The Benefits and Imperative of Venous Thromboembolism Risk Screening for Hospitalized Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Risk of Bias Assessment
2.4. Data Extraction
2.5. Data Analysis
- Narrative synthesis: The data from the included studies will be qualitatively synthesized through a narrative approach. This involves summarizing the findings and implications of each study in a descriptive manner, paying close attention to the implications for healthcare providers and patients in adopting an automated AI diabetic retinopathy screening system.
- Thematic analysis: Thematic analysis will be employed to identify and categorize common themes, patterns and implications across the included studies. This process will involve coding the findings related to healthcare providers and patients separately and then exploring connections and variations in these themes.
3. Results
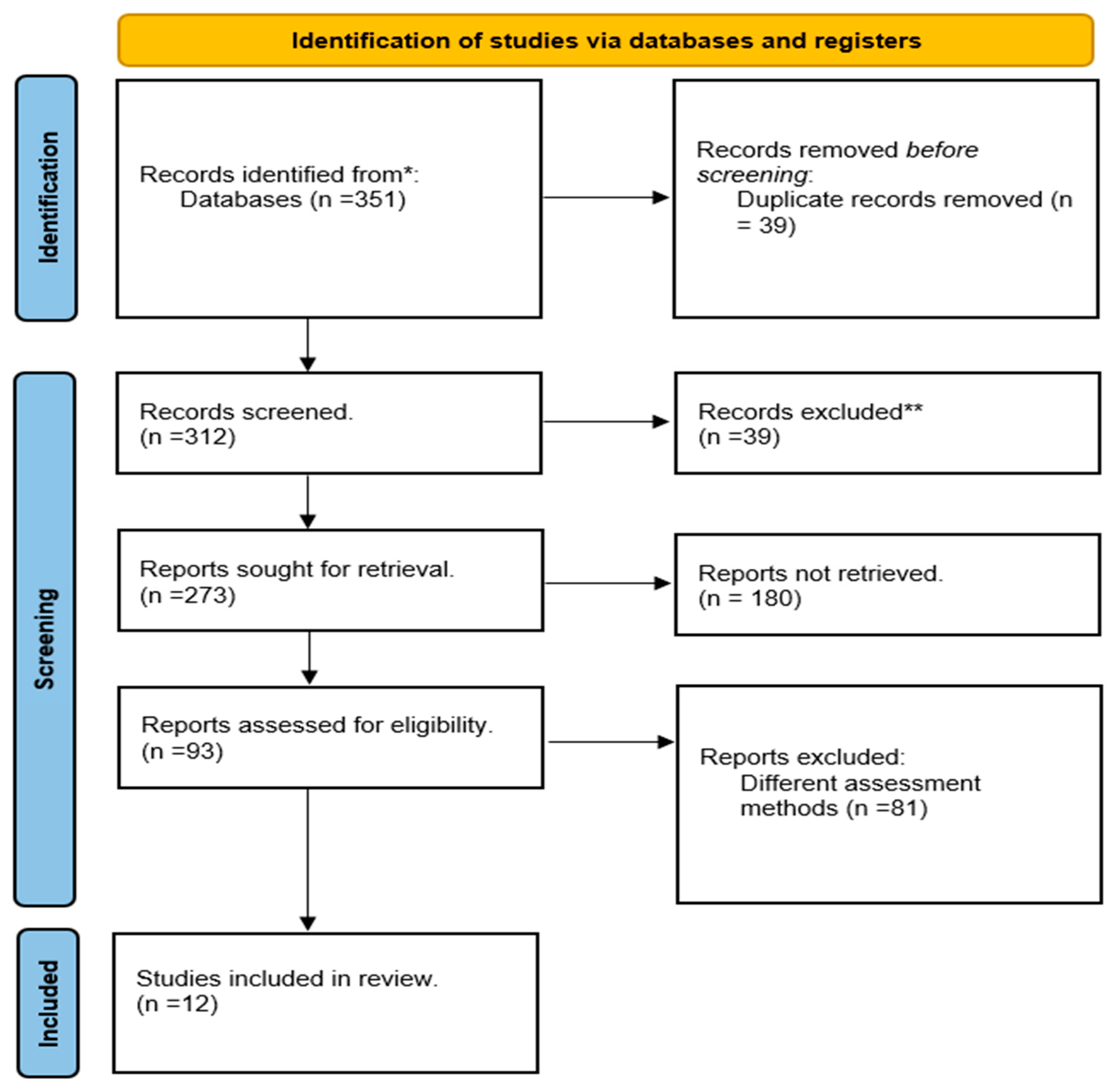
3.1. Study Selection Process
3.2. The Quality Assessment
3.3. Extraction Results
4. Discussion
4.1. Reducing Preventable Harm from Hospital-Associated VTE
4.2. Cost-Effectiveness of Targeted Thromboprophylaxis
4.3. Boosting Guideline Concordance through Standardized Approaches
4.4. Limitations of Current Risk Prediction Models
4.5. The Need for Individualized Approaches
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stone, J.; Hangge, P.; Albadawi, H.; Wallace, A.; Shamoun, F.; Knuttien, M.G.; Naidu, S.; Oklu, R. Deep Vein Thrombosis: Pathogenesis, Diagnosis, and Medical Management. Cardiovasc. Diagn. Ther. 2017, 7, S276–S284. [Google Scholar] [CrossRef]
- Ambra, N.; Mohammad, O.H.; Naushad, V.A.; Purayil, N.K.; Mohamedali, M.G.; Elzouki, A.N.; Khalid, M.K.; Illahi, M.N.; Palol, A.; Barman, M.; et al. Venous Thromboembolism Among Hospitalized Patients: Incidence and Adequacy of Thromboprophylaxis—A Retrospective Study. Vasc. Health Risk Manag. 2022, 18, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.G.; Sheaff, R.; Child, S.; Boiko, O.; Ukoumunne, O.C.; Nokes, T.; Copplestone, A.; Gericke, C.A. The Implementation of Nice Guidance on Venous Thromboembolism Risk Assessment and Prophylaxis: A before-after Observational Study to Assess the Impact on Patient Safety across Four Hospitals in England. BMC Health Serv. Res. 2013, 13, 203. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Zakai, N.A. Epidemiology and Prevention of Venous Thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Laryea, J.; Champagne, B. Venous Thromboembolism Prophylaxis. Clin. Colon Rectal Surg. 2013, 26, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.-P.; Schernthaner, G.H.; Lang, I.M. Chronic Complications of Venous Thromboembolism. J. Thromb. Haemost. 2017, 15, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, J.; Billmyer, E.; Mu, F.; Yarur, A.; Zichlin, M.L.; Yang, H.; Downes, N.; Azimi, N.; Strand, V. The Economic Burden of Thromboembolic Events Among Patients with Immune-Mediated Diseases. Adv. Ther. 2022, 39, 767–778. [Google Scholar] [CrossRef]
- Martini, W.Z. Coagulation Complications Following Trauma. Mil. Med. Res. 2016, 3, 35. [Google Scholar] [CrossRef]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef]
- Turetz, M.; Sideris, A.; Friedman, O.; Triphathi, N.; Horowitz, J. Epidemiology, Pathophysiology, and Natural History of Pulmonary Embolism. Semin. Intervent. Radiol. 2018, 35, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Bell, L.N.; Mazza, J.; Lee, A.; Yale, S.H. Variation in Definitions of Immobility in Pharmacological Thromboprophylaxis Clinical Trials in Medical Inpatients: A Systematic Review. Clin. Appl. Thromb. 2018, 24, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A.; Spencer, F.A. Risk Factors for Venous Thromboembolism. Circulation 2003, 107, I-9–I-16. [Google Scholar] [CrossRef]
- Cionac Florescu, S.; Anastase, D.-M.; Munteanu, A.-M.; Stoica, I.C.; Antonescu, D. Venous Thromboembolism Following Major Orthopedic Surgery. Maedica 2013, 8, 189–194. [Google Scholar] [PubMed]
- Heit, J.A. Epidemiology of Venous Thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Spencer, F.A.; White, R.H. The Epidemiology of Venous Thromboembolism. J. Thromb. Thrombolysis 2016, 41, 3–14. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-Associated Thrombosis: The When, How and Why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Schneeweiss, S.; Liu, J.; Solomon, D.H. Risk of Venous Thromboembolism in Patients with Rheumatoid Arthritis. Arthritis Care Res. 2013, 65, 1600–1607. [Google Scholar] [CrossRef]
- O’Donnell, M.; Weitz, J.I. Thromboprophylaxis in Surgical Patients. Can. J. Surg. 2003, 46, 129–135. [Google Scholar]
- Jones, A.; Al-Horani, R.A. Venous Thromboembolism Prophylaxis in Major Orthopedic Surgeries and Factor XIa Inhibitors. Med. Sci. 2023, 11, 49. [Google Scholar] [CrossRef]
- Rader, C.P.; Kramer, C.; König, A.; Hendrich, C.; Eulert, J. Low-Molecular-Weight Heparin and Partial Thromboplastin Time-Adjusted Unfractionated Heparin in Thromboprophylaxis after Total Knee and Total Hip Arthroplasty. J. Arthroplast. 1998, 13, 180–185. [Google Scholar] [CrossRef]
- Henke, P.K.; Pannucci, C.J. Venous Thromboembolism Risk Factor Assessment and Prophylaxis. Phlebol. J. Venous Dis. 2010, 25, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Koonarat, A.; Rattarittamrong, E.; Tantiworawit, A.; Rattanathammethee, T.; Hantrakool, S.; Chai-adisaksopha, C.; Norasetthada, L. Clinical Characteristics, Risk Factors, and Outcomes of Usual and Unusual Site Venous Thromboembolism. Blood Coagul. Fibrinolysis 2018, 29, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Choffat, D.; Farhoumand, P.D.; Jaccard, E.; de la Harpe, R.; Kraege, V.; Benmachiche, M.; Gerber, C.; Leuzinger, S.; Podmore, C.; Truong, M.K.; et al. Risk Stratification for Hospital-Acquired Venous Thromboembolism in Medical Patients (RISE): Protocol for a Prospective Cohort Study. PLoS ONE 2022, 17, e0268833. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, C.J.; Laird, S.; Dimick, J.B.; Campbell, D.A.; Henke, P.K. A Validated Risk Model to Predict 90-Day VTE Events in Postsurgical Patients. Chest 2014, 145, 567–573. [Google Scholar] [CrossRef]
- White, A.J.; Kanapathy, M.; Nikkhah, D.; Akhavani, M. Systematic Review of the Venous Thromboembolism Risk Assessment Models Used in Aesthetic Plastic Surgery. JPRAS Open 2021, 30, 116–127. [Google Scholar] [CrossRef]
- Barbar, S.; Prandoni, P. Scoring Systems for Estimating Risk of Venous Thromboembolism in Hospitalized Medical Patients. Semin. Thromb. Hemost. 2017, 43, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Cushman, M.; Burnett, A.E.; Kahn, S.R.; Beyer-Westendorf, J.; Spencer, F.A.; Rezende, S.M.; Zakai, N.A.; Bauer, K.A.; Dentali, F.; et al. American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Prophylaxis for Hospitalized and Nonhospitalized Medical Patients. Blood Adv. 2018, 2, 3198–3225. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Elmasry, Y.; van Poelgeest, E.; Welsh, T.J. Anticoagulant Use in Older Persons at Risk for Falls: Therapeutic Dilemmas—A Clinical Review. Eur. Geriatr. Med. 2023, 14, 683–696. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Chandler, J.; McKenzie, J.; Boutron, I.; Welch, V. (Eds.) Cochrane Methods 2016. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD201601/full (accessed on 7 October 2023).
- Rosenberg, D.; Eichorn, A.; Alarcon, M.; McCullagh, L.; McGinn, T.; Spyropoulos, A.C. External Validation of the Risk Assessment Model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for Medical Patients in a Tertiary Health System. J. Am. Heart Assoc. 2014, 3, e001152. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.J.; Greene, M.T.; Chopra, V.; Bernstein, S.J.; Hofer, T.P.; Flanders, S.A. Assessing the Caprini Score for Risk Assessment of Venous Thromboembolism in Hospitalized Medical Patients. Am. J. Med. 2016, 129, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Moumneh, T.; Riou, J.; Douillet, D.; Henni, S.; Mottier, D.; Tritschler, T.; Le Gal, G.; Roy, P. Validation of Risk Assessment Models Predicting Venous Thromboembolism in Acutely Ill Medical Inpatients: A Cohort Study. J. Thromb. Haemost. 2020, 18, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.H.; Edward, L. Venous Thromboembolism Prophylaxis in Hospitalized Elderly Patients: Time to Consider a MUST Strategy. J. Geriatr. Cardiol. 2011, 8, 114–120. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Li, X.; Wang, L.; Wang, M.; Xiao, J.; Yi, Q. Assessment of the Risk of Venous Thromboembolism in Medical Inpatients Using the Padua Prediction Score and Caprini Risk Assessment Model. J. Atheroscler. Thromb. 2018, 25, 1091–1104. [Google Scholar] [CrossRef]
- Mahlab-Guri, K.; Otman, M.S.; Replianski, N.; Rosenberg-Bezalel, S.; Rabinovich, I.; Sthoeger, Z. Venous Thromboembolism Prophylaxis in Patients Hospitalized in Medical Wards. Medicine 2020, 99, e19127. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Mi, J.; Wang, X.; Zou, Y.; Chen, X.; Nie, Z.; Luo, X.; Gan, R. The Cumulative Venous Thromboembolism Incidence and Risk Factors in Intensive Care Patients Receiving the Guideline-Recommended Thromboprophylaxis. Medicine 2019, 98, e15833. [Google Scholar] [CrossRef]
- Modi, S.; Deisler, R.; Gozel, K.; Reicks, P.; Irwin, E.; Brunsvold, M.; Banton, K.; Beilman, G.J. Wells Criteria for DVT Is a Reliable Clinical Tool to Assess the Risk of Deep Venous Thrombosis in Trauma Patients. World J. Emerg. Surg. 2016, 11, 24. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, A.; Lu, Y.; Huang, W. Deep Vein Thrombosis and Validation of the Caprini Risk Assessment Model in Chinese Orthopaedic Trauma Patients: A Multi-Center Retrospective Cohort Study Enrolling 34,893 Patients. Eur. J. Trauma Emerg. Surg. 2023, 49, 1863–1871. [Google Scholar] [CrossRef]
- Abukhalil, A.D.; Nasser, A.; Khader, H.; Albandak, M.; Madia, R.; Al-Shami, N.; Naseef, H.A. VTE Prophylaxis Therapy: Clinical Practice vs Clinical Guidelines. Vasc. Health Risk Manag. 2022, 18, 701–710. [Google Scholar] [CrossRef]
- Depietri, L.; Marietta, M.; Scarlini, S.; Marcacci, M.; Corradini, E.; Pietrangelo, A.; Ventura, P. Clinical Impact of Application of Risk Assessment Models (Padua Prediction Score and Improve Bleeding Score) on Venous Thromboembolism, Major Hemorrhage and Health Expenditure Associated with Pharmacologic VTE Prophylaxis: A “Real Life” Prospective and Re. Intern. Emerg. Med. 2018, 13, 527–534. [Google Scholar] [CrossRef]
- Silveira, P.C.; Ip, I.K.; Goldhaber, S.Z.; Piazza, G.; Benson, C.B.; Khorasani, R. Performance of Wells Score for Deep Vein Thrombosis in the Inpatient Setting. JAMA Intern. Med. 2015, 175, 1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Y.; Liu, Q.; Dong, F.; Pang, W.; Zhe, K.; Wan, J.; Xie, W.; Wang, W.; Yang, P.; et al. Patient-Completed Caprini Risk Score for Venous Thromboembolism Risk Assessment: Developed and Validated from 1017 Medical and Surgical Patients. TH Open 2022, 06, e184–e193. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Y.; Cheng, Y.; Du, H.; Sun, J.; Wang, Y.; Xu, M.; Guo, X. Comparison of VTE Risk Scores in Guidelines for VTE Diagnosis in Nonsurgical Hospitalized Patients with Suspected VTE. Thromb. J. 2023, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liang, Z.; Xie, J.; Ye, G.; Guan, P.; Huang, Y.; Li, X. Development and Validation of Machine Learning Models for Postoperative Venous Thromboembolism Prediction in Colorectal Cancer Inpatients: A Retrospective Study. J. Gastrointest. Oncol. 2023, 14, 220–232. [Google Scholar] [CrossRef]
- Henke, P.K.; Kahn, S.R.; Pannucci, C.J.; Secemksy, E.A.; Evans, N.S.; Khorana, A.A.; Creager, M.A.; Pradhan, A.D. Call to Action to Prevent Venous Thromboembolism in Hospitalized Patients: A Policy Statement from the American Heart Association. Circulation 2020, 141, e914–e931. [Google Scholar] [CrossRef]
- Bĕlohlávek, J.; Dytrych, V.; Linhart, A. Pulmonary Embolism, Part I: Epidemiology, Risk Factors and Risk Stratification, Pathophysiology, Clinical Presentation, Diagnosis and Nonthrombotic Pulmonary Embolism. Exp. Clin. Cardiol. 2013, 18, 129–138. [Google Scholar] [PubMed]
- Nicholson, M.; Chan, N.; Bhagirath, V.; Ginsberg, J. Prevention of Venous Thromboembolism in 2020 and Beyond. J. Clin. Med. 2020, 9, 2467. [Google Scholar] [CrossRef]
- Roberts, L.N.; Porter, G.; Barker, R.D.; Yorke, R.; Bonner, L.; Patel, R.K.; Arya, R. Comprehensive VTE Prevention Program Incorporating Mandatory Risk Assessment Reduces the Incidence of Hospital-Associated Thrombosis. Chest 2013, 144, 1276–1281. [Google Scholar] [CrossRef]
- Lau, B.D.; Haut, E.R. Practices to Prevent Venous Thromboembolism: A Brief Review. BMJ Qual. Saf. 2014, 23, 187–195. [Google Scholar] [CrossRef]
- Wilson, S.; Chen, X.; Cronin, M.; Dengler, N.; Enker, P.; Krauss, E.S.; Laberko, L.; Lobastov, K.; Obi, A.T.; Powell, C.A.; et al. Thrombosis Prophylaxis in Surgical Patients Using the Caprini Risk Score. Curr. Probl. Surg. 2022, 59, 101221. [Google Scholar] [CrossRef] [PubMed]
- Lobastov, K.; Barinov, V.; Schastlivtsev, I.; Laberko, L.; Rodoman, G.; Boyarintsev, V. Validation of the Caprini Risk Assessment Model for Venous Thromboembolism in High-Risk Surgical Patients in the Background of Standard Prophylaxis. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Clapham, R.E.; Roberts, L.N. A Systematic Approach to Venous Thromboembolism Prevention: A Focus on UK Experience. Res. Pract. Thromb. Haemost. 2023, 7, 100030. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Morrison, D.R.; Diendéré, G.; Piché, A.; Filion, K.B.; Klil-Drori, A.J.; Douketis, J.D.; Emed, J.; Roussin, A.; Tagalakis, V.; et al. Interventions for Implementation of Thromboprophylaxis in Hospitalized Patients at Risk for Venous Thromboembolism. Cochrane Database Syst. Rev. 2018, 2018, CD008201. [Google Scholar] [CrossRef]
- Grosse, S.D.; Nelson, R.E.; Nyarko, K.A.; Richardson, L.C.; Raskob, G.E. The Economic Burden of Incident Venous Thromboembolism in the United States: A Review of Estimated Attributable Healthcare Costs. Thromb. Res. 2016, 137, 3–10. [Google Scholar] [CrossRef]
- Trocio, J.; Rosen, V.M.; Gupta, A.; Dina, O.; Vo, L.; Hlavacek, P.; Rosenblatt, L. Systematic Literature Review of Treatment Patterns for Venous Thromboembolism Patients during Transitions from Inpatient to Post-Discharge Settings. Clin. Outcomes Res. 2018, 11, 23–49. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Lin, J. Direct Medical Costs of Venous Thromboembolism and Subsequent Hospital Readmission Rates: An Administrative Claims Analysis From 30 Managed Care Organizations. J. Manag. Care Pharm. 2007, 13, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Catterick, D.; Hunt, B.J. Impact of the National Venous Thromboembolism Risk Assessment Tool in Secondary Care in England. Blood Coagul. Fibrinolysis 2014, 25, 571–576. [Google Scholar] [CrossRef]
- Abboud, J.; Abdel Rahman, A.; Kahale, L.; Dempster, M.; Adair, P. Prevention of Health Care Associated Venous Thromboembolism through Implementing VTE Prevention Clinical Practice Guidelines in Hospitalized Medical Patients: A Systematic Review and Meta-Analysis. Implement. Sci. 2020, 15, 49. [Google Scholar] [CrossRef]
- Pereira, V.C.; Silva, S.N.; Carvalho, V.K.S.; Zanghelini, F.; Barreto, J.O.M. Strategies for the Implementation of Clinical Practice Guidelines in Public Health: An Overview of Systematic Reviews. Health Res. Policy Syst. 2022, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Keeley, J.; Kingman, M.; McDevitt, S.; Brewer, J.; Rogers, F.; Hill, W.; Rideman, Z.; Broderick, M. Clinical Application of Risk Assessment in PAH: Expert Center APRN Recommendations. Pulm. Circ. 2022, 12, e12106. [Google Scholar] [CrossRef]
- Mabey, E.; Ismail, S.; Tailor, F. Improving Venous Thromboembolism Risk Assessment Rates in a Tertiary Urology Department. BMJ Open Qual. 2017, 6, e000171. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, N.; Matta, S.; Aderian, S.S.; Salibi, R.; Salameh, P.; Tayeh, G.; Haddad, E.; Ghanem, H. A Prospective Observational Cohort of Clinical Outcomes in Medical Inpatients Prescribed Pharmacological Thromboprophylaxis Using Different Clinical Risk Assessment Models (COMPT RAMs). Sci. Rep. 2019, 9, 18366. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Koo, S.; Quiroz, R.; Cooper, J.M.; Paterno, M.D.; Soukonnikov, B.; Goldhaber, S.Z. Electronic Alerts to Prevent Venous Thromboembolism among Hospitalized Patients. N. Engl. J. Med. 2005, 352, 969–977. [Google Scholar] [CrossRef]
- Pandor, A.; Tonkins, M.; Goodacre, S.; Sworn, K.; Clowes, M.; Griffin, X.L.; Holland, M.; Hunt, B.J.; de Wit, K.; Horner, D. Risk Assessment Models for Venous Thromboembolism in Hospitalised Adult Patients: A Systematic Review. BMJ Open 2021, 11, e045672. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Raji, S.J.; Ellahham, S.; Bashir, N.; Al Hanaee, M.; Boharoon, H.; AlFalahi, M. Improving Venous Thromboembolism Risk Assessment Compliance Using the Electronic Tool in Admitted Medical Patients. BMJ Qual. Improv. Rep. 2015, 4, u209593.w3965. [Google Scholar] [CrossRef]
- Lavon, O.; Tamir, T. Evaluation of the Padua Prediction Score Ability to Predict Venous Thromboembolism in Israeli Non-Surgical Hospitalized Patients Using Electronic Medical Records. Sci. Rep. 2022, 12, 6121. [Google Scholar] [CrossRef]
- Moik, F.; Englisch, C.; Pabinger, I.; Ay, C. Risk Assessment Models of Cancer-Associated Thrombosis—Potentials and Perspectives. Thromb. Updat. 2021, 5, 100075. [Google Scholar] [CrossRef]
- Shaban, M.; Habib, N.; Helmy, I.; Mohammed, H.H. Dehydration Risk Factors and Outcomes in Older People in Rural Areas. Front. Nurs. 2022, 9, 395–403. [Google Scholar] [CrossRef]
- Li, A.; Kuderer, N.M.; Hsu, C.-Y.; Shyr, Y.; Warner, J.L.; Shah, D.P.; Kumar, V.; Shah, S.; Kulkarni, A.A.; Fu, J.; et al. The CoVID-TE Risk Assessment Model for Venous Thromboembolism in Hospitalized Patients with Cancer and COVID-19. J. Thromb. Haemost. 2021, 19, 2522–2532. [Google Scholar] [CrossRef]
- Shaban, M.; Shaban, M.M.; Ramadan, O.; Mohammed, H.H. Omicron: Egyptian Nurses’ Knowledge and Attitudes. J. Integr. Nurs. 2022, 4, 15. [Google Scholar] [CrossRef]
- Maynard, G. Preventing Hospital-Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement, 2nd ed.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2016.
- Van Calster, B.; Wynants, L.; Timmerman, D.; Steyerberg, E.W.; Collins, G.S. Predictive Analytics in Health Care: How Can We Know It Works? J. Am. Med. Inform. Assoc. 2019, 26, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Chiasakul, T.; Lam, B.D.; McNichol, M.; Robertson, W.; Rosovsky, R.P.; Lake, L.; Vlachos, I.S.; Adamski, A.; Reyes, N.; Abe, K.; et al. Artificial Intelligence in the Prediction of Venous Thromboembolism: A Systematic Review and Pooled Analysis. Eur. J. Haematol. 2023. [Google Scholar] [CrossRef]
- Whittington, R.; Hockenhull, J.; McGuire, J.; Leitner, M.; Barr, W.; Cherry, M.; Flentje, R.; Quinn, B.; Dundar, Y.; Dickson, R. A Systematic Review of Risk Assessment Strategies for Populations at High Risk of Engaging in Violent Behaviour: Update 2002–8. Health Technol. Assess. 2013, 17, 1–128. [Google Scholar] [CrossRef]
- Shaban, M.; Mohammed, H.H.; Hassan, S. Role of Community Health Nurse in the Prevention of Elderly Dehydration: A Mini-Review. J. Integr. Nurs. 2022, 4, 166–171. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Panagopoulos, A.; Samant, S.; Ghalib, N.; Kadillari, J.; Daniilidis, A.; Samartzis, N.; Makadia, J.; Palaiodimos, L.; Kokkinidis, D.G.; et al. Management of Venous Thromboembolism in Pregnancy. Thromb. Res. 2022, 211, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S.; et al. Shared Decision Making: A Model for Clinical Practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef]
- Zander, A.L.; Van Gent, J.-M.; Olson, E.J.; Shackford, S.R.; Badiee, J.; Dunne, C.E.; Sise, C.B.; Sise, M.J. Venous Thromboembolic Risk Assessment Models Should Not Solely Guide Prophylaxis and Surveillance in Trauma Patients. J. Trauma Acute Care Surg. 2015, 79, 194–198. [Google Scholar] [CrossRef]
- Buist, D.S.M.; Knight Ross, N.; Reid, R.J.; Grossman, D.C. Electronic Health Risk Assessment Adoption in an Integrated Healthcare System. Am. J. Manag. Care 2014, 20, 62–69. [Google Scholar] [PubMed]
| Study | Study Design | Participants | Risk Assessment Tool | Primary Outcomes | Secondary Outcomes | Results |
|---|---|---|---|---|---|---|
| (Grant et al., 2016) [33] | Case-Control | 63,548 | Caprini Score | VTE Incidence | Length of Hospital Stay, Mortality | Reduced VTE incidence, shorter hospital stay, lower mortality. |
| (Zhou et al., 2018) [36] | Retrospective case-control | 902 | Padua Score | Examined and compared how well the Padua Prediction Score (PPS) and the Caprini RAM stratify VTE risk in medical inpatients. | Healthcare Resource Utilization | Identify patients who may benefit from prophylaxis, and potential for prediction of mortality. |
| (Rosenberg et al., 2014) [32] | Cohort | 19,217 | IMPROVE Score | VTE-related Complications | Bleeding risk | Discrimination and calibration for both the overall VTE risk model and the identification of low-risk and at-risk medical patient groups. |
| (C. Zhang et al., 2019) [38] | Prospective observational | 281 | Caprini Score | VTE Incidence, Symptomatic Thromboembolic Events | Length of ICU Stay | Decreased VTE incidence, lower rates of symptomatic events, shorter ICU stay. |
| (Mahlab-Guri et al., 2020) [37] | Retrospective case-control | 4000 | Padua Score | Rate of VTE risk assessment in routine medical department practice | Cost-effectiveness | Thromboprophylaxis did not have significant effect on the low number of VTE events. No major bleeding was observed. |
| (Modi et al., 2016) [39] | Retrospective | 298 | Wells Score | Evaluated the application of the Wells scoring system in trauma population | Mortality | Lower VTE incidence, decreased mortality rates. |
| (X. Zhang et al., 2023) [40] | Multi-center retrospective cohort study | 34,893 | Caprini Score | Determine the incidence of DVT and then validate the Caprini RAM in orthopedic trauma patients. | Length of Hospital Stay | Prevalence of DVT and higher Caprini score were significantly associated with increased all-cause mortality among orthopedic trauma patients after discharge. |
| (Abukhalil et al., 2022) [41] | Cross-Sectional | 408 | IMPROVE Score | Evaluate the adherence of current clinical practice to the established guidelines at a Palestinian teaching hospital | Patient-reported Outcomes | Adapting assessment models or checklists in clinical practice based on clinical guidelines for VTE risk stratification is a practical and effective method to improve VTE prophylaxis management. |
| (Depietri et al., 2018) [42] | Observational, single-centre study | 450 | Padua Score | VTE Incidence, Symptomatic Thromboembolic Events | Quality of Life | Lower VTE incidence, decreased symptomatic events, improved quality of life. |
| (Silveira et al., 2015) [43] | Cohort | 793 | Wells Score | The Wells score’s utility for risk stratification among inpatients with suspected DVT as measured by the difference in incidence of proximal DVT among the 3 Wells score categories (low, moderate, and high pretest probability) | Healthcare Resource Utilization | The Wells score risk stratification is not sufficient to rule out DVT or influence management decisions in the inpatient setting. |
| (Moumneh et al., 2020) [34] | Retrospective analysis | 14,660 | Caprini, IMPROVE, and Padua | Externally assess the Caprini, IMPROVE, and Padua VTE risk scores and to compare their performance to advanced age as a stand-alone predictor. | Length of ICU Stay | Caprini, IMPROVE, and Padua VTE risk scores have poor discriminative ability to identify not critically ill medical inpatients at risk of VTE, and do not perform better than a risk evaluation based on patient’s age alone. |
| (Xiong, et al., 2023) [45] | Retrospective study | 3168 | IMPROVE Score | Compare the predictive power for VTE diagnosis among the Wells, Geneva, YEARS, PERC, Padua, and IMPROVE scores in the leading authoritative guidelines in nonsurgical hospitalized patients with suspected VTE. | Mortality, Length of Hospital Stay | Comparison of predictive power for VTE diagnosis among six VTE risk scores in guidelines indicates that the Geneva and Wells scores perform best is prediction of VTE. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhsh, E. The Benefits and Imperative of Venous Thromboembolism Risk Screening for Hospitalized Patients: A Systematic Review. J. Clin. Med. 2023, 12, 7009. https://doi.org/10.3390/jcm12227009
Bakhsh E. The Benefits and Imperative of Venous Thromboembolism Risk Screening for Hospitalized Patients: A Systematic Review. Journal of Clinical Medicine. 2023; 12(22):7009. https://doi.org/10.3390/jcm12227009
Chicago/Turabian StyleBakhsh, Ebtisam. 2023. "The Benefits and Imperative of Venous Thromboembolism Risk Screening for Hospitalized Patients: A Systematic Review" Journal of Clinical Medicine 12, no. 22: 7009. https://doi.org/10.3390/jcm12227009
APA StyleBakhsh, E. (2023). The Benefits and Imperative of Venous Thromboembolism Risk Screening for Hospitalized Patients: A Systematic Review. Journal of Clinical Medicine, 12(22), 7009. https://doi.org/10.3390/jcm12227009







