vakil, K.; Roukoz, H.; Sarraf, M.; Krishnan, B.; Reisman, M.; Levy, W.C.; Adabag, S. Safety and efficacy of the MitraClip ® system for severe mitral regurgitation: A Systematic Review. Catheter. Cardiovasc. Interv. 2014, 84, 129–136. [Google Scholar] [CrossRef]
- Muraru, D.; Badano, L.P.; Piccoli, G.; Gianfagna, P.; Del Mestre, L.; Ermacora, D.; Proclemer, A. Validation of a novel automated border-detection algorithm for rapid and accurate quantitation of left ventricular volumes based on three-dimensional echocardiography. Eur. J. Echocardiogr. 2010, 11, 359–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nedadur, R.; Tsang, W. Automated Three-Dimensional Left Ventricular Volumes: Rise of the Machines? J. Am. Soc. Echocardiogr. 2019, 32, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Tsang, W. Three-dimensional echocardiographic acquisition and validity of left ventricular volumes and ejection fraction. Echocardiography 2020, 37, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Kuno, T.; Takagi, H.; Krishnamoorthy, P.; Prandi, F.R.; Palazzuoli, A.; Sharma, S.K.; Kini, A.; Lerakis, S. Prognostic value of left ventricular global longitudinal strain in mitral regurgitation: A systematic review. Heart Fail. Rev. 2023, 28, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Marsan, N.A.; Delgado, V.; Shah, D.J.; Pellikka, P.; Bax, J.J.; Treibel, T.; Cavalcante, J.L. Valvular heart disease: Shifting the focus to the myocardium. Eur. Heart J. 2023, 44, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Wang, T.K.M.; Popović, Z.B. Put a Strain on Secondary Mitral Regurgitation. J. Am. Coll. Cardiol. 2020, 75, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Mandoli, G.E.; Dokollari, A.; Bisleri, G.; D’ascenzi, F.; Santoro, C.; Miglioranza, M.H.; Focardi, M.; Cavigli, L.; Patti, G.; et al. Speckle tracking echocardiography in primary mitral regurgitation: Should we reconsider the time for intervention? Heart Fail. Rev. 2022, 27, 1247–1260. [Google Scholar] [CrossRef]
- Gomes, D.A.; Lopes, P.M.; Freitas, P.; Albuquerque, F.; Reis, C.; Guerreiro, S.; Abecasis, J.; Trabulo, M.; Ferreira, A.M.; Ferreira, J.; et al. Peak left atrial longitudinal strain is associated with all-cause mortality in patients with ventricular functional mitral regurgitation. Cardiovasc. Ultrasound 2023, 21, 9. [Google Scholar] [CrossRef]
- Citro, R.; Bursi, F.; Bellino, M.; Picano, E. The Role of Stress Echocardiography in Valvular Heart Disease. Curr. Cardiol. Rep. 2022, 24, 1477–1485. [Google Scholar] [CrossRef]
- Lancellotti, P.; Dulgheru, R.; Go, Y.Y.; Sugimoto, T.; Marchetta, S.; Oury, C.; Garbi, M. Stress echocardiography in patients with native valvular heart disease. Heart 2018, 104, 807–813. [Google Scholar] [CrossRef]
- Lancellotti, P.; Garbi, M. Exercise Stress Echocardiography in Degenerative Mitral Regurgitation: Back to the Origins. Circ. Cardiovasc. Imaging 2018, 11, e008263. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Mahjoub, H.; Dulgheru, R.; Pibarot, P.; Pierard, L.A.; Lancellotti, P. Left ventricular contractile reserve in asymptomatic primary mitral regurgitation. Eur. Heart J. 2014, 35, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Griffin, B.P.; Stewart, W.J.; Cosgrove, D.M.; Thomas, J.D.; Marwick, T.H. Left ventricular function after valve repair for chronic mitral regurgitation: Predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J. Am. Coll. Cardiol. 1996, 28, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine Learning and the Future of Cardiovascular Care. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Attia, Z.; Arruda-Olson, A.M.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; Luong, C.; Medina-Inojosa, J.R.; et al. Artificial Intelligence in Cardiology: Present and Future. Mayo Clin. Proc. 2020, 95, 1015–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, M.; Chang, S.; Chen, Z. Artificial intelligence in echocardiography: Detection, functional evaluation, and disease diagnosis. Cardiovasc. Ultrasound 2021, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Thomas, J.D. Characterizing Mitral Regurgitation with Precision Phenotyping and Unsupervised Learning. JACC Cardiovasc. Imaging 2021, 14, 2301–2302. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- González-Gómez, A.; Fernández-Santos, S.; Fernández-Golfín, C.; Zamorano, J.L. Mitral valve anatomy: Pre-procedural screening and imaging techniques. EuroIntervention 2015, 14, W32–W36. [Google Scholar] [CrossRef]
- Faletra, F.F.; Pozzoli, A.; Agricola, E.; Guidotti, A.; Biasco, L.; Leo, L.A.; Taramasso, M.; Pasotti, E.; Kuwata, S.; Moccetti, M.; et al. Echocardiographic-fluoroscopic fusion imaging for transcatheter mitral valve repair guidance. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 715–726. [Google Scholar] [CrossRef]
- Gheorghe, L.L.; Mobasseri, S.; Agricola, E.; Wang, D.D.; Milla, F.; Swaans, M.; Pandis, D.; Adams, D.H.; Yadav, P.; Sievert, H.; et al. Imaging for Native Mitral Valve Surgical and Transcatheter Interventions. JACC Cardiovasc. Imaging 2021, 14, 112–127. [Google Scholar] [CrossRef]
- Feldman, T.; Wasserman, H.S.; Herrmann, H.C.; Gray, W.; Block, P.C.; Whitlow, P.; Goar, F.S.; Rodriguez, L.; Silvestry, F.; Schwartz, A.; et al. Percutaneous Mitral Valve Repair Using the Edge-to-Edge Technique. J. Am. Coll. Cardiol. 2005, 46, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Loureiro, R.; Franzen, O.; Winter, R.; Sondergaard, L.; Jacobsen, P.; Cheung, G.; Moat, N.; Ihlemann, N.; Ghione, M.; Price, S.; et al. Echocardiographic and Clinical Outcomes of Central Versus Noncentral Percutaneous Edge-to-Edge Repair of Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2013, 62, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.; Asch, F.M.; Ruf, T.; Petrescu, A.; von Bardeleben, S.; Lim, D.S.; Maisano, F.; Kar, S.; Price, M.J. Clinical Outcomes with Transcatheter Edge-to-Edge Repair in Atrial Functional MR From the EXPAND Study. JACC Cardiovasc. Interv. 2022, 15, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Smith, R.L.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Meucci, F.; Ancona, F.; Pardo Sanz, A.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. EuroIntervention 2022, 17, 1205–1226. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Guarracino, F.; Valenti, V.; Frati, G.; Sciarretta, S.; Miraldi, F.; Agati, L.; Greco, E. Echocardiography and Correction of Mitral Regurgitation: An Unbreakable Link. Cardiology 2020, 145, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Perez, M.; Estévez-Loureiro, R.; Puga, L.; Caneiro-Queija, B.; Baz, J.A.; Iñiguez-Romo, A. Real-Time Echocardiography-Fluoroscopy Fusion Imaging with Automated 3D Heart Segmentation during Transcatheter Structural Heart Interventions. JACC Cardiovasc. Interv. 2022, 15, e155–e158. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, K.; Izumo, M. Role of 3D Transesophageal Echocardiography for Transcatheter Mitral Valve Repair—A Mini Review. Front. Cardiovasc. Med. 2022, 9, 815304. [Google Scholar] [CrossRef]
- Caballero, A.; Qin, T.; Hahn, R.T.; McKay, R.; Sun, W. Quantification of mitral regurgitation after transcatheter edge-to-edge repair: Comparison of echocardiography and patient-specific in silico models. Comput. Biol. Med. 2022, 148, 105855. [Google Scholar] [CrossRef]
- Avenatti, E.; Mackensen, G.B.; El-Tallawi, K.C.; Reisman, M.; Gruye, L.; Barker, C.M.; Little, S.H. Diagnostic Value of 3-Dimensional Vena Contracta Area for the Quantification of Residual Mitral Regurgitation after MitraClip Procedure. JACC Cardiovasc. Interv. 2019, 12, 582–591. [Google Scholar] [CrossRef]
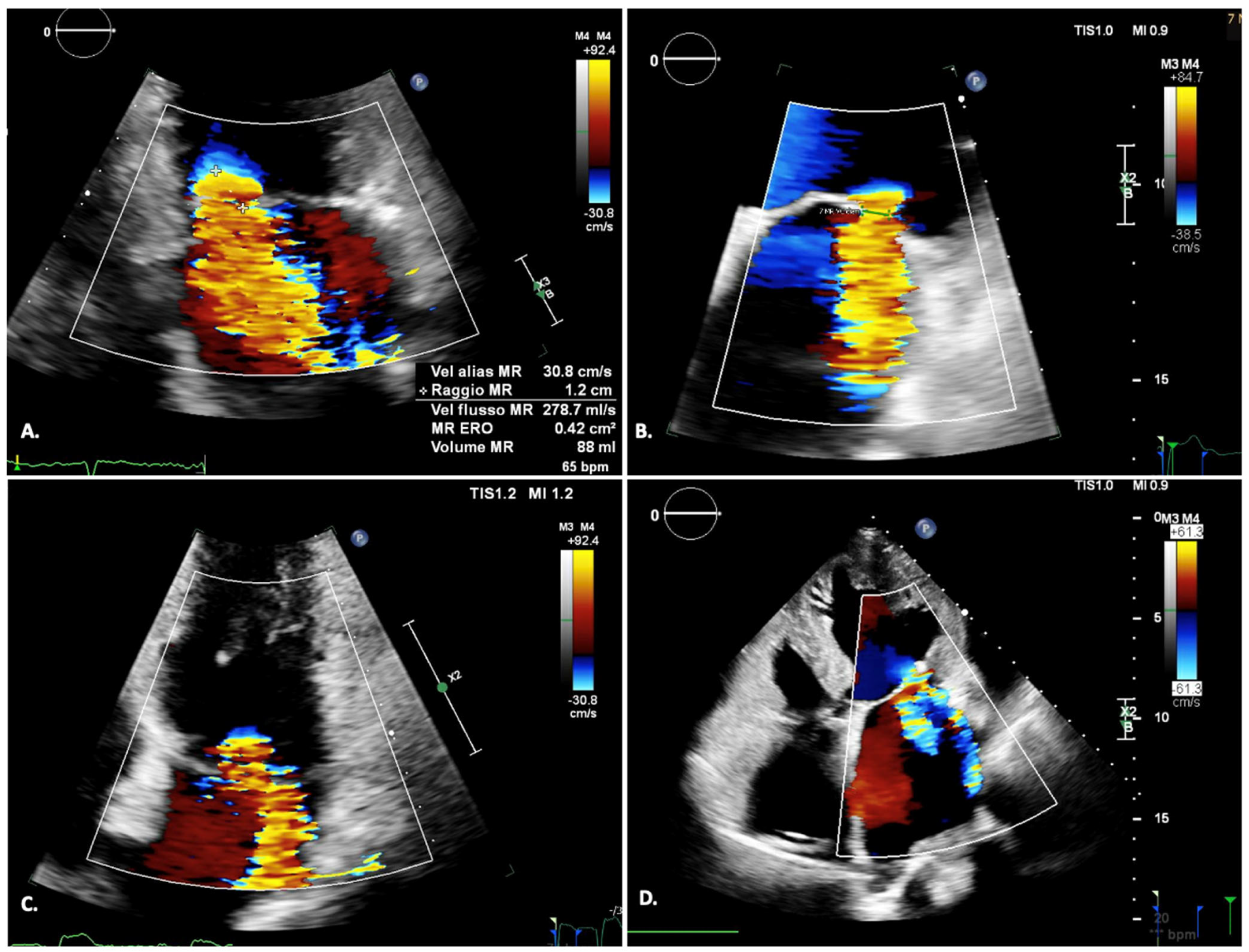

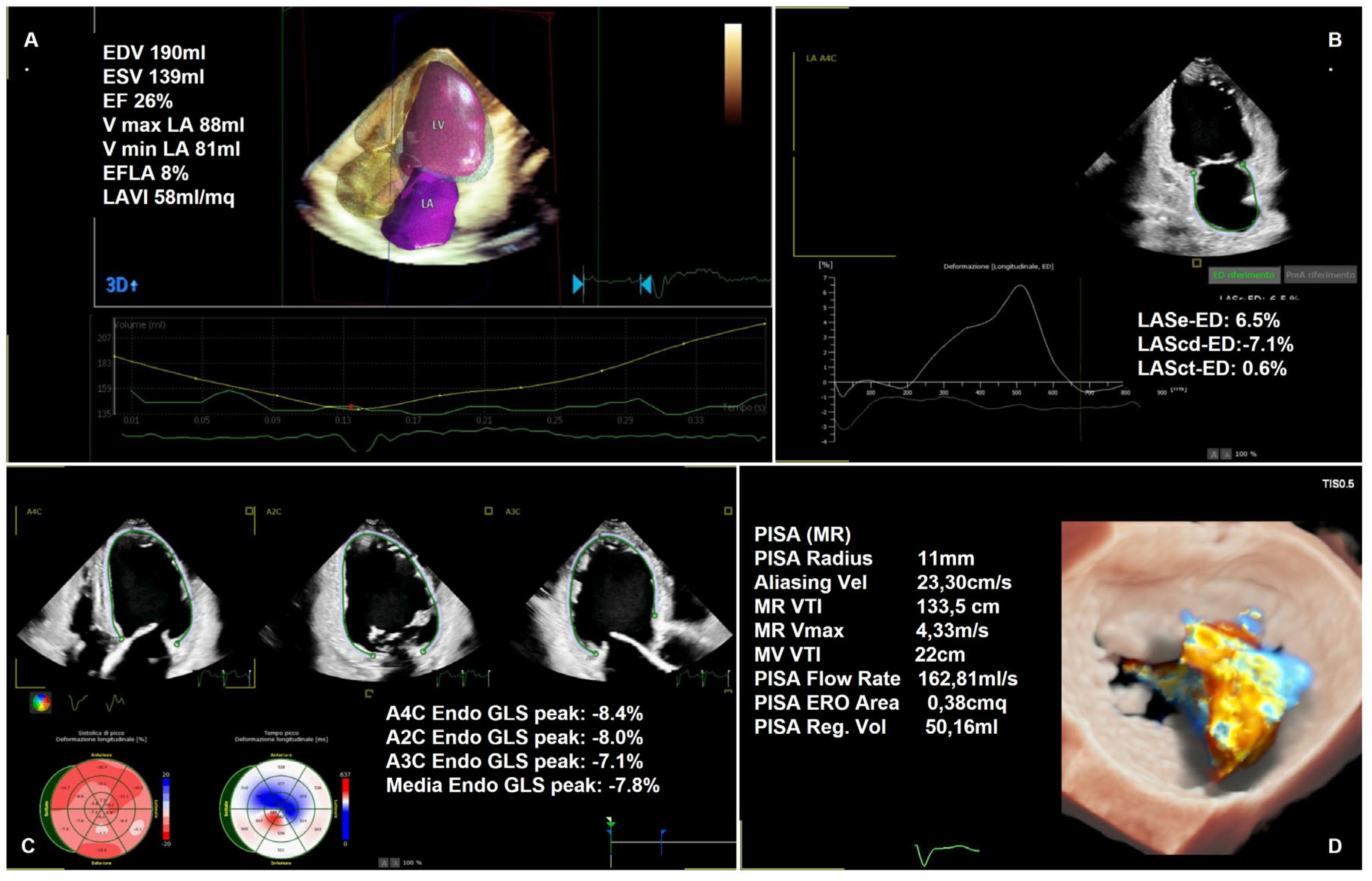
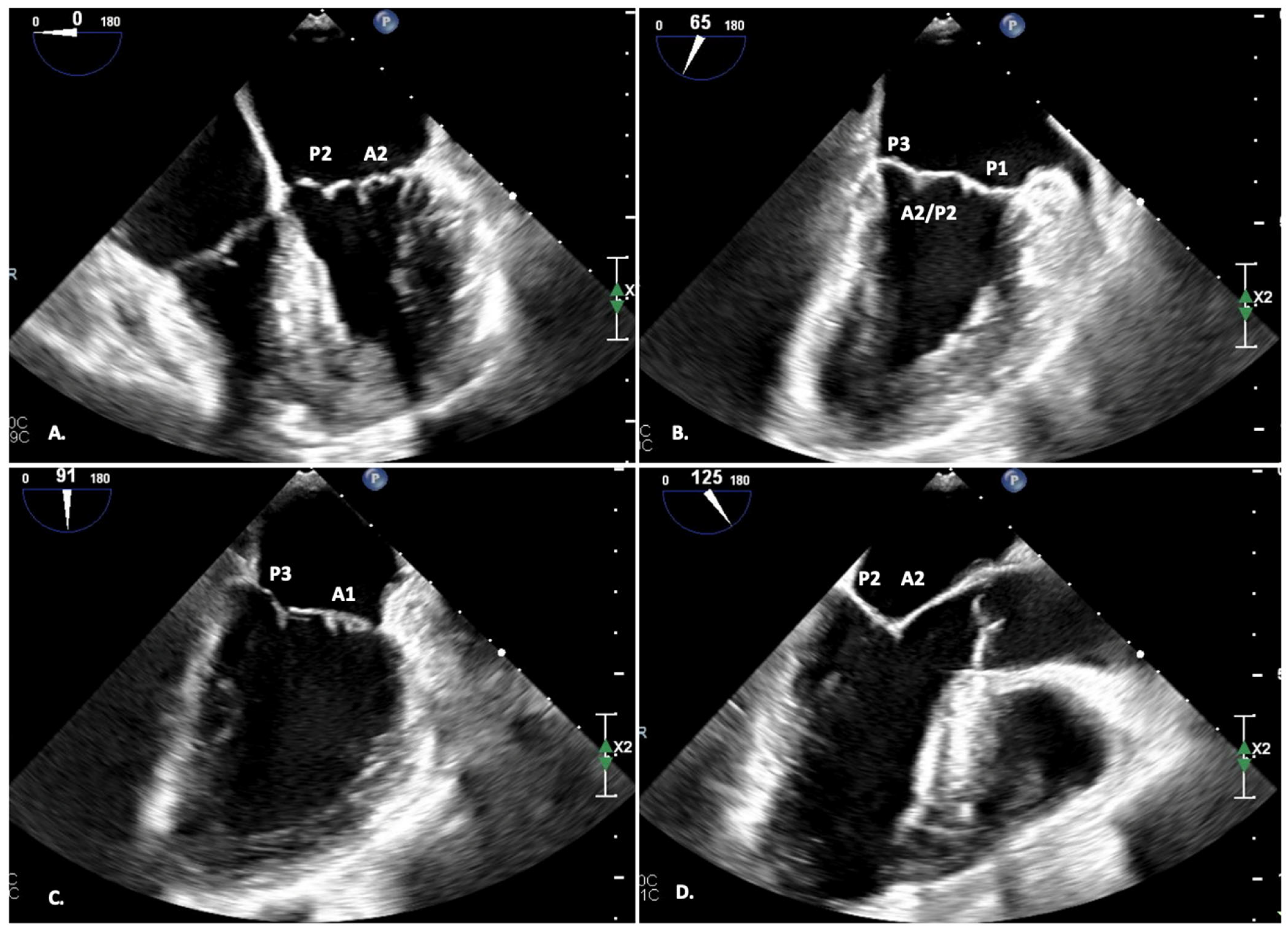

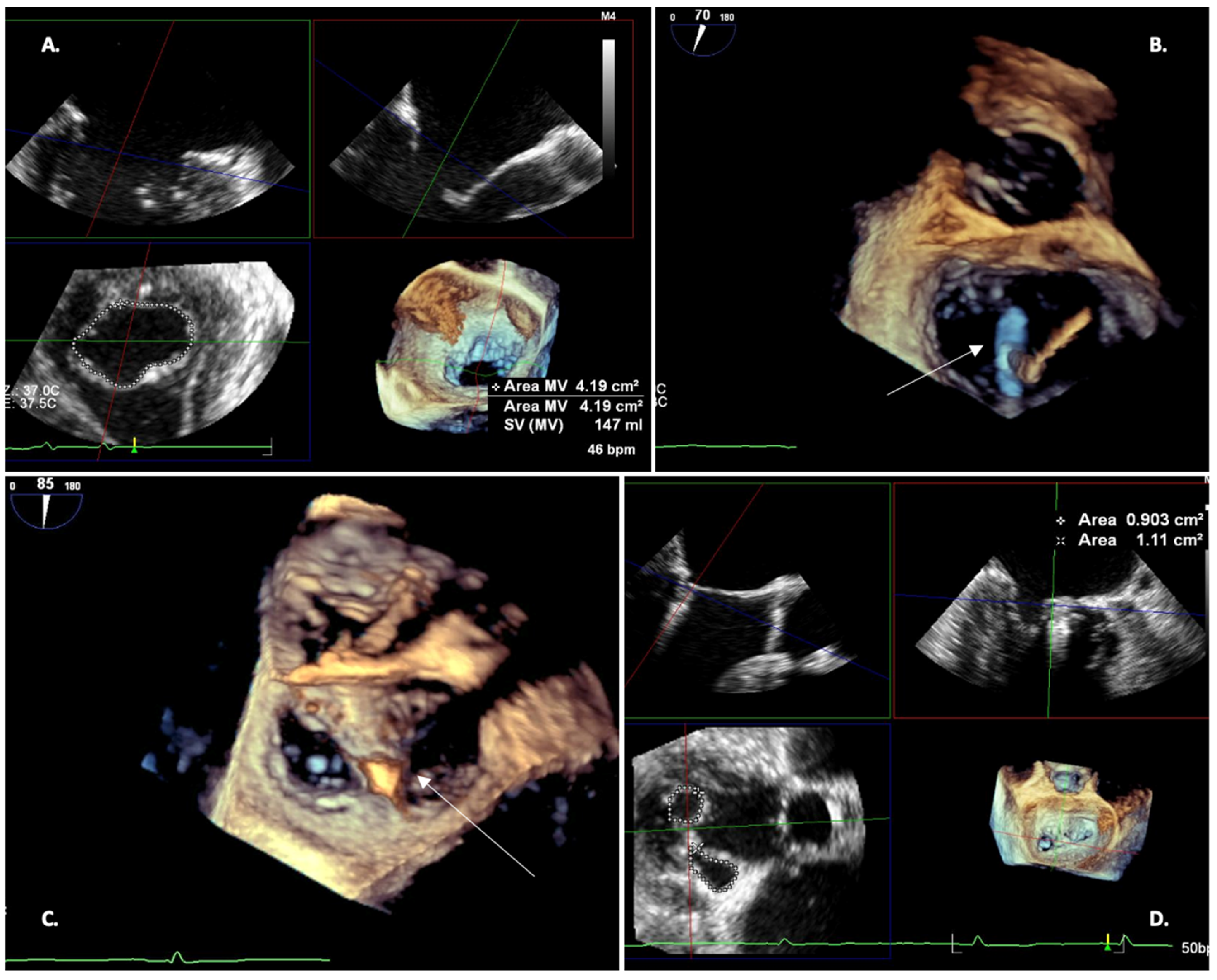
| Settings | |||
|---|---|---|---|
| Primary MR | Secondary MR | Interventional Echo | |
| 2D transthoracic | MR quantification | - | |
| Acute vs. chronic MR | |||
| Etiology: prolapse (AML-PML)/flail/rheumatic disease/degenerative disease | Etiology: ischemic vs. non ischemic (atrial MR/ventricular MR | ||
| Associated valve/heart disease | |||
| LV/LA function | |||
| Hemodynamic consequences | |||
| 3D transthoracic | MR quantification (especially eccentric jets) | - | |
| Better anatomical description | |||
| 2D/3D transesophageal | MR quantification and confirmation of severity (eccentric jets/poor transthoracic window) |
| |
| Etiology: Exclusion of thrombi/infective endocarditis; flail gap and width; leaflet-to-annulus index; calcifications | Etiology: coaptation depth/length; annular dimensions | ||
| Exclusion of contraindications for planned procedure | |||
| 3D transesophageal | Determination of morphological suitability for a specific transcatheter procedure | ||
| Better view of valve’s anatomy and possibility of obtaining ‘en-face’ surgical view | |||
| Stress echo | Confirmation/exclusion absence of symptoms during exercise | - | |
| Latent MR disclosure | |||
| Prognosis assessment analyzing contractile reserve | |||
| New automated analysis | Peak atrial longitudinal strain for early assessment of atrial dysfunction | - | |
| GLS as early assessment of ventricular damage | |||
| Heart volumes for prognosis | |||
| Optimal | Reasonable | Inappropriate | |
|---|---|---|---|
| Lesion location | Central (A2-P2 scallop) | Medial (A3-P3) Lateral (A1-P1) | Commissure, Cleft, Perforation, Vegetation |
| Lesion Extension Primary MR:
| <15 mm <10 mm <11 mm <3 mm | >15 >11 mm 1–3 mm | Complex Barlow disease No coaptation |
| Calcification | None | Not involving grasping area | Extensive |
| MV Area | >4 cm2 | >3 cm2 if good leaflet mobility and normal mean gradient | <3 cm2 or MG > 5 mmHg |
| Posterior leaflet length | >10 mm | 7–10 mm | <7 mm |
| Leaflet mobility | Normal | Systolic restriction (Carpentier IIIb) | Systo-diastolic restriction (Carpentier IIIa) |
| Leaflet Thickness | Normal | <5 mm | >5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, D.; Di Lenarda, F.; Novembre, M.L.; Paolisso, P.; Schillaci, M.; Melotti, E.; Doldi, M.; Terzi, R.; Gallazzi, M.; Conte, E.; et al. Contemporary Echocardiographic Evaluation of Mitral Regurgitation and Guidance for Percutaneous Mitral Valve Repair. J. Clin. Med. 2023, 12, 7121. https://doi.org/10.3390/jcm12227121
Marchetti D, Di Lenarda F, Novembre ML, Paolisso P, Schillaci M, Melotti E, Doldi M, Terzi R, Gallazzi M, Conte E, et al. Contemporary Echocardiographic Evaluation of Mitral Regurgitation and Guidance for Percutaneous Mitral Valve Repair. Journal of Clinical Medicine. 2023; 12(22):7121. https://doi.org/10.3390/jcm12227121
Chicago/Turabian StyleMarchetti, Davide, Francesca Di Lenarda, Maria Laura Novembre, Pasquale Paolisso, Matteo Schillaci, Eleonora Melotti, Marco Doldi, Riccardo Terzi, Michele Gallazzi, Edoardo Conte, and et al. 2023. "Contemporary Echocardiographic Evaluation of Mitral Regurgitation and Guidance for Percutaneous Mitral Valve Repair" Journal of Clinical Medicine 12, no. 22: 7121. https://doi.org/10.3390/jcm12227121
APA StyleMarchetti, D., Di Lenarda, F., Novembre, M. L., Paolisso, P., Schillaci, M., Melotti, E., Doldi, M., Terzi, R., Gallazzi, M., Conte, E., Volpato, V., Bartorelli, A., & Andreini, D. (2023). Contemporary Echocardiographic Evaluation of Mitral Regurgitation and Guidance for Percutaneous Mitral Valve Repair. Journal of Clinical Medicine, 12(22), 7121. https://doi.org/10.3390/jcm12227121








