Primary Biliary Cholangitis (PBC)-Autoimmune Hepatitis (AIH) Variant Syndrome: Clinical Features, Response to Therapy and Long-Term Outcome
Abstract
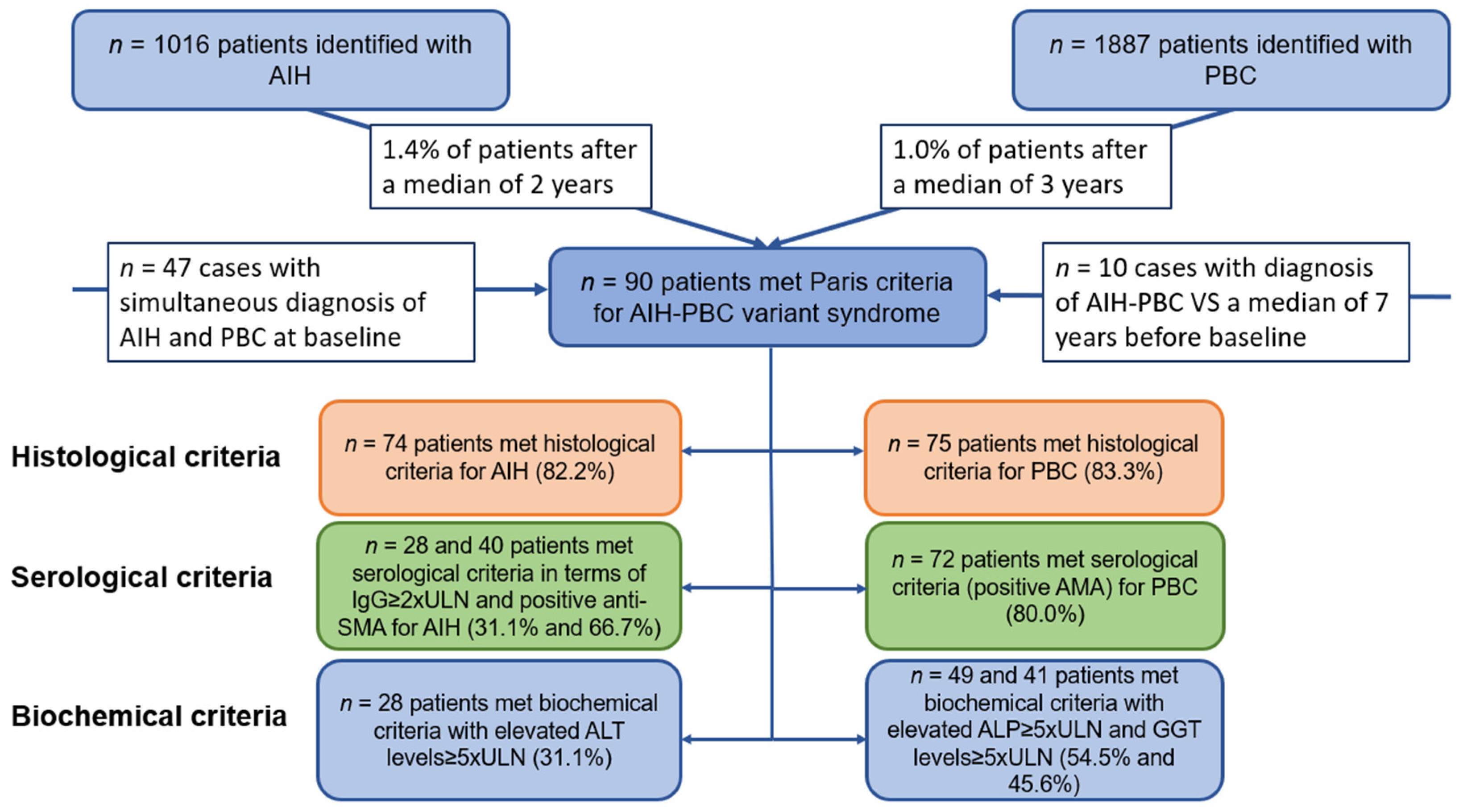
:1. Introduction
2. Materials and Methods
2.1. Study Population and Diagnostic Criteria
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline and Clinical Characteristics
3.2. Serological Findings
3.3. Response to Therapy
3.3.1. Outcome of Patients Treated with UDCA Alone (n = 21)
3.3.2. Outcome of the IS + UDCA Group (n = 63)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Material and Methods
Appendix A.1.1. Data Collection
Appendix A.1.2. Methods Applied for Testing on Autoantibodies
References
- Czaja, A.J. Frequency and nature of the variant syndromes of autoimmune liver disease. Hepatology 1998, 28, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.S.; Washington, K. Primary Biliary Cholangitis and Autoimmune Hepatitis. Surg. Pathol. Clin. 2018, 11, 329–349. [Google Scholar] [PubMed]
- Bonder, A.; Retana, A.; Winston, D.M.; Leung, J.; Kaplan, M.M. Prevalence of primary biliary cirrhosis–autoimmune hepatitis overlap syndrome. Clin. Gastroenterol. Hepatol. 2011, 9, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Poupon, R.; Chazouilleres, O.; Corpechot, C.; Chrétien, Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology 2006, 44, 85–90. [Google Scholar] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar]
- Couto, C.A.; Bittencourt, P.L.; Porta, G.; Abrantes-Lemos, C.P.; Carrilho, F.J.; Guardia, B.D.; Cançado, E.L. Antismooth muscle and antiactin antibodies are indirect markers of histological and biochemical activity of autoimmune hepatitis. Hepatology 2014, 59, 592–600. [Google Scholar]
- Kirstein, M.M.; Metzler, F.; Geiger, E.; Heinrich, E.; Hallensleben, M.; Manns, M.P.; Vogel, A. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology 2015, 62, 1524–1535. [Google Scholar]
- Park, Y.; Cho, Y.; Cho, E.J.; Kim, Y.J. Retrospective analysis of autoimmune hepatitis-primary biliary cirrhosis overlap syndrome in Korea: Characteristics, treatments, and outcomes. Clin. Mol. Hepatol. 2015, 21, 150–157. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Q.; Wang, Z.; Miao, Q.; Xiao, X.; Tang, R.; Chen, X.; Bian, Z.; Zhang, H.; Yang, Y.; et al. The Natural History and Prognosis of Primary Biliary Cirrhosis with Clinical Features of Autoimmune Hepatitis. Clin. Rev. Allergy Immunol. 2015, 50, 114–123. [Google Scholar]
- Neuhauser, M.; Bjornsson, E.; Treeprasertsuk, S.; Enders, F.; Silveira, M.; Talwalkar, J.; Lindor, K. Autoimmune hepatitis–PBC overlap syndrome: A simplified scoring system may assist in the diagnosis. Am. J. Gastroenterol. 2010, 105, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.G.; A Talwalkar, J.; Angulo, P.; Lindor, K.D. Overlap of Autoimmune Hepatitis and Primary Biliary Cirrhosis: Long-Term Outcomes. Am. J. Gastroenterol. 2007, 102, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef] [PubMed]
- Hennes, E.M.; Zeniya, M.; Czaja, A.J.; Parés, A.; Dalekos, G.N.; Krawitt, E.L.; Bittencourt, P.L.; Porta, G.; Boberg, K.M.; Hofer, H.; et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008, 48, 169–176. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis-2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Pape, S.; Snijders, R.J.; Gevers, T.J.; Chazouilleres, O.; Dalekos, G.N.; Hirschfield, G.M.; Lenzi, M.; Trauner, M.; Manns, M.P.; Vierling, J.M.; et al. Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J. Hepatol. 2022, 76, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; Hirschfield, G.M.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812. [Google Scholar] [CrossRef]
- Liu, F.; Pan, Z.G.; Ye, J.; Xu, D.; Guo, H.; Li, G.P.; Xu, K.S.; Hou, X.H.; Song, Y.H. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Simplified criteria may be effective in the diagnosis in Chinese patients. J. Dig. Dis. 2014, 15, 660–668. [Google Scholar] [CrossRef]
- Chazouillères, O.; Wendum, D.; Serfaty, L.; Montembault, S.; Rosmorduc, O.; Poupon, R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features and response to therapy. Hepatology 1998, 28, 296–301. [Google Scholar] [CrossRef]
- Joshi, S.; Cauch-Dudek, K.; Wanless, I.R.; Lindor, K.D.; Jorgensen, R.; Batts, K.; Heathcote, E.J. Primary biliary cirrhosis with additional features of autoimmune hepatitis: Response to therapy with ursodeoxycholic acid. Hepatology 2002, 35, 409–413. [Google Scholar] [CrossRef]
- Muratori, P.; Granito, A.; Pappas, G.; Pendino, G.M.; Quarneti, C.; Cicola, R.; Menichella, R.; Ferri, S.; Cassani, F.; Bianchi, F.B.; et al. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am. J. Gastroenterol. 2009, 104, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Talwalkar, J.A.; Keach, J.C.; Angulo, P.; Lindor, K.D. Overlap of autoimmune hepatitis and primary biliary cirrhosis: An evaluation of a modified scoring system. Am. J. Gastroenterol. 2002, 97, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Boberg, K.M.; Chapman, R.W.; Hirschfield, G.M.; Lohse, A.W.; Manns, M.P.; Schrumpf, E. Overlap syndromes: The International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J. Hepatol. 2011, 54, 374–385. [Google Scholar] [CrossRef] [PubMed]
- De Luca-Johnson, J.; Wangensteen, K.J.; Hanson, J.; Krawitt, E.; Wilcox, R. Natural History of Patients Presenting with Autoimmune Hepatitis and Coincident Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 2710–2720. [Google Scholar] [CrossRef]
- Lohse, A.W.; Büschenfelde, K.H.; Franz, B.; Kanzler, S.; Gerken, G.; Dienes, H.P. Characterization of the overlap syndrome of primary biliary cirrhosis (PBC) and autoimmune hepatitis: Evidence for it being a hepatitic form of PBC in genetically susceptible individuals. Hepatology 1999, 29, 1078–1084. [Google Scholar] [CrossRef]
- Chazouillères, O.; Wendum, D.; Serfaty, L.; Rosmorduc, O.; Poupon, R. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J. Hepatol. 2006, 44, 400–406. [Google Scholar] [CrossRef]
- Ozaslan, E.; Efe, C.; Heurgué-Berlot, A.; Kav, T.; Masi, C.; Purnak, T.; Muratori, L.; Ustündag, Y.; Bresson-Hadni, S.; Thiéfin, G.; et al. Factors associated with response to therapy and outcome of patients with primary biliary cirrhosis with features of autoimmune hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 863–869. [Google Scholar] [CrossRef]
- Laschtowitz, A.; Zachou, K.; Lygoura, V.; Pape, S.; Derben, F.; Jaeckel, E.; Oller-Moreno, S.; Weidemann, S.; Krech, T.; Piecha, F.; et al. Histological activity despite normal ALT and IgG serum levels in patients with autoimmunhepatitis and cirrhosis. JHEP Rep. 2021, 12, 100321. [Google Scholar] [CrossRef]
| Characteristics | AIH-PBC Cohort (n = 90) | AIH Control Cohort (n = 100) | PBC Control Cohort (n = 100) |
|---|---|---|---|
| Patient age at diagnosis (years), median (range) | 49 (19–77) | 64 (25–86) | 76 (47–96) |
| Male/female, n (%) | 10 (11)/80 (89) | 29 (29)/71 (71) | 0 (0)/100 (100) |
| Caucasian, n (%) | 67 (74) | 93 (93) | 88 (88) |
| BMI (kg/m2), median (range) | 26 (19–40) | 23 (17.7–28.3) | 22 (17.6–27.7) |
| Length of follow-up (years), median (range) | 7 (0.5–21) | 7 (0–20) | 8 (0–19) |
| Liver biochemical tests, n (%) | |||
| ALT level, ×ULN | 2.9 (1.1–55.7) | 6.1 (0.3–50.3) | 1 (0.2–11.2) |
| AST level, ×ULN | 4.2 (1.2–51.1) | 8.1 (0.5–85.0) | 1 (0.2–16.6) |
| ALP level, ×ULN | 1.4 (0.3–6.3) | 1.2 (0.3–8.0) | 1.5 (0.5–6.1) |
| GGT level, ×ULN | 3.5 (0.2–19.6) | 2.3 (0.2–18.1) | 1.5 (0.2–18.8) |
| Bilirubin level, ×ULN | 0.6 (0.2–18.6) | 0.9 (0.1–20.6) | 0.4 (0.1–5.6) |
| Albumin level, ×LLN | 0.9 (0.8–1.8) | 0.9 (0.7–1.5) | 0.8 (0.7–1.9) |
| IgM level, ×ULN | 1.3 (0–3.9) | 0.6 (0.1–1.6) | 1.2 (0.2–2.9) |
| IgG level, ×ULN | 1 (0.3–2.3) | 1.1 (0.4–2.9) | 0.7 (0.5–1.4) |
| Histological features | |||
| of AIH * | 15 (16.7) | 100 (100) | |
| of PBC ** | 16 (17.8) | ||
| of AIH and PBC | 59 (65.6) | 100 (100) | |
| Clinical manifestation of autoimmune diseases | |||
| Initial manifestation of AIH | 14 (15.6) | 100 (100) | |
| Initial manifestation of PBC | 19 (21.1) | 100 (100) | |
| Simultaneous manifestation of AIH and PBC | 47 (52.2) | ||
| n. a. | 10 (11.1) | ||
| Significant liver fibrosis, n (%) | 18 (20.0) | 8 (8.0) | 13 (13.0) |
| Liver cirrhosis, n (%) | 16 (17.8) | 5 (5.0) | 11 (11.0) |
| MELD, median (range) | 10 (5–23) | 11 (11–27) | 10 (6–20) |
| Child class A/B/C, n (%) | 9 (18.9)/6 (6.7)/1 (1.1) | 3 (3.0)/1 (1.0)/1 (1.0) | 7 (7.0)/3 (3.0)/1 (1.0) |
| GLOBE Score pretreatment, median (range) | −3.56 (−8.99–2.66) | −6.24 (−9.44–4.29) |
| Characteristics | AIH-PBC Cohort (n = 90) | AIH Control Cohort (n = 100) | PBC Control Cohort (n = 100) |
|---|---|---|---|
| ANA, n (%) | 60/84 (71.4) | 83/100 (83) | 23/95 (24.2) |
| Non-specific subtypes of ANA | |||
| Anti-Ro/SSA | 3/17 (17.6) | 4/22 (18.2) | 3/15 (15.8) |
| SSA 52-60 | 4/14 (28.6) | 5/20 (25.0) | 3/16 (18.8) |
| Anti-La/SSB | 0/14 (0) | 4/18 (22.2) | 1/17 (5.9) |
| Anti-Scl 70 | 0/16 (0) | 0/8 (0) | 0/10 (0) |
| ACA | 3/10 (30.0) | 1/21 (4.7) | 2/16 (12.5) |
| Anti-gp210 | 2/15 (13.3) | 0/12 (0) | 3/12 (25.0) |
| Anti-Sp100 | 3/14 (21.4) | 1/12 (8.3) | 4/12 (33.3) |
| Anti-CENP-A | 2/8 (25.0) | 0/13 (0) | 1/17 (5.9) |
| Anti-CENP-B | 4/12 (33.3) | 0/17 (0) | 2/17 (11.8) |
| Anti-dsDNA | 3/27 (11.1) | 6/40 (15.0) | 8/36 (22.2) |
| U1-RNP | 0/9 (0) | 0/8 (0) | 0/10 (0) |
| Sm-RNP | 0/15 (0) | 1/8 (12.5) | 0/6 (0) |
| Anti-PML | 2/14 (14.3) | 0/14 (0) | 7/15 (46.7) |
| ANCA | |||
| pANCA | 4/20 (20.0) | 3/30 (10.0) | 0/32 (0) |
| Anti-MPO | 2/13 (15.4) | 3/29 (10.3) | 0/32 (0) |
| Anti-PR 3 | 2/11 (18.2) | 3/8 (37.5) | 0/15 (0) |
| AMA | 72/90 (80.0) | 6/100 (6.0) | 91/100 (91.0) |
| AMA-M2 | 26/36 (72.2) | 1/16 (6.3) | 8/36 (22.2) |
| AMA-M2-3E | 14/20 (70.0) | 0/8 (0) | 6/14 (42.9) |
| Anti-SMA | 40/60 (66.7) | 24/71 (33.8) | 0/32 (0) |
| Anti-LKM-1 | 2/55 (3.6) | 9/63 (14.3) | 0/32 (0) |
| Anti-LC1 | 1/17 (5.9) | 0/31 (0) | 0/35 (0) |
| Anti-SLA/LP | 5/62 (8.1) | 12/68 (17.6) | 0/38 (0) |
| Anti-Jo1 | 0/17 (0) | 0/12 (0) | 0/29 (0) |
| Parameters | UDCA Responders (n = 4) | UDCA Partial Responders (n = 12) | p | UDCA Responders (n = 4) | UDCA Non-Responders (n = 5) | p |
|---|---|---|---|---|---|---|
| Female gender | 4/4 (100) | 11/12 (92) | 1.00 | 4/4 (100) | 4/5 (80) | 1.00 |
| Age | 46 (29–74) | 51 (38–69) | 0.60 | 46 (29–74) | 54 (33–72) | 0.56 |
| ALT × ULN | 1.5 (0.8–2.1) | 1.7 (1.4–9) | 0.26 | 1.5 (0.8–2.1) | 3.0 (1.5–22) | 0.09 |
| AST × ULN | 1.4 (0.7–3.2) | 2.3 (0.8–4.3) | 0.36 | 1.4 (0.7–3.2) | 3.8 (0.9–12) | 0.11 |
| ALP × ULN | 1.1 (0.5–2.8) | 1.5 (1.3–4.7) | 0.26 | 1.1 (0.5–3.1) | 1.4 (1.8–3.5) | 0.42 |
| GGT × ULN | 2.2 (0.4–4.3) | 3.0 (1.9–13) | 0.38 | 2.2 (0.4–4.3) | 3.5 (1.0–19) | 0.29 |
| IgG × ULN | 0.8 (0.6–1.1) | 0.9 (0.7–1.1) | 0.90 | 0.8 (0.6–1.1) | 1.2 (0.7–1.5) | 0.11 |
| IgM × ULN | 0.9 (0.2–3.9) | 1.3 (0.4–3.5) | 0.87 | 0.9 (0.2–3.9) | 1.4 (0.5–4.1) | 0.56 |
| ANA | 3/4 (60) | 7/10 (70) | 1.00 | 3/4 (60) | 3/5 (60) | 1.00 |
| SMA | 3/4 (75) | 7/9 (67) | 1.00 | 3/4 (75) | 3/4 (75) | 1.00 |
| AMA | 4/4 (100) | 9/12 (75) | 0.53 | 4/4 (100) | 4/5 (80) | 1.00 |
| AMA-M2 | 2/3 (67) | 4/6 (66.7) | 1.00 | 2/3 (67) | 1/2 (50) | 1.00 |
| Significant fibrosis | 1/4 (25) | 2/12 (17) | 1.00 | 1/4 (25) | 1/5 (20) | 1.00 |
| Liver cirrhosis | 2/4 (50) | 3/12 (25) | 0.55 | 2/4 (50) | 3/5 (60) | 1.00 |
| Severe histological interface hepatitis | 0/4 (0) | 4/12 (33) | 0.52 | 0/4 (0) | 3/5 (40) | 0.17 |
| Parameters | Therapy Responders (n = 37) | Partial Responders (n = 18) | p | Therapy Responders (n = 37) | Therapy Non-Responders (n = 8) | p |
|---|---|---|---|---|---|---|
| Female gender | 31/37 (84) | 16/18 (89) | 1.00 | 31/37 (84) | 6/8 (75) | 0.62 |
| Age | 51 (26–72) | 50 (26–77) | 0.82 | 51 (26–72) | 53 (18–72) | 0.61 |
| ALT × ULN | 4.6 (0.2–41.3) | 4.2 (0.3–12.6) | 0.18 | 4.6 (0.2–41.3) | 5.1 (0.4–15.9) | 0.53 |
| AST × ULN | 4.4 (0.5–36.8) | 4.1 (0.6–11) | 0.19 | 4.4 (0.5–36.8) | 5.0 (0.4–17.9) | 0.63 |
| ALP × ULN | 1.1 (0.4–5.1) | 1.6 (0.3–6.3) | 0.34 | 1.1 (0.4–5.1) | 1.2 (0.6–4.6) | 0.80 |
| GGT × ULN | 3.3 (0.3–12.1) | 3.6 (0.7–16.4) | 0.45 | 3.3 (0.3–12.1) | 2.2 (0.2–5.5) | 0.16 |
| IgG × ULN | 0.9 (0.4–1.9) | 0.8 (0.4–2.3) | 0.93 | 0.9 (0.4–1.9) | 1.1 (0.8–1.5) | 0.37 |
| IgM × ULN | 1.2 (0.4–2.6) | 1.3 (0.5–3.2) | 0.39 | 1.2 (0.4–2.6) | 1.8 (0.7–3.1) | 0.07 |
| ANA | 24/35 (69) | 12/17 (71) | 1.00 | 24/35 (69) | 6/8 (75) | 1.00 |
| SMA | 17/25 (68) | 7/11 (64) | 1.00 | 17/25 (68) | 4/6 (67) | 1.00 |
| AMA | 30/37 (81) | 14/18 (78) | 1.00 | 30/37 (81) | 6/8 (75) | 0.65 |
| AMA-M2 | 10/13 (77) | 6/8 (75) | 1.00 | 10/13 (77) | 3/4 (75) | 1.00 |
| Significant fibrosis | 11/37 (30) | 3/18 (17) | 0.35 | 11/37 (30) | 0/8 (0) | 0.17 |
| Liver cirrhosis | 5/37 (11) | 2/18 (17) | 1.00 | 5/37 (11) | 1/8 (13) | 1.00 |
| Severe histological interface hepatitis | 4/37 (11) | 6/18 (33) | 0.06 | 4/37 (11) | 4/8 (50) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, M.; Lange, C.M.; Langer, M.M.; Schattenberg, J.M.; Seessle, J.; Dietz, J.; Vermehren, A.; Michael, F.A.; Mondorf, A.; Zeuzem, S.; et al. Primary Biliary Cholangitis (PBC)-Autoimmune Hepatitis (AIH) Variant Syndrome: Clinical Features, Response to Therapy and Long-Term Outcome. J. Clin. Med. 2023, 12, 7047. https://doi.org/10.3390/jcm12227047
Graf M, Lange CM, Langer MM, Schattenberg JM, Seessle J, Dietz J, Vermehren A, Michael FA, Mondorf A, Zeuzem S, et al. Primary Biliary Cholangitis (PBC)-Autoimmune Hepatitis (AIH) Variant Syndrome: Clinical Features, Response to Therapy and Long-Term Outcome. Journal of Clinical Medicine. 2023; 12(22):7047. https://doi.org/10.3390/jcm12227047
Chicago/Turabian StyleGraf, Markus, Christian M. Lange, Mona M. Langer, Jörn M. Schattenberg, Jessica Seessle, Julia Dietz, Annika Vermehren, Florian A. Michael, Antonia Mondorf, Stefan Zeuzem, and et al. 2023. "Primary Biliary Cholangitis (PBC)-Autoimmune Hepatitis (AIH) Variant Syndrome: Clinical Features, Response to Therapy and Long-Term Outcome" Journal of Clinical Medicine 12, no. 22: 7047. https://doi.org/10.3390/jcm12227047
APA StyleGraf, M., Lange, C. M., Langer, M. M., Schattenberg, J. M., Seessle, J., Dietz, J., Vermehren, A., Michael, F. A., Mondorf, A., Zeuzem, S., Pathil, A., & Graf, C. (2023). Primary Biliary Cholangitis (PBC)-Autoimmune Hepatitis (AIH) Variant Syndrome: Clinical Features, Response to Therapy and Long-Term Outcome. Journal of Clinical Medicine, 12(22), 7047. https://doi.org/10.3390/jcm12227047






