Efficacy of Omega-3 Intake in Managing Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Guidelines
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy and Study Selection
2.4. Evaluation of Methodological Quality
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
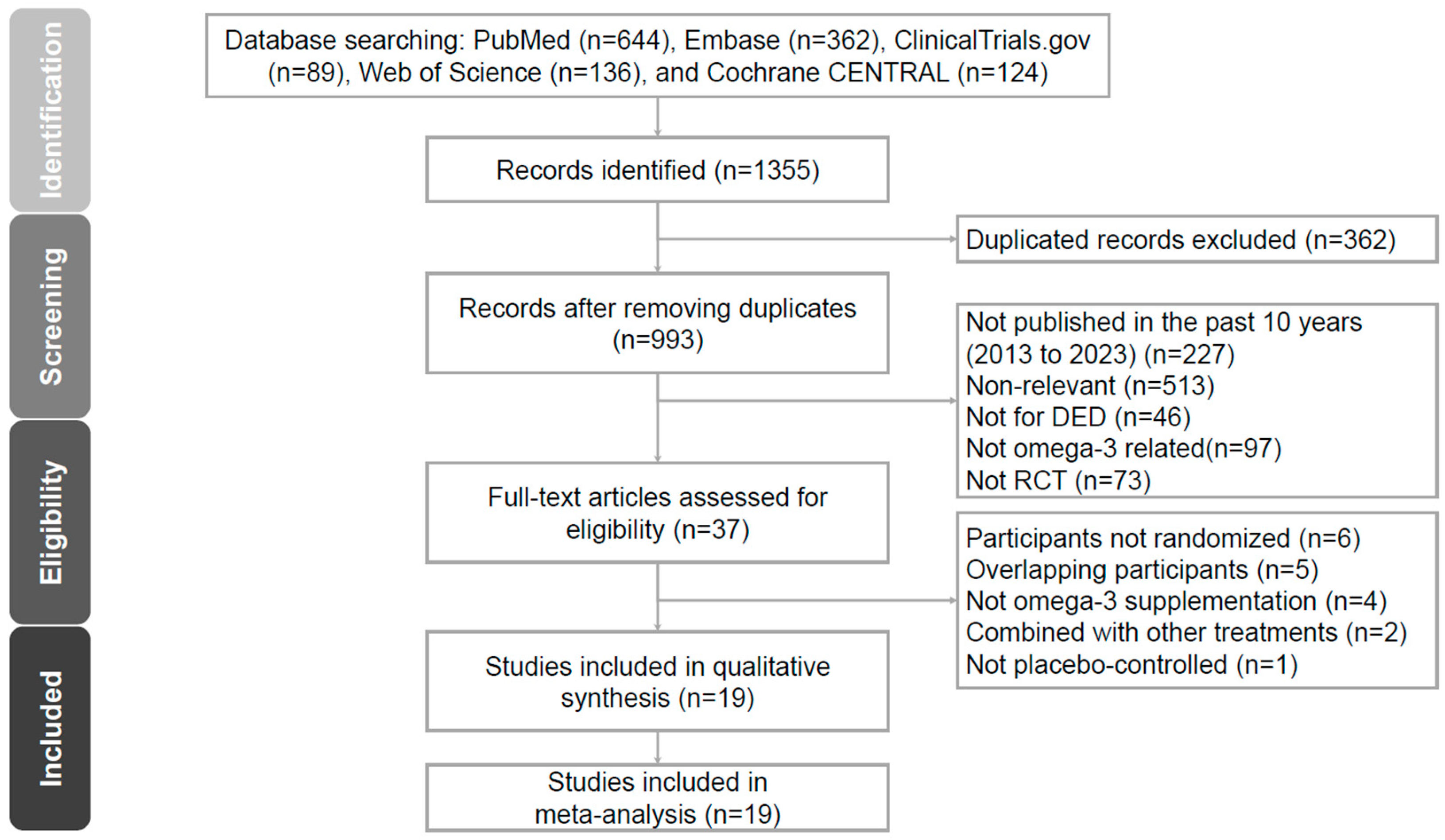
3.1. Study Selection and Characteristics
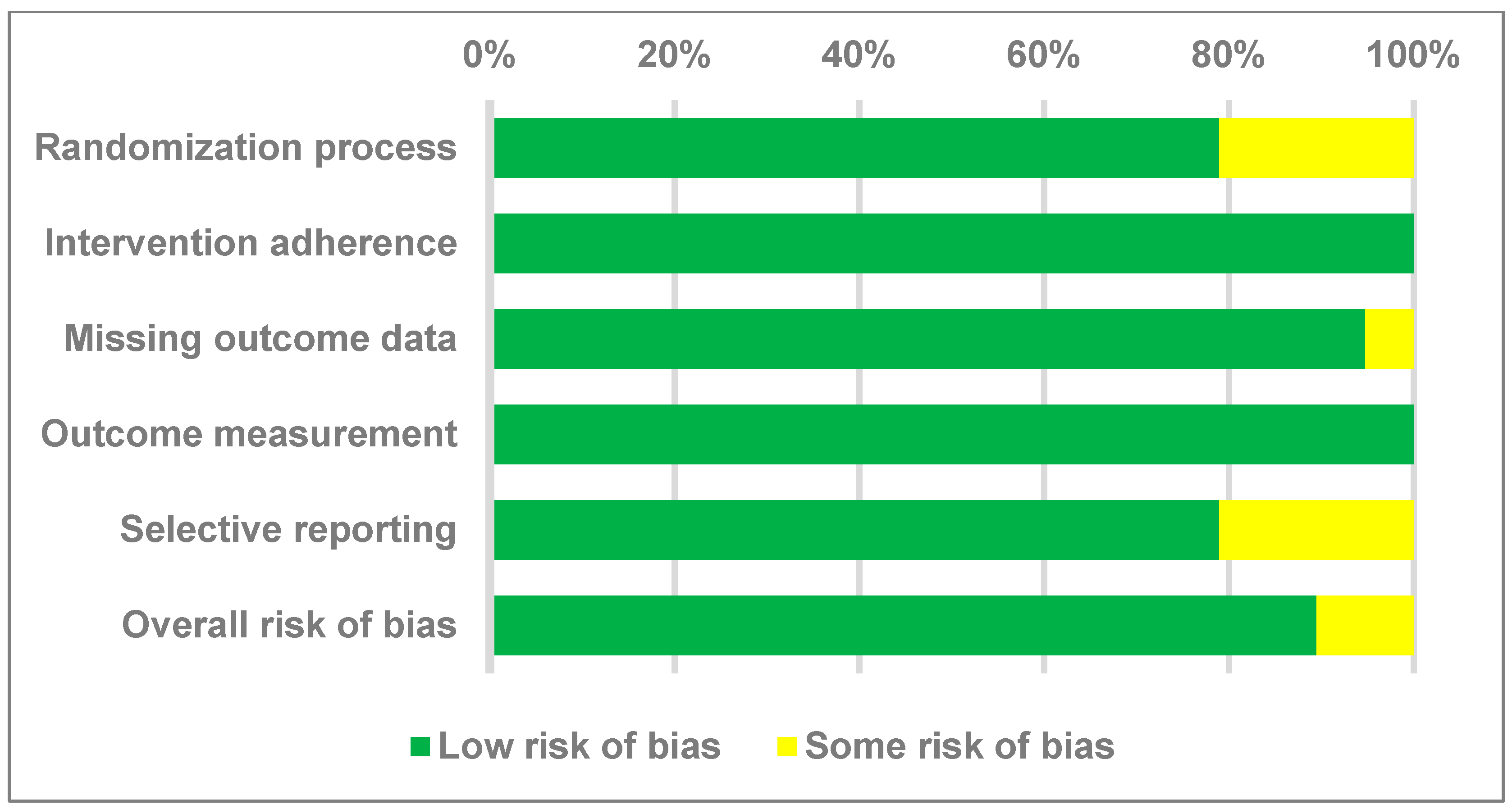
3.2. Quality Assessment of the Selected Studies
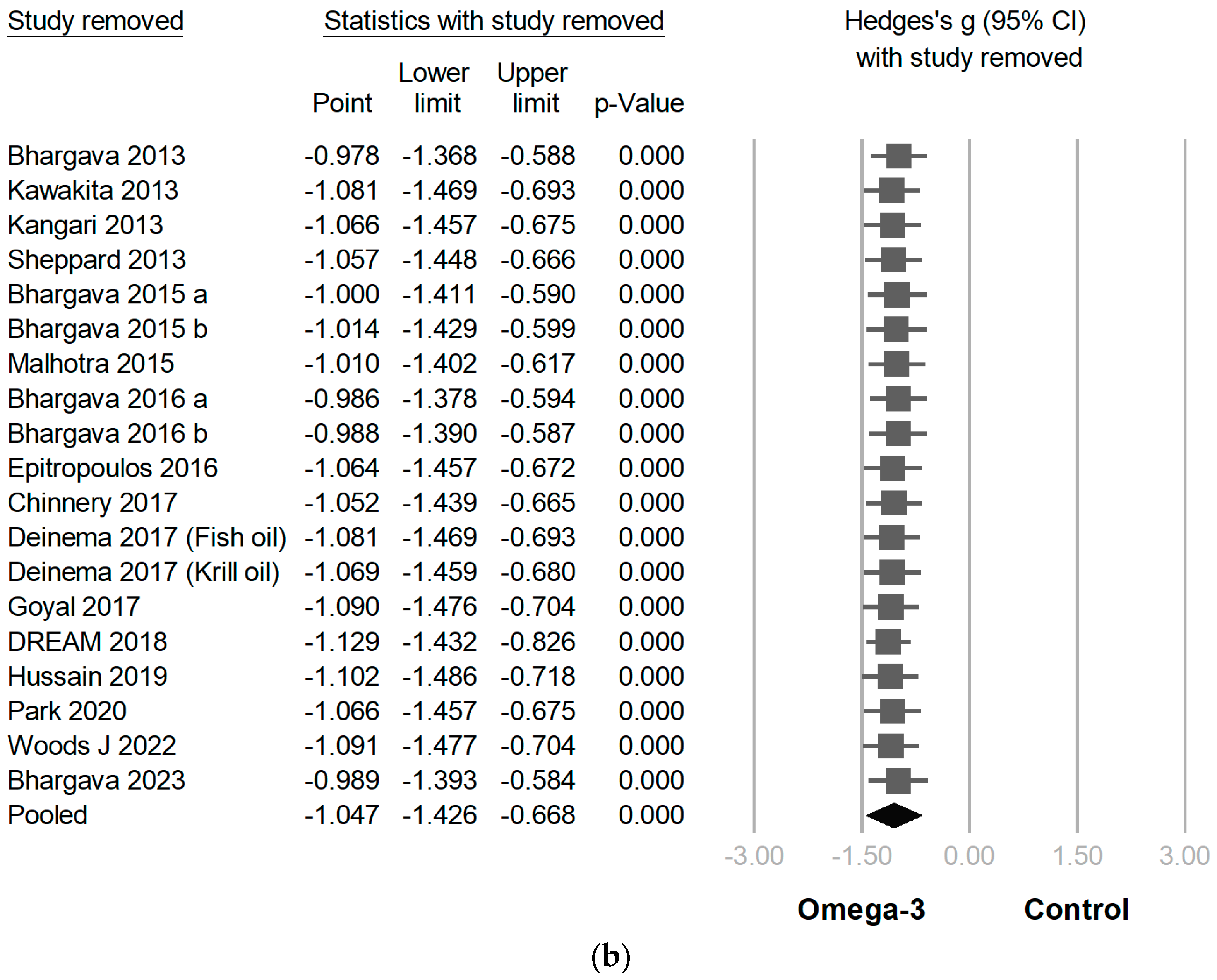
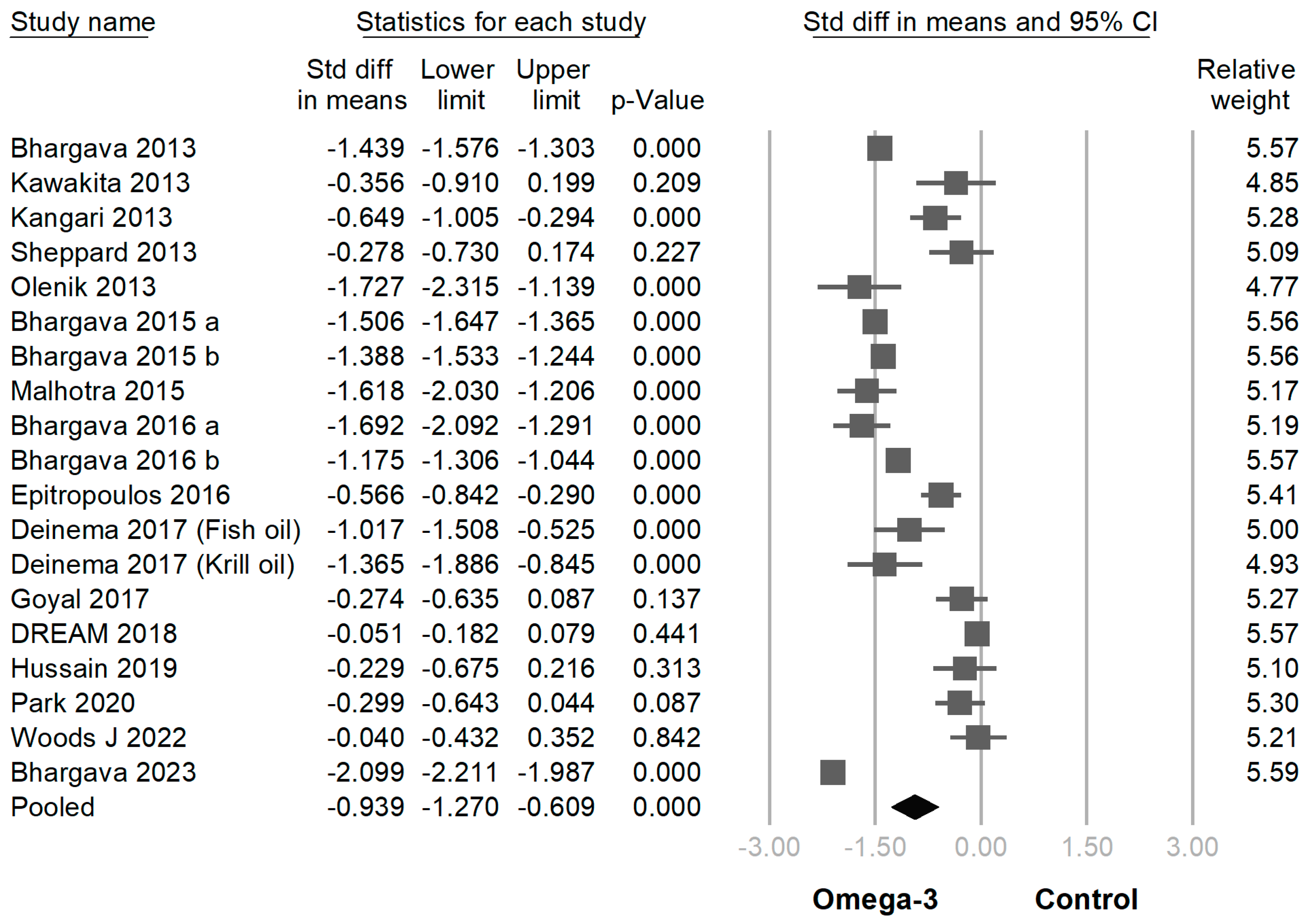
3.3. Efficacy of Omega-3 Supplementation on Different Outcomes
3.4. Publication Bias and Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P.; Kumar, M.; Mehra, N.; Mishra, A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int. J. Ophthalmol. 2013, 6, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. The pathology of dry eye: The interaction between the ocular surface and lacrimal glands. Cornea 1998, 17, 584–589. [Google Scholar] [CrossRef]
- Sacchetti, M.; Mantelli, F.; Lambiase, A.; Mastropasqua, A.; Merlo, D.; Bonini, S. Systematic review of randomised clinical trials on topical ciclosporin A for the treatment of dry eye disease. Br. J. Ophthalmol. 2014, 98, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea 2015, 34, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kangari, H.; Eftekhari, M.H.; Sardari, S.; Hashemi, H.; Salamzadeh, J.; Ghassemi-Broumand, M.; Khabazkhoob, M. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology 2013, 120, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Harris, W. Omega-6 and omega-3 fatty acids: Partners in prevention. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 125–129. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Asbell, P.A.; Maguire, M.G.; Pistilli, M.; Ying, G.S.; Szczotka-Flynn, L.B.; Hardten, D.R.; Lin, M.C.; Shtein, R.M. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.C.; Tuan, H.I.; Kang, Y.N. Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Li, T.; Deeks, J. Chapter 6: Choosing effect measures and computing estimates of effect. Version 6.2, 2021. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2021; Available online: https://training.cochrane.org/handbook/current (accessed on 16 January 2022).
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including variants on randomized trials. In Cochrane Handbook for Systematic Reviews of Interventions v6; The Cochrane Collaboration: London, UK, 2021; p. 2. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2019; pp. 241–284. [Google Scholar]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2019; pp. 349–374. [Google Scholar]
- Kawakita, T.; Kawabata, F.; Tsuji, T.; Kawashima, M.; Shimmura, S.; Tsubota, K. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: Randomized controlled trial. Biomed. Res. 2013, 34, 215–220. [Google Scholar] [CrossRef]
- Sheppard, J.D., Jr.; Singh, R.; McClellan, A.J.; Weikert, M.P.; Scoper, S.V.; Joly, T.J.; Whitley, W.O.; Kakkar, E.; Pflugfelder, S.C. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea 2013, 32, 1297–1304. [Google Scholar] [CrossRef]
- Oleñik, A.; Jiménez-Alfaro, I.; Alejandre-Alba, N.; Mahillo-Fernández, I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin. Intervig. Aging 2013, 8, 1133–1138. [Google Scholar] [CrossRef]
- Bhargava, R.; Kumar, P.; Phogat, H.; Kaur, A.; Kumar, M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Contact Lens Anterior Eye 2015, 38, 206–210. [Google Scholar] [CrossRef]
- Malhotra, C.; Singh, S.; Chakma, P.; Jain, A.K. Effect of oral omega-3 Fatty Acid supplementation on contrast sensitivity in patients with moderate meibomian gland dysfunction: A prospective placebo-controlled study. Cornea 2015, 34, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Chandra, M.; Bansal, U.; Singh, D.; Ranjan, S.; Sharma, S. A Randomized Controlled Trial of Omega 3 Fatty Acids in Rosacea Patients with Dry Eye Symptoms. Curr. Eye Res. 2016, 41, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Kumar, P.; Arora, Y. Short-Term Omega 3 Fatty Acids Treatment for Dry Eye in Young and Middle-Aged Visual Display Terminal Users. Eye Contact Lens 2016, 42, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Epitropoulos, A.T.; Donnenfeld, E.D.; Shah, Z.A.; Holland, E.J.; Gross, M.; Faulkner, W.J.; Matossian, C.; Lane, S.S.; Toyos, M.; Bucci, F.A., Jr.; et al. Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea 2016, 35, 1185–1191. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Naranjo Golborne, C.; Downie, L.E. Omega-3 supplementation is neuroprotective to corneal nerves in dry eye disease: A pilot study. Ophthalmic Physiol. Opt. 2017, 37, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Deinema, L.A.; Vingrys, A.J.; Wong, C.Y.; Jackson, D.C.; Chinnery, H.R.; Downie, L.E. A Randomized, Double-Masked, Placebo-Controlled Clinical Trial of Two Forms of Omega-3 Supplements for Treating Dry Eye Disease. Ophthalmology 2017, 124, 43–52. [Google Scholar] [CrossRef]
- Goyal, P.; Jain, A.K.; Malhotra, C. Oral Omega-3 Fatty Acid Supplementation for Laser In Situ Keratomileusis-Associated Dry Eye. Cornea 2017, 36, 169–175. [Google Scholar] [CrossRef]
- Hussain, M.; Shtein, R.M.; Pistilli, M.; Maguire, M.G.; Oydanich, M.; Asbell, P.A. The Dry Eye Assessment and Management (DREAM) extension study—A randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease. Ocul. Surf. 2020, 18, 47–55. [Google Scholar] [CrossRef]
- Park, J.; Yoo, Y.S.; Shin, E.; Han, G.; Shin, K.; Lim, D.H.; Chung, T.Y. Effects of the re-esterified triglyceride (rTG) form of omega-3 supplements on dry eye following cataract surgery. Br. J. Ophthalmol. 2021, 105, 1504–1509. [Google Scholar] [CrossRef]
- Ng, A.; Woods, J.; Jahn, T.; Jones, L.W.; Sullivan Ritter, J. Effect of a Novel Omega-3 and Omega-6 Fatty Acid Supplement on Dry Eye Disease: A 3-month Randomized Controlled Trial. Optom. Vis. Sci. 2022, 99, 67–75. [Google Scholar] [CrossRef]
- Bhargava, R.; Pandey, K.; Ranjan, S.; Mehta, B.; Malik, A. Omega-3 fatty acids supplements for dry eye—Are they effective or ineffective? Indian J. Ophthalmol. 2023, 71, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Ji, J. Omega-3 essential fatty acids therapy for dry eye syndrome: A meta-analysis of randomized controlled studies. Med. Sci. Monit. 2014, 20, 1583–1589. [Google Scholar] [CrossRef] [PubMed]

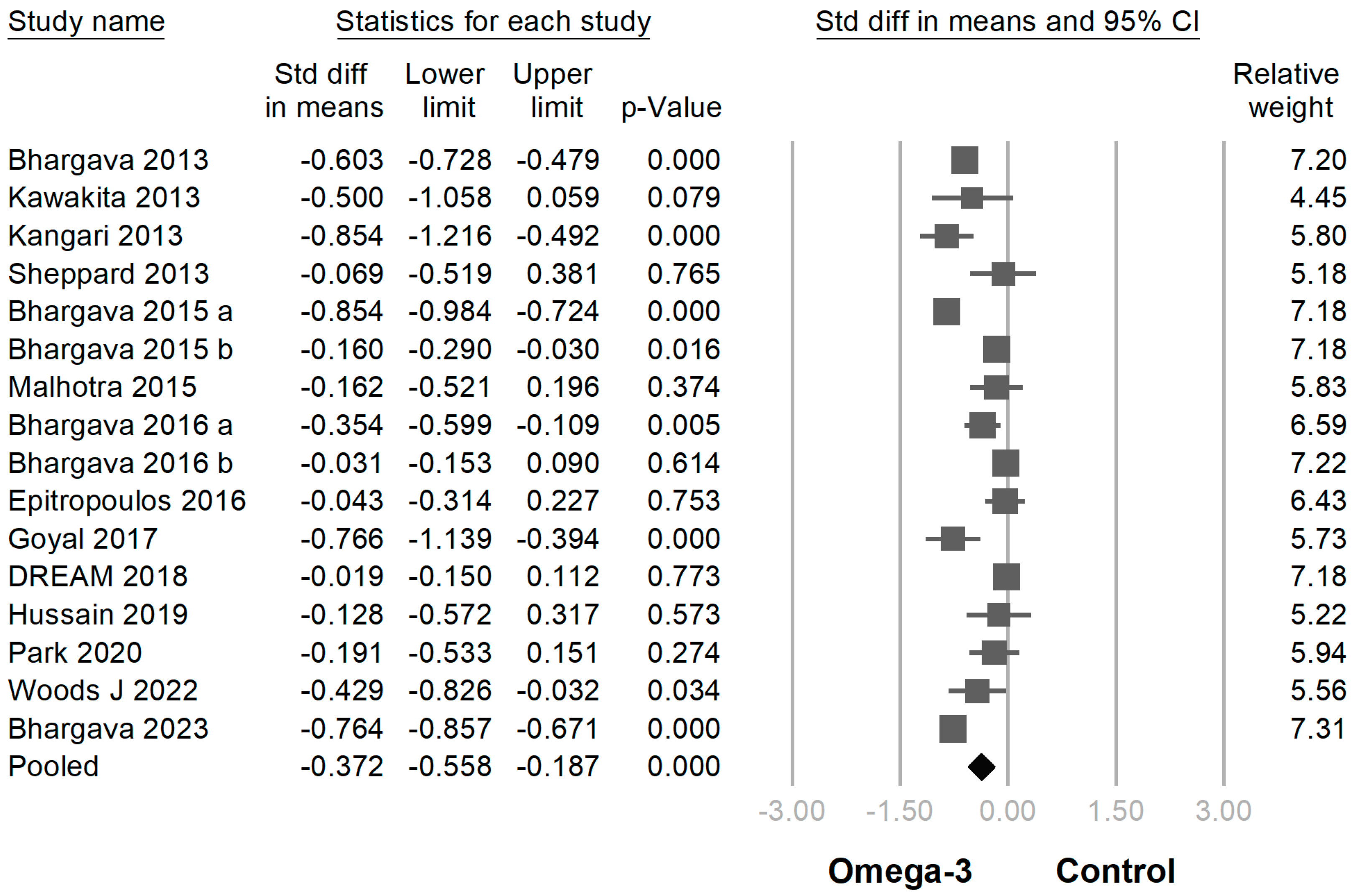
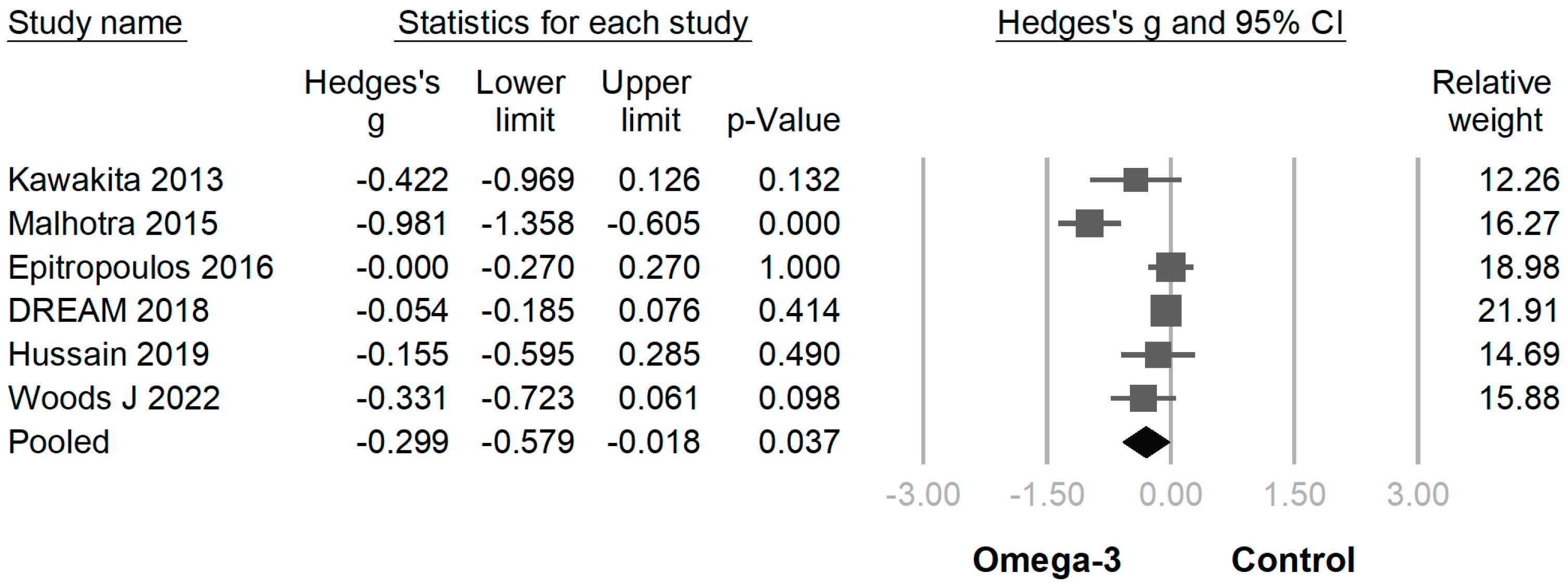
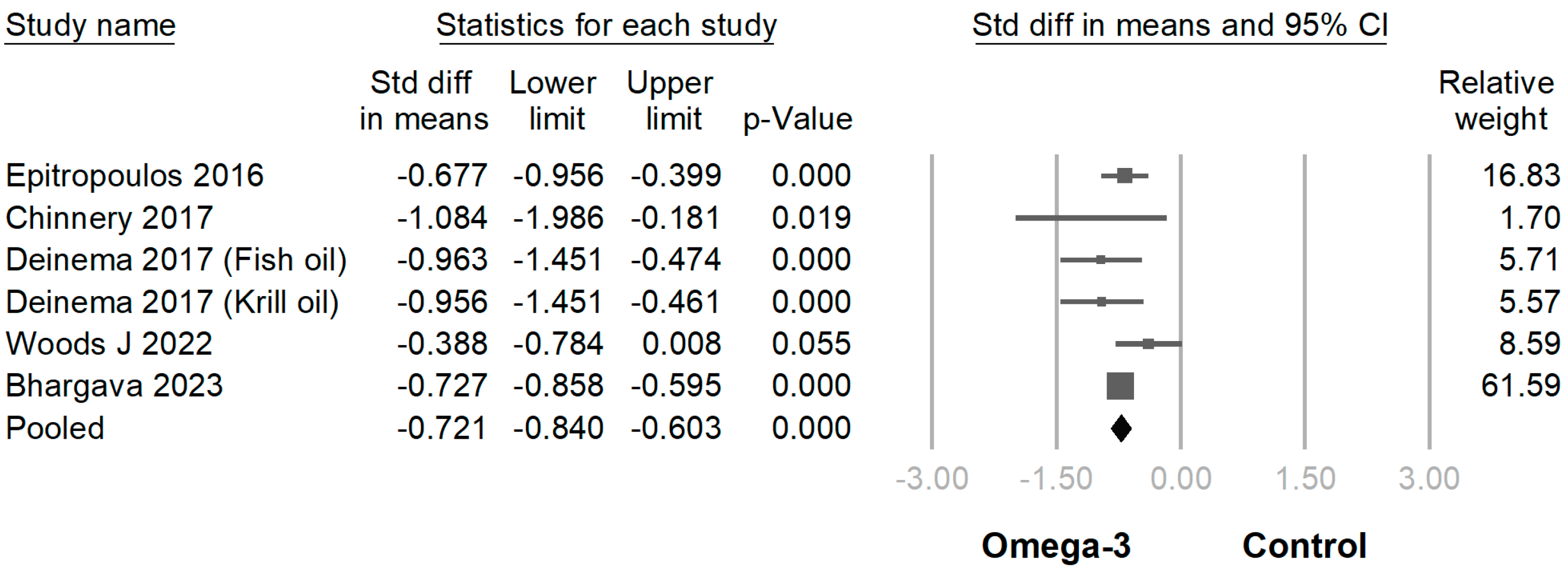
| Author (Year of Publication) | Etiology of Dry Eye Disease | Sample Size | Age | Sex (M/F) | Duration (Month) | Omega-3 Fatty Acid Daily Dose | EPA Percentage (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Omega-3 | Placebo | Omega-3 | Placebo | Omega-3 | Placebo | |||||
| Bhargava 2013 [2] | Not specified | 264 | 254 | 38.8 | 40.1 | Total: 254/268 | 3 | EPA 650 mg + DHA 350 mg | 65 | |
| Kawakita 2013 [21] | Not specified | 15 | 11 | 52.5 | 51.9 | 5/10 | 1/10 | 4 | EPA 1245 mg + DHA 540 mg | 70 |
| Kangari 2013 [6] | Not specified | 33 | 31 | 60.6 | 61.8 | 15/18 | 11/20 | 1 | EPA 360 mg + DHA 240 mg | 60 |
| Sheppard 2013 [22] | Not specified | 19 | 19 | 62 | 61 | 0/19 | 0/19 | 6 | EPA 128 mg + DHA 99 mg + other fatty acid 1185 mg | 9 |
| Oleñik 2013 [23] | MGD | 30 | 31 | 58 | 54 | 9/24 | 9/22 | 3 | EPA 127.5 mg + DHA 1050 mg | 11 |
| Bhargava 2015 a [5] | Contact lens | 240 | 256 | Not specified | Not specified | 6 | EPA 720 mg + DHA 480 mg | 60 | ||
| Bhargava 2015 b [24] | Visual display terminal users | 220 | 236 | 22.8 | 23.7 | Total: 219/237 | 3 | EPA 360 mg + DHA 240 mg | 60 | |
| Malhotra 2015 [25] | MGD | 30 | 30 | 53.3 | 53.6 | 13/17 | 19/11 | 3 | EPA 720 mg + DHA 480 mg | 60 |
| Bhargava 2016 a [26] | Rosacea | 65 | 65 | 47.7 | 48.9 | 25/40 | 27/38 | 6 | EPA 720 mg + DHA 480 mg | 60 |
| Bhargava 2016 b [27] | Visual display terminal users | 256 | 266 | 28.9 | 29.6 | Not specified | 1.5 | EPA 1440 mg + DHA 960 mg | 60 | |
| Epitropoulos 2016 [28] | MGD | 54 | 51 | 57 | 56.5 | 16/38 | 14/37 | 3 | EPA 1680 mg + DHA 560 mg | 75 |
| Chinnery 2017 [29] | Not specified | 8 | 4 | 42 | 46 | 2/6 | 1/3 | 3 | EPA 1000 mg + DHA 500 mg | 67 |
| Deinema 2017 [30] | Not specified | 37 | 17 | 40.8 | 46.2 | 15/22 | 3/14 | 3 | Fish oil (EPA 1000 mg + DHA 500 mg) | 67 |
| Krill oil (EPA 945 mg + DHA 510 mg) | 65 | |||||||||
| Goyal 2017 [31] | LASIK-associated | 30 | 30 | 23.6 | 23.6 | Total: 27/33 | 3 | EPA 720 mg + DHA 480 mg | 60 | |
| DREAM 2018 [9] | Not specified | 349 | 186 | 58.3 | 57.5 | 65/284 | 36/150 | 12 | EPA 2000 mg + DHA 1000 mg | 67 |
| Hussain 2019 [32] | Not specified | 22 | 21 | 58.2 | 58.4 | 3/19 | 4/17 | 12 | EPA 2000 mg + DHA 1000 mg | 67 |
| Park 2020 [33] | Cataract surgery-associated | 32 | 34 | 64.6 | 66.3 | 12/20 | 12/22 | 2 | EPA 1680 mg + DHA 560 mg | 75 |
| Woods J 2022 [34] | Not specified | 24 | 26 | 32 | 35 | 3/21 | 11/15 | 3 | EPA 1200 mg + DHA 300 mg | 80 |
| Bhargava 2023 [35] | Visual display terminal users | 470 | 480 | 6.5 | 25.8 | 225/255 | 242/238 | 6 | EPA 1440 mg + DHA 960 mg | 60 |
| Outcome | Publication Bias | Meta-Regression by Duration (Month) | Meta-Regression by Omega-3 Daily Dose (mg) | Meta-Regression by EPA Percentage | |||
|---|---|---|---|---|---|---|---|
| Egger’s Test p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Score of Dry Eye Symptoms | 0.101 | −0.1399 | 0.021 | −0.0005 | 0.002 | −0.0154 | <0.001 |
| TBUT | 0.169 | −0.1297 | 0.009 | −0.0004 | 0.001 | −0.0138 | <0.001 |
| Schirmer Test | 0.513 | −0.0476 | 0.018 | −0.0002 | 0.015 | −0.0058 | <0.001 |
| CFS | 0.205 | −0.0224 | 0.337 | −0.0001 | 0.205 | −0.004 | 0.05 |
| Osmolarity | 0.629 | −0.1799 | <0.001 | −0.0004 | <0.001 | −0.0104 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-X.; Ko, M.-L. Efficacy of Omega-3 Intake in Managing Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 7026. https://doi.org/10.3390/jcm12227026
Wang W-X, Ko M-L. Efficacy of Omega-3 Intake in Managing Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2023; 12(22):7026. https://doi.org/10.3390/jcm12227026
Chicago/Turabian StyleWang, Wei-Xiang, and Mei-Lan Ko. 2023. "Efficacy of Omega-3 Intake in Managing Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 12, no. 22: 7026. https://doi.org/10.3390/jcm12227026
APA StyleWang, W.-X., & Ko, M.-L. (2023). Efficacy of Omega-3 Intake in Managing Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 12(22), 7026. https://doi.org/10.3390/jcm12227026






