Risk for Esophageal Cancer Based on Lifestyle Factors–Smoking, Alcohol Consumption, and Body Mass Index: Insight from a South Korean Population Study in a Low-Incidence Area
Abstract
:1. Introduction
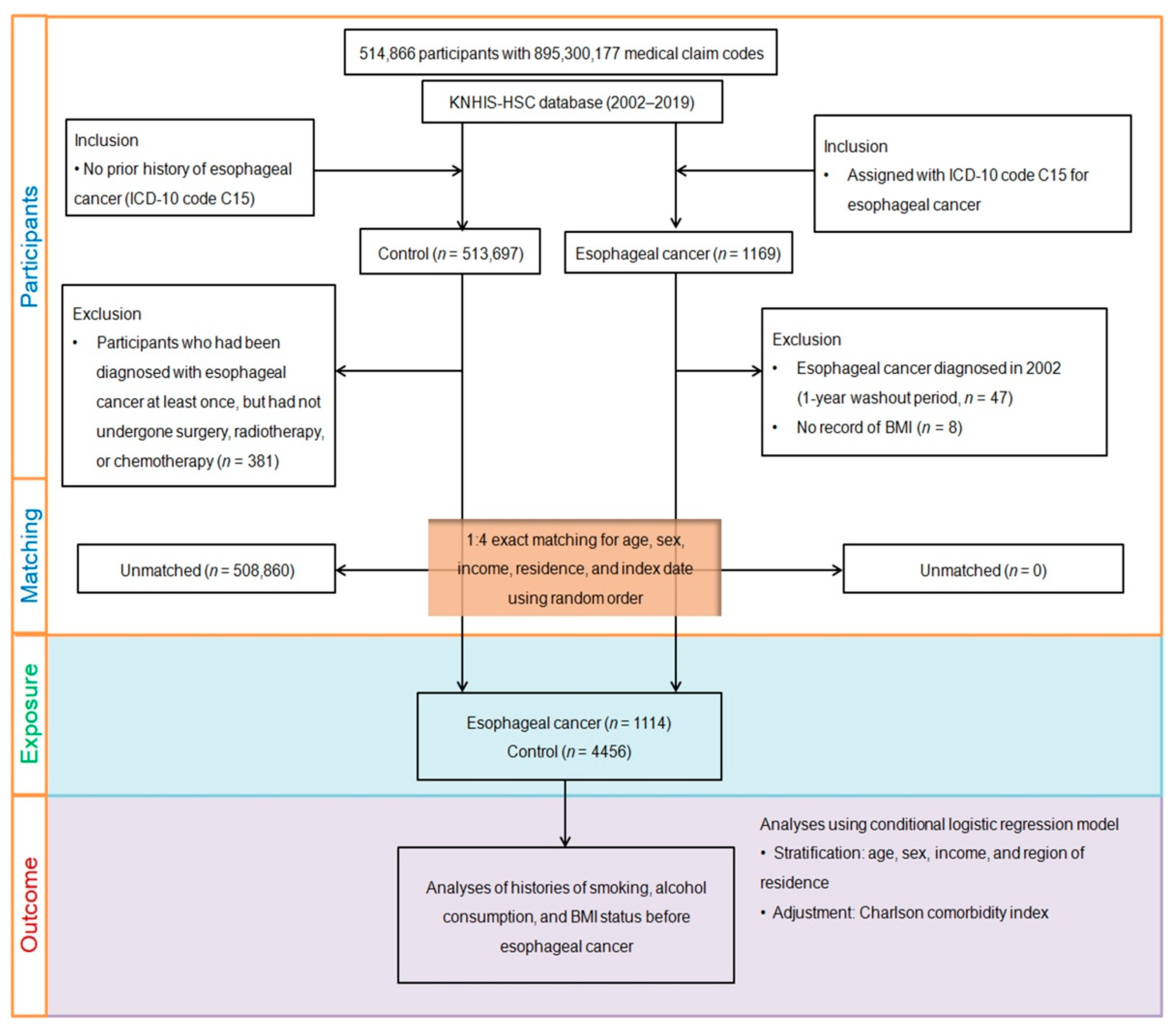
2. Materials and Methods
2.1. Exposure (Smoking, Alcohol Consumption, and Obesity)
2.2. Outcome (Esophageal Cancer)
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Relations of Smoking, Alcohol, and Obesity Status with Incident Esophageal Cancer
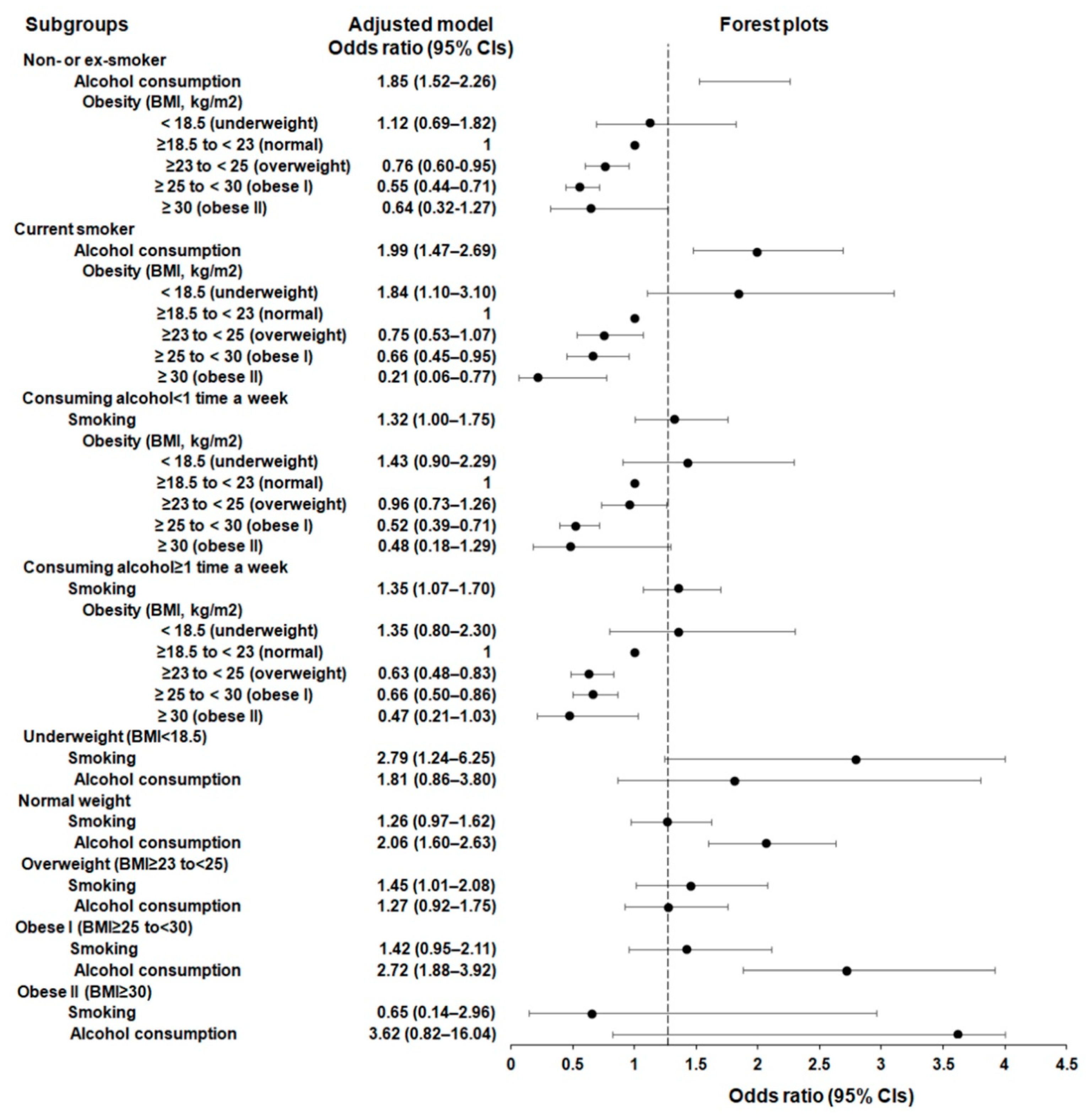
3.2. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, Y. Low body mass index is associated with poor treatment outcome following radiotherapy in esophageal squamous cell carcinoma. Radiat. Oncol. J. 2023, 41, 40–47. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.J. Esophageal Cancer in Korea: Epidemiology and Treatment Patterns. J. Chest Surg. 2021, 54, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kang, M.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2022. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2022, 54, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Won, Y.J.; Jung, H.K.; Kong, H.J.; Jung, K.W.; Oh, C.M.; Choe, S.; Lee, J. Trends in incidence and survival of esophageal cancer in Korea: Analysis of the Korea Central Cancer Registry Database. J. Gastroenterol. Hepatol. 2018, 33, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer 2023, 14, 3–11. [Google Scholar] [CrossRef]
- Lin, Y.; Totsuka, Y.; He, Y.; Kikuchi, S.; Qiao, Y.; Ueda, J.; Wei, W.; Inoue, M.; Tanaka, H. Epidemiology of esophageal cancer in Japan and China. J. Epidemiol. 2013, 23, 233–242. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Song, G.; Li, Y.; Liang, D.; Jin, J.; Wen, D.; Shan, B. Incidence and mortality rate of esophageal cancer has decreased during past 40 years in Hebei Province, China. Chin. J. Cancer Res. 2015, 27, 562–571. [Google Scholar] [CrossRef]
- Tsai, M.C.; Chou, Y.C.; Lee, Y.K.; Hsu, W.L.; Tang, C.S.; Chen, S.Y.; Huang, S.P.; Chen, Y.C.; Lee, J.M. Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis. Cancers 2022, 14, 5844. [Google Scholar] [CrossRef]
- Zhao, X.; Lim, F. Lifestyle Risk Factors in Esophageal Cancer: An Integrative Review. Crit. Care Nurs. Q. 2020, 43, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yuan, J.M.; Wang, R.; Gao, Y.T.; Yu, M.C. Alcohol, tobacco, and diet in relation to esophageal cancer: The Shanghai Cohort Study. Nutr. Cancer 2008, 60, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.D.; Sun, X.D.; Abnet, C.C.; Fan, J.H.; Dawsey, S.M.; Dong, Z.W.; Mark, S.D.; Qiao, Y.L.; Taylor, P.R. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int. J. Cancer 2005, 113, 456–463. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, J.K.; Hu, X.S.; Wang, P.H.; Qin, Y.; Lu, Y.C.; Yang, J.; Liu, A.M.; Wu, D.L.; Zhang, Z.F.; et al. Association of smoking, alcohol drinking and dietary factors with esophageal cancer in high- and low-risk areas of Jiangsu Province, China. World J. Gastroenterol. 2006, 12, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Yoon, S.J. Burden of Cancer Due to Cigarette Smoking and Alcohol Consumption in Korea. Int. J. Environ. Res. Public Health 2022, 19, 3493. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Han, B.D.; Han, K.; Jung, J.H.; Son, J.W.; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity Fact Sheet in Korea, 2021: Trends in Obesity Prevalence and Obesity-Related Comorbidity Incidence Stratified by Age from 2009 to 2019. J. Obes. Metab. Syndr. 2022, 31, 169–177. [Google Scholar] [CrossRef]
- Smith, M.; Zhou, M.; Whitlock, G.; Yang, G.; Offer, A.; Hui, G.; Peto, R.; Huang, Z.; Chen, Z. Esophageal cancer and body mass index: Results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int. J. Cancer 2008, 122, 1604–1610. [Google Scholar] [CrossRef]
- Cook, M.B.; Kamangar, F.; Whiteman, D.C.; Freedman, N.D.; Gammon, M.D.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: A pooled analysis from the international BEACON consortium. J. Natl. Cancer Inst. 2010, 102, 1344–1353. [Google Scholar] [CrossRef]
- Tian, J.; Zuo, C.; Liu, G.; Che, P.; Li, G.; Li, X.; Chen, H. Cumulative evidence for the relationship between body mass index and the risk of esophageal cancer: An updated meta-analysis with evidence from 25 observational studies. J. Gastroenterol. Hepatol. 2020, 35, 730–743. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, D.H.; Han, K.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, N. Joint Effects of Low Body Mass Index and Alcohol Consumption on Developing Esophageal Squamous Cell Cancer: A Korean Nationwide Population-Based Cohort Study. Asian Pac. J. Cancer Prev. 2017, 18, 1881–1887. [Google Scholar] [CrossRef]
- Kendall, B.E.; Fox, G.A.; Fujiwara, M.; Nogeire, T.M. Demographic heterogeneity, cohort selection, and population growth. Ecology 2011, 92, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Lee, E.; Jung, H.W.; Jang, I.Y. Geriatrics Fact Sheet in Korea 2021. Ann. Geriatr. Med. Res. 2021, 25, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Sung, J.; Kim, C.Y. High risk groups in health behavior defined by clustering of smoking, alcohol, and exercise habits: National Heath and Nutrition Examination Survey. J. Prev. Med. Public Health 2010, 43, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H. Network Analysis of Complex Health Risk Factors and Chronic Diseases. Health and Welfare Issue & Focus 254; Korea Institute for Health and Social Affairs: Sejong-si, Republic of Korea, 2014; pp. 1–8. [Google Scholar]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Kim, N.Y.; Cho, S.J.; Nam, E.S.; Min, K.W.; et al. Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case-Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. [Google Scholar] [CrossRef]
- Kwon, M.J.; Park, J.Y.; Kim, S.G.; Kim, J.K.; Lim, H.; Kim, J.H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Park, H.Y.; et al. Potential Association of Osteoporosis and Not Osteoporotic Fractures in Patients with Gout: A Longitudinal Follow-Up Study. Nutrients 2022, 15, 134. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.; Sundararajan, V. Practice of epidemiology: Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Yang, D.; Dalton, J.E. A unified approach to measuring the effect size between two groups using SAS. In Proceedings of the SAS Global Forum 2012: Statistics and Data Analysis, Orlando, FL, USA, 22–25 April 2012; pp. 335–2012. [Google Scholar]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Engel, L.S.; Chow, W.H.; Vaughan, T.L.; Gammon, M.D.; Risch, H.A.; Stanford, J.L.; Schoenberg, J.B.; Mayne, S.T.; Dubrow, R.; Rotterdam, H.; et al. Population attributable risks of esophageal and gastric cancers. J. Natl. Cancer Inst. 2003, 95, 1404–1413. [Google Scholar] [CrossRef]
- Lee, V.W.Y.; Li, A.; Li, J.T.S. Burden of smoking in Asia-Pacific countries. Tob. Induc. Dis. 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.J. Trends in Alcohol Consumption for Korean Adults from 1998 to 2018: Korea National Health and Nutritional Examination Survey. Nutrients 2021, 13, 609. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.; Kim, J.; Park, S.; Kim, Y.T.; Park, O.; Oh, K. Trends in health behaviors over 20 years: Findings from the 1998–2018 Korea National Health and Nutrition Examination Survey. Epidemiol. Health 2021, 43, e2021026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Xie, S.H.; Li, W.T.; Lagergren, J. Smoking Cessation and Risk of Esophageal Cancer by Histological Type: Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2017, 109, djx115. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Cook, M.B.; Pandeya, N.; Vaughan, T.L.; Abnet, C.C.; Giffen, C.; Webb, P.M.; Murray, L.J.; Casson, A.G.; Risch, H.A.; et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol. 2012, 36, 306–316. [Google Scholar] [CrossRef]
- Prabhu, A.; Obi, K.O.; Rubenstein, J.H. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: A meta-analysis. Off. J. Am. Coll. Gastroenterol.|ACG 2014, 109, 822–827. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, J.K.; Zhang, Z.F.; Han, R.Q.; Yang, J.; Zhou, J.Y.; Wang, X.S.; Zhang, X.F.; Liu, A.M.; van’ t Veer, P.; et al. Smoking and alcohol drinking increased the risk of esophageal cancer among Chinese men but not women in a high-risk population. Cancer Causes Control 2011, 22, 649–657. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, J.M.; Wu, D.C.; Hsu, H.K.; Kao, E.L.; Huang, H.L.; Wang, T.N.; Huang, M.C.; Wu, M.T. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int. J. Cancer 2005, 113, 475–482. [Google Scholar] [CrossRef]
- Vingeliene, S.; Chan, D.S.M.; Vieira, A.R.; Polemiti, E.; Stevens, C.; Abar, L.; Navarro Rosenblatt, D.; Greenwood, D.C.; Norat, T. An update of the WCRF/AICR systematic literature review and meta-analysis on dietary and anthropometric factors and esophageal cancer risk. Ann. Oncol. 2017, 28, 2409–2419. [Google Scholar] [CrossRef]
- Vioque, J.; Barber, X.; Bolumar, F.; Porta, M.; Santibanez, M.; de la Hera, M.G.; Moreno-Osset, E.; Group, P.S. Esophageal cancer risk by type of alcohol drinking and smoking: A case-control study in Spain. BMC Cancer 2008, 8, 221. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2018; Ministry of Health and Welfare: Osong-si, Republic of Korea, 2019. [Google Scholar]
- Koyanagi, Y.N.; Matsuo, K.; Ito, H.; Wang, C.; Tamakoshi, A.; Sugawara, Y.; Tsuji, I.; Ono, A.; Tsugane, S.; Sawada, N.; et al. Body mass index and esophageal and gastric cancer: A pooled analysis of 10 population-based cohort studies in Japan. Cancer Sci. 2023, 114, 2961–2972. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.; Oki, E.; Ohgaki, K.; Sakamoto, Y.; Ito, S.; Egashira, A.; Saeki, H.; Kakeji, Y.; Morita, M.; Sakaguchi, Y.; et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: Molecular mechanisms of carcinogenesis. Int. J. Clin. Oncol. 2010, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Radojicic, J.; Zaravinos, A.; Spandidos, D.A. HPV, KRAS mutations, alcohol consumption and tobacco smoking effects on esophageal squamous-cell carcinoma carcinogenesis. Int. J. Biol. Markers 2012, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Gadekar, V.P.; Gundla, P.S.; Mandarthi, S.; Jayendra, N.; Tungekar, A.; Lavanya, B.V.; Bhagavath, A.K.; Cordero, M.A.W.; Pitkaniemi, J.; et al. Global comparative transcriptomes uncover novel and population-specific gene expression in esophageal squamous cell carcinoma. Infect. Agents Cancer 2023, 18, 47. [Google Scholar] [CrossRef]
- Yokoyama, A.; Tsutsumi, E.; Imazeki, H.; Suwa, Y.; Nakamura, C.; Mizukami, T.; Yokoyama, T. Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydrogenase-2 genotype. Alcohol. Clin. Exp. Res. 2008, 32, 1607–1614. [Google Scholar] [CrossRef]
- Chang, J.S.; Hsiao, J.R.; Chen, C.H. ALDH2 polymorphism and alcohol-related cancers in Asians: A public health perspective. J. Biomed. Sci. 2017, 24, 19. [Google Scholar] [CrossRef]
- Choi, C.K.; Yang, J.; Kweon, S.S.; Cho, S.H.; Kim, H.Y.; Myung, E.; Shin, M.H. Association between ALDH2 polymorphism and esophageal cancer risk in South Koreans: A case-control study. BMC Cancer 2021, 21, 254. [Google Scholar] [CrossRef]
- Cui, R.; Kamatani, Y.; Takahashi, A.; Usami, M.; Hosono, N.; Kawaguchi, T.; Tsunoda, T.; Kamatani, N.; Kubo, M.; Nakamura, Y.; et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology 2009, 137, 1768–1775. [Google Scholar] [CrossRef]
- Tanaka, F.; Yamamoto, K.; Suzuki, S.; Inoue, H.; Tsurumaru, M.; Kajiyama, Y.; Kato, H.; Igaki, H.; Furuta, K.; Fujita, H.; et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut 2010, 59, 1457–1464. [Google Scholar] [CrossRef]
- Mokhlesi, A.; Sharifi, Z.; Berimipour, A.; Taleahmad, S.; Talkhabi, M. Identification of hub genes and microRNAs with prognostic values in esophageal cancer by integrated analysis. Noncoding RNA Res. 2023, 8, 459–470. [Google Scholar] [CrossRef]
- Liu, J.H.; Wu, Q.F.; Fu, J.K.; Che, X.M.; Li, H.J. Obesity Potentiates Esophageal Squamous Cell Carcinoma Growth and Invasion by AMPK-YAP Pathway. J. Immunol. Res. 2020, 2020, 6765474. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Obianyo, O.; Du, Y.; Fu, H.; Li, S.; Ye, K. Blockade of Asparagine Endopeptidase Inhibits Cancer Metastasis. J. Med. Chem. 2017, 60, 7244–7255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, F.; Ma, G.; Wei, W.; Wu, N.; Liu, Z. Dysregulated ceramides metabolism by fatty acid 2-hydroxylase exposes a metabolic vulnerability to target cancer metastasis. Signal Transduct. Target. Ther. 2022, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Shao, Y.; Nakyeyune, R.; Shen, Y.; Niu, C.; Zhu, L.; Ruan, X.; Wei, T.; Wei, P.; Liu, F. Association of Body Mass Index and the Risk of Gastro-Esophageal Cancer: A Mendelian Randomization Study in a Japanese Population. Nutr. Cancer 2023, 75, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Launoy, G.; Milan, C.; Day, N.E.; Faivre, J.; Pienkowski, P.; Gignoux, M. Oesophageal cancer in France: Potential importance of hot alcoholic drinks. Int. J. Cancer 1997, 71, 917–923. [Google Scholar] [CrossRef]


| Characteristics | Total Participants | |||
|---|---|---|---|---|
| Esophageal Cancer | Control | Standardized Difference | ||
| Age (years; n, %) | 0.00 | |||
| 40–44 | 2 (0.18) | 8 (0.18) | ||
| 45–49 | 15 (1.35) | 60 (1.35) | ||
| 50–54 | 62 (5.57) | 248 (5.57) | ||
| 55–59 | 131 (11.76) | 524 (11.76) | ||
| 60–64 | 187 (16.79) | 748 (16.79) | ||
| 65–69 | 239 (21.45) | 956 (21.45) | ||
| 70–74 | 210 (18.85) | 840 (18.85) | ||
| 75–79 | 164 (14.72) | 656 (14.72) | ||
| 80–84 | 87 (7.81) | 348 (7.81) | ||
| 85+ | 17 (1.53) | 68 (1.53) | ||
| Sex (n, %) | 0.00 | |||
| Male | 1035 (92.91) | 4140 (92.91) | ||
| Female | 79 (7.09) | 316 (7.09) | ||
| Income (n, %) | 0.00 | |||
| 1 (lowest) | 190 (17.06) | 760 (17.06) | ||
| 2 | 142 (12.75) | 568 (12.75) | ||
| 3 | 195 (17.50) | 780 (17.50) | ||
| 4 | 235 (21.10) | 940 (21.10) | ||
| 5 (highest) | 352 (31.60) | 1408 (31.60) | ||
| Region of residence (n, %) | 0.00 | |||
| Urban | 420 (37.70) | 1680 (37.70) | ||
| Rural | 694 (62.30) | 2776 (62.30) | ||
| CCI score (n, %) | 1.81 | |||
| 0 | 23 (2.06) | 2174 (48.79) | ||
| 1 | 24 (2.15) | 826 (18.54) | ||
| 2 | 300 (26.93) | 534 (11.98) | ||
| 3 | 214 (19.21) | 382 (8.57) | ||
| ≥4 | 553 (49.64) | 540 (12.12) | ||
| Obesity status (BMI, kg/m2) | 0.31 | |||
| <18.5 (underweight) | 78 (7.00) | 156 (3.50) | ||
| ≥18.5 to <23 (normal) | 528 (47.40) | 1657 (37.19) | ||
| ≥23 to <25 (overweight) | 270 (24.24) | 1213 (27.22) | ||
| ≥25 to <30 (obese I) | 220 (19.75) | 1335 (29.96) | ||
| ≥30 (obese II) | 18 (1.62) | 95 (2.13) | ||
| Smoking status (n, %) | 0.28 | |||
| Non-smoker or ex-smoker | 701 (62.93) | 3370 (75.63) | ||
| Current smoker | 413 (37.07) | 1086 (24.37) | ||
| Alcohol consumption (n, %) | 0.22 | |||
| <1 time a week | 461 (41.38) | 2332 (52.33) | ||
| ≥1 time a week | 653 (58.62) | 2124 (47.67) | ||
| N of Esophageal Cancer (Exposure/Total, %) | N of Control (Exposure/Total, %) | Odd Ratios for Esophageal Cancer (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|
| Crude † | p | Adjusted †‡ | p | |||
| Smoking status | 413/1114 (37.07) | 1086/4456 (24.37) | 1.92 (1.66–2.23) | <0.001 * | 1.37 (1.15–1.63) | 0.001 * |
| Alcohol consumption | 653/1114 (58.62) | 2124/4456 (47.67) | 1.60 (1.40–1.84) | <0.001 * | 1.89 (1.60–2.23) | <0.001 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 78/1114 (7.00) | 156/4456 (3.50) | 1.59 (1.19–2.12) | 0.002 * | 1.42 (1.00–2.01) | 0.050 |
| ≥18.5 to <23 (normal) | 528/1114 (47.40) | 1657/4456 (37.19) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 270/1114 (24.24) | 1213/4456 (27.22) | 0.69 (0.59–0.82) | <0.001 * | 0.76 (0.62–0.92) | 0.004 * |
| ≥25 to <30 (obese I) | 220/1114 (19.75) | 1335/4456 (29.96) | 0.51 (0.43–0.61) | <0.001 * | 0.59 (0.48–0.72) | <0.001 * |
| ≥30 (obese II) | 18/1114 (1.62) | 95/4456 (2.13) | 0.59 (0.35–0.98) | 0.041 | 0.47 (0.26–0.85) | 0.013 * |
| Characteristics | N of Esophageal Cancer | N of Control | ORs of Esophageal Cancer | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude † | p | Adjusted †‡ | p | |
| <55 years old (n = 395) | ||||||
| Smoking | 42/79 (53.2) | 119/316 (37.7) | 2.04 (1.20–3.48) | 0.009 * | 1.40 (0.62–3.14) | 0.421 |
| Alcohol consumption | 55/79 (69.6) | 163/316 (51.6) | 2.38 (1.34–4.20) | 0.003 * | 2.56 (1.10–5.95) | 0.029 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 3/79 (3.8) | 4/316 (1.3) | 2.40 (0.51–11.42) | 0.271 | 2.04 (0.10–40.00) | 0.640 |
| ≥18.5 to <23 (normal) | 31/79 (39.2) | 102/316 (32.3) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 19/79 (24.1) | 80/316 (25.3) | 0.78 (0.41–1.47) | 0.438 | 1.79 (0.69–4.59) | 0.229 |
| ≥25 to <30 (obese I) | 25/79 (31.7) | 121/316 (38.3) | 0.67 (0.37–1.22) | 0.191 | 1.28 (0.53–3.09) | 0.586 |
| ≥30 (obese II) | 1/79 (1.3) | 9/316 (2.9) | 0.37 (0.05–3.02) | 0.353 | 0.08 (0.01–0.88) | 0.039 * |
| ≥55 years old (n = 5175) | ||||||
| Smoking | 371/1035 (35.9) | 967/4140 (23.4) | 1.92 (1.65–2.23) | <0.001 * | 1.37 (1.14–1.64) | 0.001 * |
| Alcohol consumption | 598/1035 (57.8) | 1961/4140 (47.4) | 1.56 (1.36–1.80) | <0.001 * | 1.87 (1.57–2.21) | <0.001 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 75/1035 (7.3) | 152/4140 (3.7) | 1.56 (1.16–2.09) | 0.003 * | 1.39 (0.98–1.98) | 0.066 |
| ≥18.5 to <23 (normal) | 497/1035 (48) | 1555/4140 (37.6) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 251/1035 (24.3) | 1133/4140 (27.4) | 0.69 (0.58–0.81) | <0.001 * | 0.73 (0.60–0.89) | 0.002 * |
| ≥25 to <30 (obese I) | 195/1035 (18.8) | 1214/4140 (29.3) | 0.50 (0.41–0.60) | <0.001 * | 0.56 (0.46–0.69) | <0.001 * |
| ≥30 (obese II) | 17/1035 (1.6) | 86/4140 (2.1) | 0.61 (0.36–1.04) | 0.069 | 0.55 (0.30–1.01) | 0.052 |
| Men (n = 5175) | ||||||
| Smoking | 410/1035 (39.6) | 1079/4140 (26.1) | 1.93 (1.66–2.23) | <0.001 * | 1.38 (1.16–1.65) | 0.001 * |
| Alcohol consumption | 639/1035 (61.7) | 2080/4140 (50.2) | 1.61 (1.40–1.86) | <0.001 * | 1.91 (1.61–2.26) | <0.001 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 73/1035 (7.1) | 149/4140 (3.6) | 1.56 (1.16–2.10) | 0.004 * | 1.37 (0.95–1.96) | 0.088 |
| ≥18.5 to <23 (normal) | 488/1035 (47.2) | 1535/4140 (37.1) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 248/1035 (24) | 1131/4140 (27.3) | 0.68 (0.57–0.81) | <0.001 * | 0.75 (0.62–0.92) | 0.006 * |
| ≥25 to <30 (obese I) | 210/1035 (20.3) | 1242/4140 (30) | 0.52 (0.44–0.63) | <0.001 * | 0.62 (0.50–0.77) | <0.001 * |
| ≥30 (obese II) | 16/1035 (1.6) | 83/4140 (2) | 0.60 (0.35–1.03) | 0.062 | 0.55 (0.29–1.02) | 0.058 |
| Women (n = 395) | ||||||
| Smoking | 3/79 (3.8) | 7/316 (2.2) | 1.74 (0.44–6.87) | 0.430 | 0.88 (0.12–6.52) | 0.898 |
| Alcohol consumption | 14/79 (17.7) | 44/316 (13.9) | 1.39 (0.68–2.81) | 0.365 | 1.39 (0.54–3.57) | 0.495 |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 5/79 (6.3) | 7/316 (2.2) | 2.13 (0.63–7.15) | 0.223 | 3.47 (0.75–16.14) | 0.112 |
| ≥18.5 to <23 (normal) | 40/79 (50.6) | 122/316 (38.6) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 22/79 (27.9) | 82/316 (26) | 0.81 (0.45–1.47) | 0.495 | 0.82 (0.41–1.66) | 0.584 |
| ≥25 to <30 (obese I) | 10/79 (12.7) | 93/316 (29.4) | 0.34 (0.16–0.71) | 0.004 * | 0.28 (0.12–0.65) | 0.004 * |
| ≥30 (obese II) | 2/79 (2.5) | 12/316 (3.8) | 0.52 (0.11–2.46) | 0.412 | 0.17 (0.03–1.08) | 0.060 |
| Characteristics | N of Esophageal Cancer | N of Control | ORs of Esophageal Cancer | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p | Adjusted † | p | |
| Non- or ex-smoker (n = 4071) | ||||||
| Alcohol consumption | 353/701 (50.4) | 1461/3370 (43.4) | 1.33 (1.13–1.56) | 0.001 * | 1.85 (1.52–2.26) | <0.001 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 34/701 (4.9) | 100/3370 (3.0) | 1.30 (0.87–1.96) | 0.205 | 1.12 (0.69–1.82) | 0.653 |
| ≥18.5 to <23 (normal) | 308/701 (43.9) | 1180/3370 (35.0) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 189/701 (27.0) | 950/3370 (28.2) | 0.76 (0.62–0.93) | 0.008 | 0.76 (0.60–0.95) | 0.018 * |
| ≥25 to <30 (obese I) | 156/701 (22.3) | 1074/3370 (31.9) | 0.56 (0.45–0.69) | <0.001 * | 0.55 (0.44–0.71) | <0.001 * |
| ≥30 (obese II) | 14/701 (2.0) | 66/3370 (2.0) | 0.81 (0.45–1.47) | 0.491 | 0.64 (0.32–1.27) | 0.200 |
| Current smoker (n = 1499) | ||||||
| Alcohol consumption | 300/413 (72.6) | 663/1086 (61.1) | 1.69 (1.32–2.17) | <0.001 * | 1.99 (1.47–2.69) | <0.001 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 44/413 (10.7) | 56/1086 (5.2) | 1.70 (1.11–2.61) | 0.014 * | 1.84 (1.10–3.10) | 0.020 * |
| ≥18.5 to <23 (normal) | 220/413 (53.3) | 477/1086 (43.9) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 81/413 (19.6) | 263/1086 (24.2) | 0.67 (0.50–0.90) | 0.008 * | 0.75 (0.53–1.07) | 0.112 |
| ≥25 to <30 (obese I) | 64/413 (15.5) | 261/1086 (24.0) | 0.53 (0.39–0.73) | <0.001 * | 0.66 (0.45–0.95) | 0.027 * |
| ≥30 (obese II) | 4/413 (1.0) | 29/1086 (2.7) | 0.30 (0.10–0.86) | 0.025 * | 0.21 (0.06–0.77) | 0.018 * |
| Consuming alcohol < 1 time a week (n = 2793) | ||||||
| Smoking | 113/461 (24.5) | 423/2332 (18.1) | 1.47 (1.16–1.86) | 0.002 * | 1.32 (1.00–1.75) | 0.051 |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 34/461 (7.4) | 96/2332 (4.1) | 1.53 (1.01–2.33) | 0.047 * | 1.43 (0.90–2.29) | 0.134 |
| ≥18.5 to <23 (normal) | 205/461 (44.5) | 886/2332 (38.0) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 134/461 (29.1) | 596/2332 (25.6) | 0.97 (0.76–1.24) | 0.816 | 0.96 (0.73–1.26) | 0.771 |
| ≥25 to <30 (obese I) | 83/461 (18.0) | 710/2332 (30.5) | 0.51 (0.38–0.66) | <0.001 * | 0.52 (0.39–0.71) | 0.816 |
| ≥30 (obese II) | 5/461 (1.1) | 44/2332 (1.9) | 0.49 (0.19–1.25) | 0.137 | 0.48 (0.18–1.29) | 0.143 |
| Consuming alcohol ≥ 1 time a week (n = 2777) | ||||||
| Smoking | 300/653 (45.9) | 663/2124 (31.2) | 1.87 (1.57–2.24) | <0.001 * | 1.35 (1.07–1.70) | 0.011 * |
| Obesity status (BMI, kg/m2) | ||||||
| <18.5 (underweight) | 44/653 (6.7) | 60/2124 (2.8) | 1.75 (1.16–2.64) | 0.008 * | 1.35 (0.80–2.30) | 0.265 |
| ≥18.5 to <23 (normal) | 323/653 (49.5) | 771/2124 (36.3) | 1.00 | 1.00 | ||
| ≥23 to <25 (overweight) | 136/653 (20.8) | 617/2124 (29.1) | 0.53 (0.42–0.66) | <0.001 * | 0.63 (0.48–0.83) | 0.001 * |
| ≥25 to <30 (obese I) | 137/653 (21.0) | 625/2124 (29.4) | 0.52 (0.42–0.66) | <0.001 * | 0.66 (0.50–0.86) | 0.003 * |
| ≥30 (obese II) | 13/653 (2.0) | 51/2124 (2.4) | 0.61 (0.33–1.13) | 0.118 | 0.47 (0.21–1.03) | 0.058 |
| Underweight (BMI < 18.5 kg/m2, n = 234) | ||||||
| Smoking | 44/78 (56.4) | 56/156 (35.9) | 2.31 (1.33–4.02) | 0.003 * | 2.79 (1.24–6.25) | 0.013 * |
| Alcohol consumption | 44/78 (56.4) | 60/156 (38.5) | 2.07 (1.19–3.60) | 0.010 * | 1.81 (0.86–3.80) | 0.119 |
| Normal weight (BMI ≥ 18.5 to <23 kg/m2, n = 2185) | ||||||
| Smoking | 220/528 (41.7) | 477/1657 (28.8) | 1.77 (1.44–2.17) | <0.001 * | 1.26 (0.97–1.62) | 0.081 |
| Alcohol consumption | 323/528 (61.2) | 771/1657 (46.5) | 1.81 (1.48–2.21) | <0.001 * | 2.06 (1.60–2.63) | <0.001 * |
| Overweight (BMI ≥ 23 to <25 kg/m2, n = 1483) | ||||||
| Smoking | 81/270 (30.0) | 263/1213 (21.7) | 1.55 (1.15–2.08) | 0.004 * | 1.45 (1.01–2.08) | 0.042 * |
| Alcohol consumption | 136/270 (50.4) | 617/1213 (50.9) | 0.98 (0.75–1.28) | 0.883 | 1.27 (0.92–1.75) | 0.148 |
| Obese I (BMI ≥ 25 to <30 kg/m2, n = 1555) | ||||||
| Smoking | 64/220 (29.1) | 261/1335 (19.6) | 1.69 (1.23–2.33) | 0.001 * | 1.42 (0.95–2.11) | 0.087 |
| Alcohol consumption | 137/220 (62.3) | 625/1335 (46.8) | 1.88 (1.40–2.51) | <0.001 * | 2.72 (1.88–3.92) | <0.001 * |
| Obese II (BMI ≥ 30 kg/m2, n = 113) | ||||||
| Smoking | 4/18 (22.2) | 29/95 (30.5) | 0.65 (0.20–2.15) | 0.480 | 0.65 (0.14–2.96) | 0.579 |
| Alcohol consumption | 13/18 (72.2) | 51/95 (53.7) | 2.24 (0.74–6.79) | 0.153 | 3.62 (0.82–16.04) | 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.J.; Kang, H.S.; Choi, H.G.; Kim, J.-H.; Kim, J.H.; Bang, W.J.; Hong, S.K.; Kim, N.Y.; Hong, S.; Lee, H.K. Risk for Esophageal Cancer Based on Lifestyle Factors–Smoking, Alcohol Consumption, and Body Mass Index: Insight from a South Korean Population Study in a Low-Incidence Area. J. Clin. Med. 2023, 12, 7086. https://doi.org/10.3390/jcm12227086
Kwon MJ, Kang HS, Choi HG, Kim J-H, Kim JH, Bang WJ, Hong SK, Kim NY, Hong S, Lee HK. Risk for Esophageal Cancer Based on Lifestyle Factors–Smoking, Alcohol Consumption, and Body Mass Index: Insight from a South Korean Population Study in a Low-Incidence Area. Journal of Clinical Medicine. 2023; 12(22):7086. https://doi.org/10.3390/jcm12227086
Chicago/Turabian StyleKwon, Mi Jung, Ho Suk Kang, Hyo Geun Choi, Joo-Hee Kim, Ji Hee Kim, Woo Jin Bang, Sung Kwang Hong, Nan Young Kim, Sangkyoon Hong, and Hong Kyu Lee. 2023. "Risk for Esophageal Cancer Based on Lifestyle Factors–Smoking, Alcohol Consumption, and Body Mass Index: Insight from a South Korean Population Study in a Low-Incidence Area" Journal of Clinical Medicine 12, no. 22: 7086. https://doi.org/10.3390/jcm12227086
APA StyleKwon, M. J., Kang, H. S., Choi, H. G., Kim, J.-H., Kim, J. H., Bang, W. J., Hong, S. K., Kim, N. Y., Hong, S., & Lee, H. K. (2023). Risk for Esophageal Cancer Based on Lifestyle Factors–Smoking, Alcohol Consumption, and Body Mass Index: Insight from a South Korean Population Study in a Low-Incidence Area. Journal of Clinical Medicine, 12(22), 7086. https://doi.org/10.3390/jcm12227086






