Serum-Creatinine-to-Cystatin C-to-Waist-Circumference Ratios as an Indicator of Severe Airflow Limitation in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
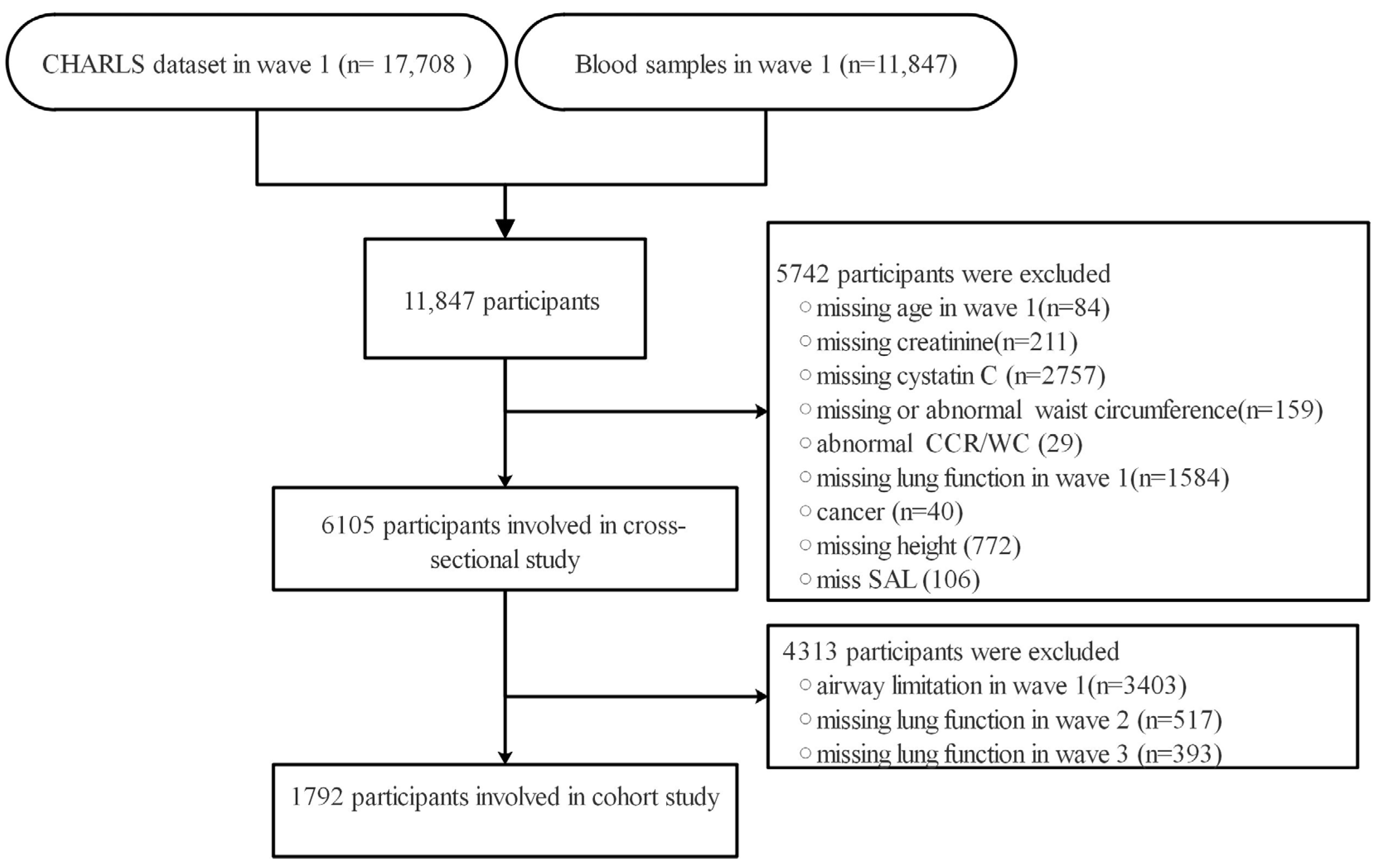
2.1. Study Population
2.2. Exposure Measurement
2.3. Lung Function Assessment
2.4. Covariates
3. Statistical Analysis
4. Results
4.1. Characteristics of Participants
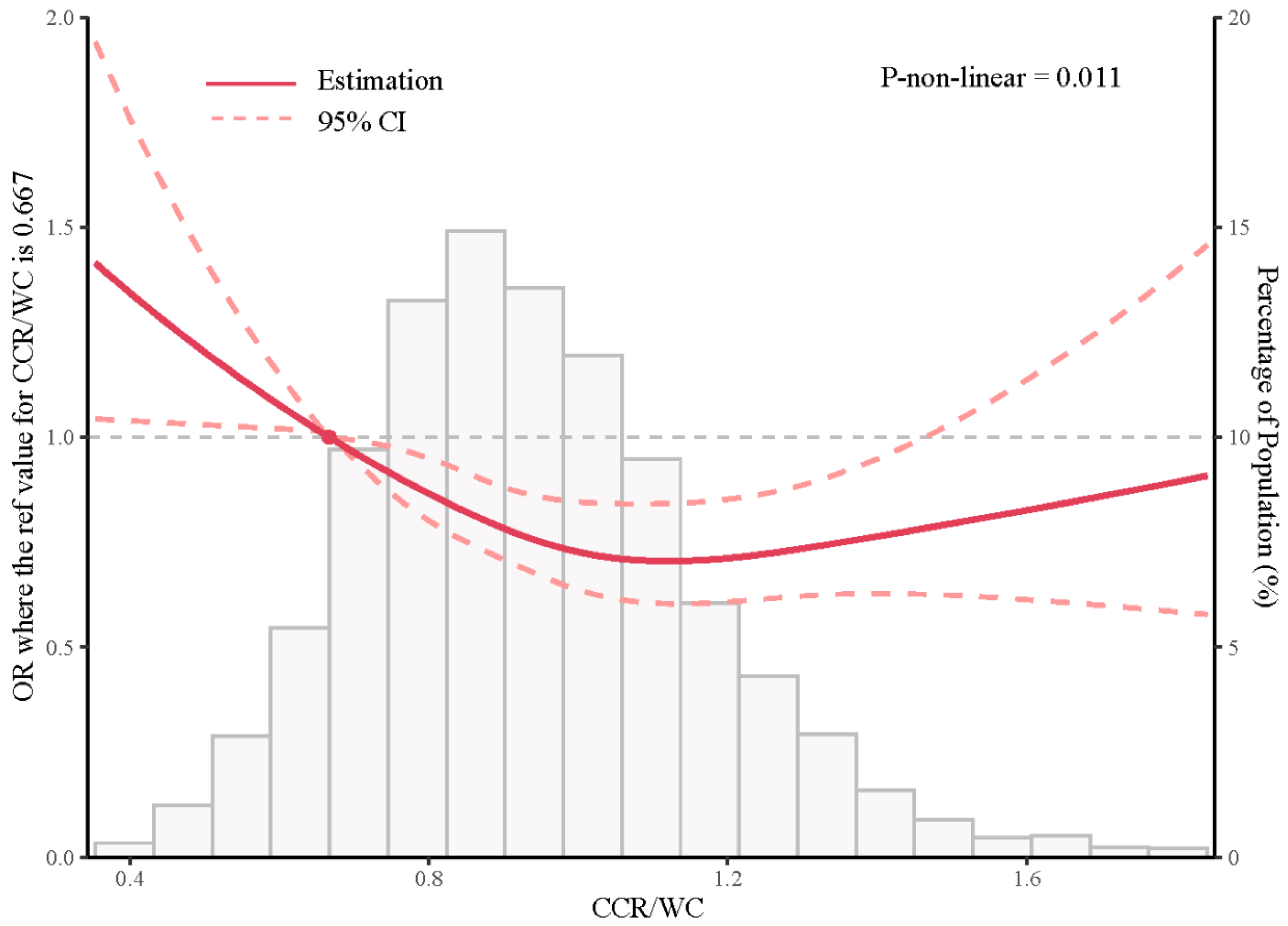
4.2. Association between CCR/WC Ratio and Lung Function at Baseline
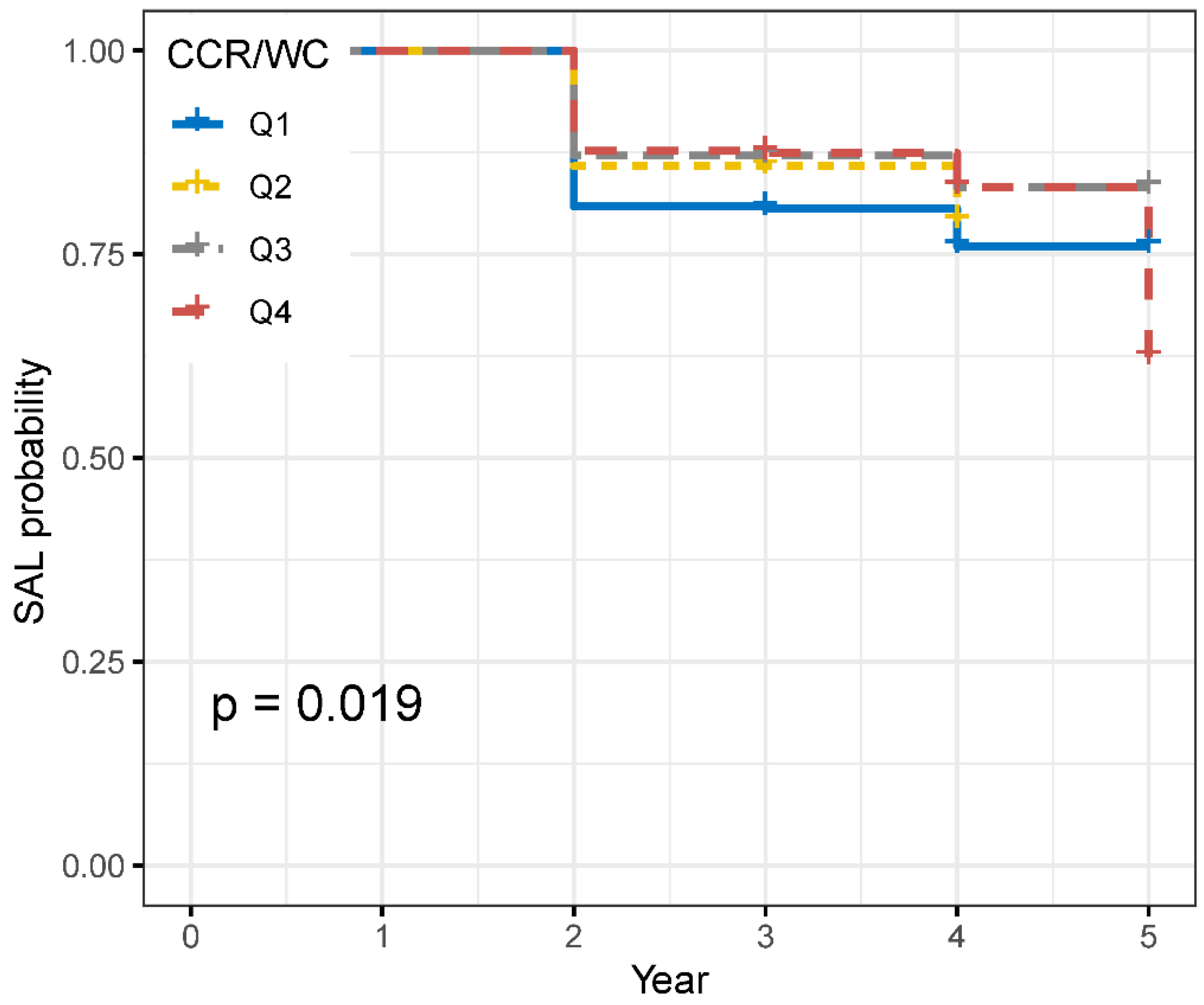
4.3. Risk of New-Onset SAL of CCR/WC Quartiles
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Wei, L.; Zhang, X.; Qi, G. Impact of demographic age structure on energy consumption structure: Evidence from population aging in mainland China. Energy 2023, 273, 127226. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Y.; Shi, L.; Zhang, S.; Chang, F. Prevalence and patterns of multimorbidity among the elderly in China: A cross-sectional study using national survey data. BMJ Open 2019, 9, e024268. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Stout-Delgado, H.W. Stout-Delgado, Aging and lung disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Donahue, P.T.; Xue, Q.-L.; Carlson, M.C. Peak Expiratory Flow Predicts Incident Dementia in a Representative Sample of US Older Adults: The National Health and Aging Trends Study (NHATS). J. Gerontol. Ser. A 2023, 78, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.; Sanchez-Rodriguez, D.; López-Escobar, J.; Meza, D.; Dávalos-Yerovi, V.; Duran, X.; Messaggi-Sartor, M.; Guillén-Solà, A.; Muniesa, J.M.; Duarte, E. Is peak expiratory flow an accurate sarcopenia screening tool in older patients referred to respiratory rehabilitation? Eur. Geriatr. Med. 2020, 11, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Luna, N.; Orea-Tejeda, A.; González-Islas, D.; Flores-Cisneros, L.; Keirns-Davis, C.; Sánchez-Santillán, R.; Pérez-García, I.; Gastelum-Ayala, Y.; Martínez-Vázquez, V.; Martínez-Reyna, Ó. Association between body composition, sarcopenia and pulmonary function in chronic obstructive pulmonary disease. BMC Pulm. Med. 2022, 22, 106. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Ihara, K.; Kim, H.; Obuchi, S. Definition of respiratory sarcopenia with peak expiratory flow rate. J. Am. Med. Dir. Assoc. 2019, 20, 1021–1025. [Google Scholar] [CrossRef]
- Hirai, K.; Tanaka, A.; Homma, T.; Goto, Y.; Akimoto, K.; Uno, T.; Yoshitaka, U.; Miyata, Y.; Inoue, H.; Ohta, S.; et al. Serum creatinine/cystatin C ratio as a surrogate marker for sarcopenia in patients with chronic obstructive pulmonary disease. Clin. Nutr. 2021, 40, 1274–1280. [Google Scholar] [CrossRef]
- Grevendonk, L.; Connell, N.J.; McCrum, C.; Fealy, C.E.; Bilet, L.; Bruls, Y.M.H.; Mevenkamp, J.; Schrauwen-Hinderling, V.B.; Jörgensen, J.A.; Moonen-Kornips, E.; et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat. Commun. 2021, 12, 4773. [Google Scholar] [CrossRef]
- Zheng, W.-H.; Zhu, Y.-B.; Yao, Y.; Huang, H.-B. Serum creatinine/cystatin C ratio as a muscle mass evaluating tool and prognostic indicator for hospitalized patients: A meta-analysis. Front. Med. 2023, 9, 1058464. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.M.; Elkhashab, K.A.E.; Ragab, T.M. Correlation of Serum Cystatin C level with Coronary Artery Disease and Its Severity. J. Med. Res. 2021, 7, 65–69. [Google Scholar] [CrossRef]
- Nishiki, K.; Nojiri, M.; Kato, R.; Shinomiya, S.; Oikawa, T.; Ishizaki, T.; Toga, H.; Mizuno, S. Serum Creatinine/Cystatin C Ratio Associated with Cross-Sectional Area of Erector Spinae Muscles and Pulmonary Function in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2021, ume 16, 3513–3524. [Google Scholar] [CrossRef]
- He, L.B.D.; Li, Y.B.D.; Gou, X.B.D.; Lei, L.B.D. The serum creatinine to cystatin C ratio predicts the risk of acute exacerbation of chronic obstructive pulmonary disease. Medicine 2023, 102, e33304. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Tsai, Y.-L.; Chou, R.-H.; Kuo, C.-S.; Chang, C.-C.; Huang, P.-H.; Lin, S.-J. Serum creatinine to cystatin C ratio is associated with major adverse cardiovascular events in patients with obstructive coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1509–1515. [Google Scholar] [CrossRef]
- Amado, C.A.; García-Unzueta, M.T.; Lavin, B.A.; Guerra, A.R.; Agüero, J.; Ramos, L.; Muñoz, P. The Ratio Serum Creatinine/Serum Cystatin C (a Surrogate Marker of Muscle Mass) as a Predictor of Hospitalization in Chronic Obstructive Pulmonary Disease Outpatients. Respiration 2019, 97, 302–309. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Chang, I.-C.; Liou, H.-H.; Wang, C.-H.; Lai, Y.-H.; Kuo, C.-H.; Hsu, B.-G. Serum indices based on creatinine and cystatin C predict mortality in patients with non-dialysis chronic kidney disease. Sci. Rep. 2021, 11, 16863. [Google Scholar] [CrossRef]
- Svartengren, M.; Cai, G.-H.; Malinovschi, A.; Theorell-Haglöw, J.; Janson, C.; Elmståhl, S.; Lind, L.; Lampa, E.; Lindberg, E. The impact of body mass index, central obesity and physical activity on lung function: Results of the EpiHealth study. ERJ Open Res. 2020, 6, 214–2020. [Google Scholar] [CrossRef]
- Hirano, T.; Doi, K.; Matsunaga, K.; Takahashi, S.; Donishi, T.; Suga, K.; Oishi, K.; Yasuda, K.; Mimura, Y.; Harada, M.; et al. A Novel Role of Growth Differentiation Factor (GDF)-15 in Overlap with Sedentary Lifestyle and Cognitive Risk in COPD. J. Clin. Med. 2020, 9, 2737. [Google Scholar] [CrossRef]
- Venkatrao, M.; Nagarathna, R.; Patil, S.S.; Singh, A.; Rajesh, S.; Nagendra, H. A composite of BMI and waist circumference may be a better obesity metric in Indians with high risk for type 2 diabetes: An analysis of NMB-2017, a nationwide cross-sectional study. Diabetes Res. Clin. Pract. 2020, 161, 108037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, W.; Mai, Z.; Wang, M.; Chen, H.; Sun, J. The serum creatinine to cystatin C to waist circumference ratios predicts risk for type 2 diabetes: A Chinese cohort study. J. Diabetes 2023, 15, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Jian, W.; Gao, Y.; Hao, C.; Wang, N.; Ai, T.; Liu, C.; Xu, Y.; Kang, J.; Yang, L.; Shen, H.; et al. Reference values for spirometry in Chinese aged 4–80 years. J. Thorac. Dis. 2017, 9, 4538–4549. [Google Scholar] [CrossRef]
- He, Y.-Y.; Jin, M.-L.; Chang, J.; Wang, X.-J. Associations of sarcopenia with peak expiratory flow among community-dwelling elderly population: Based on the China Health and Retirement Longitudinal Study (CHARLS). Eur. Geriatr. Med. 2023, 1–10. [Google Scholar] [CrossRef]
- Verma, M.; Rajput, M.; Kishore, K.; Kathirvel, S. Asian BMI criteria are better than WHO criteria in predicting Hypertension: A cross-sectional study from rural India. J. Fam. Med. Prim. Care 2019, 8, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Wang, K.; Jia, S.; Zhao, W.; Ge, M.; Dong, B. The creatinine-to-cystatin C ratio (a surrogate marker of muscle mass) as a predictor of lung function decline in older adults: A nationwide longitudinal study in China. Respir. Med. 2023, 211, 107197. [Google Scholar] [CrossRef]
- Peralta, G.P.; Granell, R.; Bédard, A.; Carsin, A.; Fuertes, E.; Howe, L.D.; Márquez, S.; Jarvis, D.L.; Garcia-Aymerich, J. Mid-childhood fat mass and airflow limitation at 15 years: The mediating role of insulin resistance and C-reactive protein. Pediatr. Allergy Immunol. 2022, 33, e13894. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Gu, K.; Jiang, X. Association of Body Mass Index and Abdominal Obesity with the Risk of Airflow Obstruction: National Health and Nutrition Examination Survey (NHANES) 2007–2012. COPD J. Chronic Obstr. Pulm. Dis. 2022, 19, 99–108. [Google Scholar] [CrossRef]
- Thaveeratitham, P.; Sonpeayung, R.; Janwantanakul, P. Impact of abdominal obesity and smoking on respiratory muscle strength and lung function. J. Med. Sci. 2020, 40, 224. [Google Scholar] [CrossRef]
- Khwanchuea, R.; Punsawad, C. Sex differences in the relationship between body composition and biomarkers of bone and fat metabolism in obese boys and girls. Bone Rep. 2021, 14, 101087. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Won, C.W. Sex-different changes of body composition in aging: A systemic review. Arch. Gerontol. Geriatr. 2022, 102, 104711. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Jung, Y. Energy Metabolism Changes and Dysregulated Lipid Metabolism in Postmenopausal Women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef]
- Cognuck, S.Q.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; de Paula, F.J.; Franci, C.R.; Elias, L.L.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar]
- Chuang, S.-Y.; Hsu, Y.-Y.; Chen, R.C.-Y.; Liu, W.-L.; Pan, W.-H. Abdominal Obesity and Low Skeletal Muscle Mass Jointly Predict Total Mortality and Cardiovascular Mortality in an Elderly Asian Population. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 1049–1055. [Google Scholar] [CrossRef]
- Tabara, Y.; Okada, Y.; Ochi, M.; Ohyagi, Y.; Igase, M. Association of Creatinine-to-Cystatin C Ratio with Myosteatosis and Physical Performance in Older Adults: The Japan Shimanami Health Promoting Program. J. Am. Med Dir. Assoc. 2021, 22, 2366–2372.e3. [Google Scholar] [CrossRef]
- Rovedder, P.M.E.; Borba, G.C.; Anderle, M.; Flores, J.; Ziegler, B.; Barreto, S.S.M.; Dalcin, P.d.T.R. Peripheral muscle strength is associated with lung function and functional capacity in patients with cystic fibrosis. Physiother. Res. Int. 2019, 24, e1771. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Fowler, J.R.; Tucker, L.A.; Bailey, B.W.; LeCheminant, J.D. Physical Activity and Insulin Resistance in 6,500 NHANES Adults: The Role of Abdominal Obesity. J. Obes. 2020, 2020, 3848256. [Google Scholar] [CrossRef]
- Wiebe, N.; Stenvinkel, P.; Tonelli, M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw. Open 2019, 2, e1910456. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall | Controls (n = 4259) | SAL (n = 1846) | p | |
|---|---|---|---|---|---|
| Age (years) | 59.52 ± 9.78 | 58.90 ± 9.68 | 60.95 ± 9.85 | <0.001 | |
| Sex | Male | 2804 (45.9) | 1965 (46.1) | 839 (45.4) | 0.640 |
| Female | 3301 (54.1) | 2294 (53.9) | 1007 (54.6) | ||
| Residence | Urban Community | 2049 (33.6) | 1463 (34.4) | 586 (31.7) | 0.051 |
| Rural Village | 4056 (66.4) | 2796 (65.6) | 1260 (68.3) | ||
| Married | Unmarried | 1026 (16.8) | 692 (16.2) | 334 (18.1) | 0.083 |
| Married | 5079 (83.2) | 3567 (83.8) | 1512 (81.9) | ||
| Educational | Less than lower secondary | 5563 (91.1) | 3831 (90.0) | 1732 (93.8) | <0.001 |
| Upper secondary and vocational training | 475 (7.8) | 370 (8.7) | 105 (5.7) | ||
| Tertiary | 67 (1.1) | 58 (1.4) | 9 (0.5) | ||
| Smoking | Never smoked | 3742 (61.5) | 2651 (62.4) | 1091 (59.5) | 0.027 |
| Former smoker | 517 (8.5) | 338 (8.0) | 179 (9.8) | ||
| Current smoker | 1825 (30.0) | 1260 (29.7) | 565 (30.8) | ||
| Drinking | No | 3748 (61.4) | 2633 (61.8) | 1115 (60.4) | 0.315 |
| Yes | 2355 (38.6) | 1625 (38.2) | 730 (39.6) | ||
| BMI (kg/m2) | <18.5 | 427 (7.0) | 229 (5.4) | 198 (10.8) | <0.001 |
| 18.5 to 23.9 | 2547 (41.9) | 1736 (40.9) | 811 (44.2) | ||
| 23 to 24.9 | 1242 (20.4) | 893 (21.0) | 349 (19.0) | ||
| 25 to 100 | 1862 (30.6) | 1387 (32.7) | 475 (25.9) | ||
| PG (mg/dL) | 110.07 ± 35.31 | 110.20 ± 34.33 | 109.76 ± 37.48 | 0.652 | |
| TC (mg/dL) | 193.33 ± 38.65 | 194.16 ± 38.33 | 191.42 ± 39.31 | 0.011 | |
| TG (mg/dL) | 131.28 ± 98.55 | 132.84 ± 99.30 | 127.71 ± 96.72 | 0.062 | |
| LDL-C (mg/dL) | 116.52 ± 35.19 | 118.05 ± 35.02 | 113.00 ± 35.35 | <0.001 | |
| HDL-C (mg/dL) | 51.20 ± 15.18 | 50.69 ± 14.85 | 52.40 ± 15.85 | <0.001 | |
| HbA1c (mg/dL) | 5.27 ± 0.80 | 5.25 ± 0.78 | 5.29 ± 0.83 | 0.074 | |
| UA (mg/dL) | 4.44 ± 1.26 | 4.46 ± 1.26 | 4.40 ± 1.27 | 0.092 | |
| Creatinine (mg/dL) | 0.78 ± 0.24 | 0.78 ± 0.20 | 0.77 ± 0.32 | 0.062 | |
| Cystatin C (mg/L) | 1.01 ± 0.27 | 1.01 ± 0.25 | 1.04 ± 0.32 | <0.001 | |
| CCR/WC | 0.94 ± 0.23 | 0.94 ± 0.23 | 0.92 ± 0.24 | 0.001 | |
| ADL | No | 5061 (83.7) | 3641 (86.3) | 1420 (77.9) | <0.001 |
| Yes | 982 (16.3) | 578 (13.7) | 404 (22.1) | ||
| Hypertension | No | 4466 (73.5) | 3153 (74.4) | 1313 (71.4) | 0.016 |
| Yes | 1610 (26.5) | 1084 (25.6) | 526 (28.6) | ||
| Diabetes | No | 5690 (93.8) | 3953 (93.5) | 1737 (94.4) | 0.239 |
| Yes | 378 (6.2) | 274 (6.5) | 104 (5.6) | ||
| Lung disease | No | 5472 (89.9) | 3960 (93.2) | 1512 (82.1) | <0.001 |
| Yes | 616 (10.1) | 287 (6.8) | 329 (17.9) | ||
| CVD | No | 5320 (87.5) | 3754 (88.5) | 1566 (85.3) | 0.001 |
| Yes | 758 (12.5) | 488 (11.5) | 270 (14.7) | ||
| Stroke | No | 5953 (97.8) | 4167 (98.1) | 1786 (97.1) | 0.019 |
| Yes | 136 (2.2) | 82 (1.9) | 54 (2.9) | ||
| Kidney | No | 5716 (94.2) | 4015 (94.8) | 1701 (92.7) | 0.003 |
| Yes | 355 (5.8) | 222 (5.2) | 133 (7.3) | ||
| Asthma | No | 5794 (95.2) | 4133 (97.4) | 1661 (90.3) | <0.001 |
| Yes | 290 (4.8) | 112 (2.6) | 178 (9.7) | ||
| PEF (L/min) | 288.22 ± 122.32 | 342.94 ± 100.52 | 161.96 ± 57.59 | <0.001 |
| PEF | PEF/PEF prediction | SAL | |||||
|---|---|---|---|---|---|---|---|
| β (95%CI) | p | β (95%CI) | p | OR (95%CI) | p | ||
| All | |||||||
| Crude model | 106.32 (93.30, 119.35) | <0.001 | 0.05 (0.02, 0.08) | 0.002 | 0.66 (0.52, 0.84) | 0.001 | |
| Adjusted model 1 | 24.78 (11.96, 37.59) | <0.001 | 0.08 (0.04, 0.11) | <0.001 | 0.69 (0.52, 0.92) | 0.011 | |
| Adjusted model 2 | 25.95 (12.72, 39.18) | <0.001 | 0.08 (0.05, 0.12) | <0.001 | 0.64 (0.47, 0.85) | 0.003 | |
| Male | |||||||
| Crude model | 73.82 (51.50, 96.14) | <0.001 | 0.05 (0.00, 0.10) | 0.033 | 0.53 (0.36, 0.77) | 0.001 | |
| Adjusted model 1 | 26.81 (5.50, 48.12) | 0.014 | 0.07 (0.03, 0.12) | 0.003 | 0.60 (0.39, 0.92) | 0.019 | |
| Adjusted model 2 | 27.86 (5.94, 49.78) | 0.013 | 0.08 (0.03, 0.13) | 0.003 | 0.58 (0.37, 0.90) | 0.017 | |
| Female | |||||||
| Crude model | 46.13 (31.55, 60.71) | <0.001 | 0.05 (0.01, 0.10) | 0.013 | 0.76 (0.54, 1.06) | 0.112 | |
| Adjusted model 1 | 21.50 (6.41, 36.58) | 0.005 | 0.08 (0.03, 0.12) | 0.001 | 0.78 (0.53, 1.15) | 0.209 | |
| Adjusted model 2 | 24.78 (9.10, 40.46) | 0.002 | 0.09 (0.04, 0.14) | <0.001 | 0.67 (0.45, 1.00) | 0.059 | |
| All | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | ||
| Crude model | |||||||
| Q1 | Ref | Ref | Ref | ||||
| Q2 | 0.85 (0.64, 1.12) | 0.253 | 0.69 (0.42, 1.14) | 0.149 | 0.93 (0.66, 1.31) | 0.663 | |
| Q3 | 0.67 (0.50, 0.90) | 0.008 | 0.47 (0.28, 0.78) | 0.004 | 0.86 (0.59, 1.26) | 0.442 | |
| Q4 | 0.67 (0.50, 0.91) | 0.010 | 0.61 (0.38, 0.97) | 0.037 | 0.65 (0.41, 1.03) | 0.069 | |
| Adjusted model 1 | |||||||
| Q1 | Ref | Ref | Ref | ||||
| Q2 | 0.75 (0.55, 1.01) | 0.055 | 0.56 (0.33, 0.94) | 0.029 | 0.74 (0.51, 1.08) | 0.117 | |
| Q3 | 0.57 (0.41, 0.79) | 0.001 | 0.36 (0.21, 0.62) | 0.000 | 0.66 (0.44, 1.00) | 0.049 | |
| Q4 | 0.49 (0.35, 0.70) | <0.001 | 0.46 (0.27, 0.77) | 0.003 | 0.41 (0.24, 0.70) | 0.001 | |
| Adjusted model 2 | |||||||
| Q1 | Ref | Ref | Ref | ||||
| Q2 | 0.73 (0.54, 0.99) | 0.043 | 0.58 (0.35, 0.99) | 0.044 | 0.73 (0.50, 1.07) | 0.104 | |
| Q3 | 0.57 (0.41, 0.79) | 0.001 | 0.40 (0.23, 0.68) | 0.001 | 0.65 (0.43, 1.00) | 0.048 | |
| Q4 | 0.49 (0.34, 0.70) | <0.001 | 0.53 (0.31, 0.90) | 0.018 | 0.40 (0.23, 0.69) | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, Q.; Zhang, H.; Li, B.; Zhang, C.; Zhao, Y.; Lu, J.; Ma, X. Serum-Creatinine-to-Cystatin C-to-Waist-Circumference Ratios as an Indicator of Severe Airflow Limitation in Older Adults. J. Clin. Med. 2023, 12, 7116. https://doi.org/10.3390/jcm12227116
Li J, Sun Q, Zhang H, Li B, Zhang C, Zhao Y, Lu J, Ma X. Serum-Creatinine-to-Cystatin C-to-Waist-Circumference Ratios as an Indicator of Severe Airflow Limitation in Older Adults. Journal of Clinical Medicine. 2023; 12(22):7116. https://doi.org/10.3390/jcm12227116
Chicago/Turabian StyleLi, Jinxuan, Qi Sun, Hongguang Zhang, Bingjie Li, Chaoyu Zhang, Yixin Zhao, Jianbo Lu, and Xu Ma. 2023. "Serum-Creatinine-to-Cystatin C-to-Waist-Circumference Ratios as an Indicator of Severe Airflow Limitation in Older Adults" Journal of Clinical Medicine 12, no. 22: 7116. https://doi.org/10.3390/jcm12227116
APA StyleLi, J., Sun, Q., Zhang, H., Li, B., Zhang, C., Zhao, Y., Lu, J., & Ma, X. (2023). Serum-Creatinine-to-Cystatin C-to-Waist-Circumference Ratios as an Indicator of Severe Airflow Limitation in Older Adults. Journal of Clinical Medicine, 12(22), 7116. https://doi.org/10.3390/jcm12227116







