18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography Findings of Polymyalgia Rheumatica in Patients with Giant Cell Arteritis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Recruitment
2.2. Study Protocol
2.2.1. Patient Disease Assessment
2.2.2. FDG-PET-CT Equipment, Protocol, and Interpretation
2.3. Statistical Analysis
3. Results
3.1. Differences in Significant Extravascular Sites of FDG Uptake between Patients with LV-GCA Clinically Diagnosed with PMR or Not
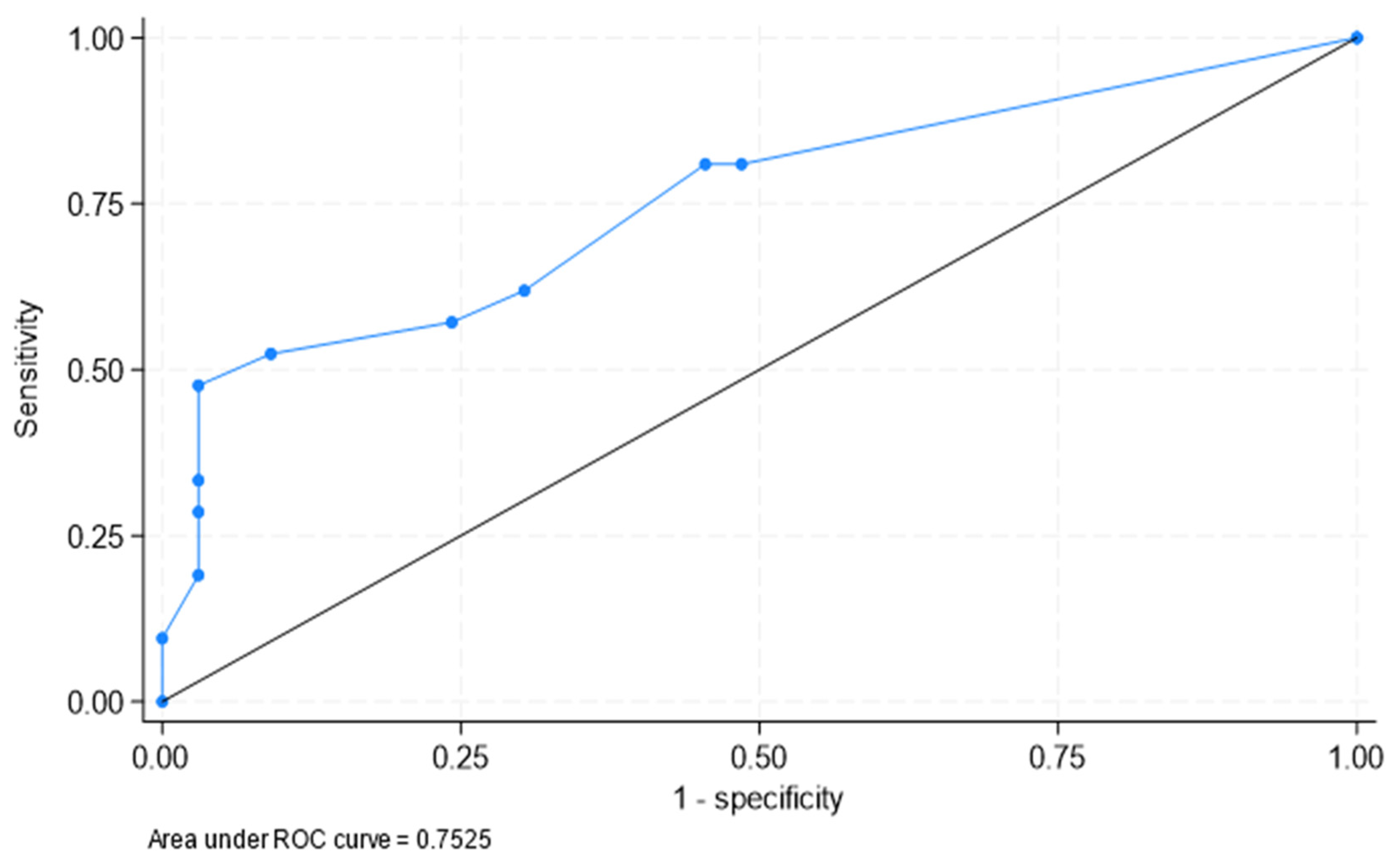
3.2. Determination of the Best Cut-Offs for the Diagnosis of PMR in Patients with LV-GCA
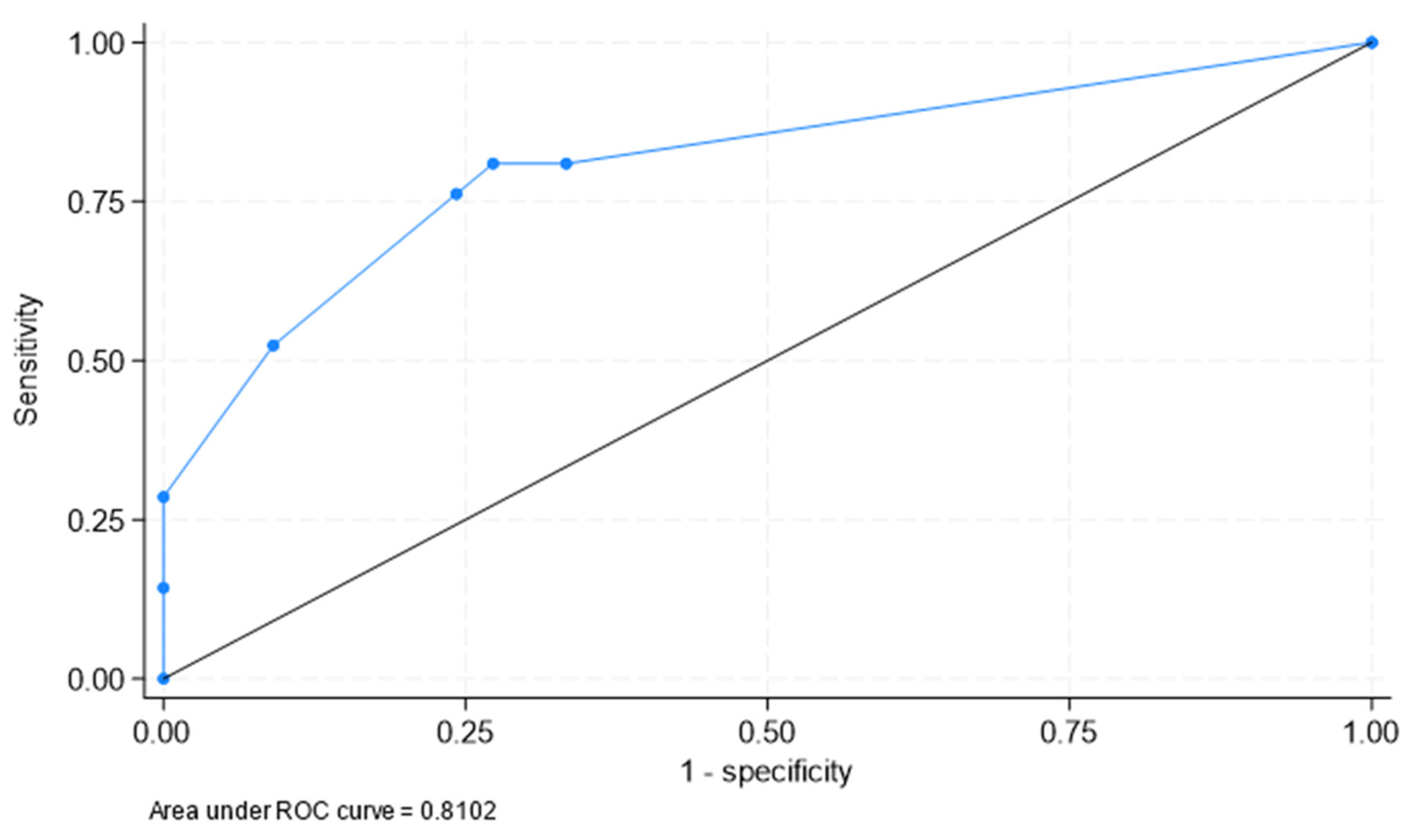
3.3. Best Areas of Significant FDG Uptake to Clinically Identify PMR in Patients with LV-GCA
4. Discussion
5. Significance and Innovation
- -
- 18F-FDG-PET-CT can be useful to identify PMR in patients presenting with LV-GCA.
- -
- In patients with LV-GCA, the presence of significant 18F-FDG-PET-CT uptake in the shoulder, greater trochanter, and lumbar interspinous areas allows PMR to be identified.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Lopez-Diaz, M.J.; Miranda-Filloy, J.A.; Gonzalez-Juanatey, C.; Martin, J.; Llorca, J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009, 61, 1454–1461. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Matteson, E.L.; Castañeda, S. Polymyalgia rheumatica. Lancet 2017, 390, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Pipitone, N.; Versari, A.; Hunder, G.G. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat. Rev. Rheumatol. 2012, 8, 509–521. [Google Scholar] [CrossRef]
- Serling-Boyd, N.; Stone, J.H. Recent advances in the diagnosis and management of giant cell arteritis. Curr. Opin. Rheumatol. 2020, 32, 201–207. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Castañeda, S.; Martínez-Rodríguez, I.; Atienza-Mateo, B.; Blanco, R.; González-Gay, M.A. Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. J. Clin. Med. 2021, 10, 3704. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Slijkhuis, B.G.C.; Tomelleri, A.; Gheysens, O.; Jiemy, W.F.; Piccolo, C.; Nienhuis, P.; Sandovici, M.; Brouwer, E.; Glaudemans, A.W.; et al. Positron Emission Tomography Imaging in Vasculitis. Cardiol. Clin. 2023, 41, 251–265. [Google Scholar] [CrossRef]
- Germanò, G.; Versari, A.; Muratore, F.; Pipitone, N.; Bajocchi, G.L.; Catanoso, M.G.; Salvarani, C. Isolated vasculitis of the lower extremities in a patient with polymyalgia rheumatica and giant cell arteritis. Clin. Exp. Rheumatol. 2011, 1 (Suppl. 64), S138–S139. [Google Scholar]
- Duftner, C.; Dejaco, C.; Sepriano, A.; Falzon, L.; Schmidt, W.A.; Ramiro, S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: A systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018, 4, e000612. [Google Scholar] [CrossRef]
- Schäfer, V.S.; Jin, L.; Schmidt, W.A. Imaging for Diagnosis, Monitoring, and Outcome Prediction of Large Vessel Vasculitides. Curr. Rheumatol. Rep. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Cantini, F.; Salvarani, C.; Niccoli, L.; Nannini, C.; Boiardi, L.; Padula, A.; Olivieri, I.; Valentino, M.; Barozzi, L. Fat suppression magnetic resonance imaging in shoulders of patients with polymyalgia rheumatica. J. Rheumatol. 2004, 31, 120–124. [Google Scholar]
- Cantini, F.; Salvarani, C.; Olivieri, I.; Niccoli, L.; Padula, A.; Macchioni, L.; Boiardi, L.; Ciancio, G.; Mastrorosato, M.; Rubini, F.; et al. Shoulder ultrasonography in the diagnosis of polymyalgia rheumatica: A case-control study. J. Rheumatol. 2001, 28, 1049–1055. [Google Scholar] [PubMed]
- Cantini, F.; Salvarani, C.; Olivieri, I.; Niccoli, L.; Padula, A.; Bozza, A. Hip bursitis in active polymyalgia rheumatica: Report of a case. Clin. Exp. Rheumatol. 1999, 17, 512–513. [Google Scholar]
- Salvarani, C.; Barozzi, L.; Cantini, F.; Niccoli, L.; Boiardi, L.; Valentino, M.; Pipitone, N.; Bajocchi, G.; Macchioni, P.; Catanoso, M.G.; et al. Cervical interspinous bursitis in active polymyalgia rheumatica. Ann. Rheum. Dis. 2008, 67, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Cimmino, M.A.; Maradit-Kremers, H.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. 2012 provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2012, 71, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Sondag, M.; Guillot, X.; Verhoeven, F.; Blagosklonov, O.; Prati, C.; Boulahdour, H.; Wendling, D. Utility of 18F-fluoro-dexoxyglucose positron emission tomography for the diagnosis of polymyalgia rheumatica: A controlled study. Rheumatology 2016, 55, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Henckaerts, L.; Gheysens, O.; Vanderschueren, S.; Goffin, K.; Blockmans, D. Use of 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of polymyalgia rheumatica-A prospective study of 99 patients. Rheumatology 2018, 57, 1908–1916. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Treglia, G.; Glaudemans, A.W.J.M.; Brouwer, E.; Jamar, F.; Slart, R.H.J.A.; Gheysens, O. Diagnostic value of [18F]FDG-PET/CT in polymyalgia rheumatica: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1876–1889. [Google Scholar] [CrossRef]
- Moya-Alvarado, P.; Fernandez Leon, A.; Corica, M.E.; Camacho Marti, V.; López-Mora, D.A.; Castellví, I.; Corominas, H. The added value of 18f-FDG PET/CT in the assessment of onset and steroid resistant polimyalgia rheumatica. PLoS ONE 2021, 16, e0255131. [Google Scholar] [CrossRef]
- Rehak, Z.; Vasina, J.; Nemec, P.; Fojtik, Z.; Koukalova, R.; Bortlicek, Z.; Rehakova, D.; Adam, J.; Vavrusova, A.; Adam, Z. Various forms of 18F-FDG PET and PET/CT findings in patients with polymyalgia rheumatica. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2015, 159, 629–636. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Martínez-Rodríguez, I.; Loricera, J.; Banzo, I.; Calderón-Goercke, M.; Calvo-Río, V.; González-Vela, C.; Corrales, A.; Castañeda, S.; Blanco, R.; et al. Predictors of positive 18F-FDG PET/CT-scan for large vessel vasculitis in patients with persistent polymyalgia rheumatica. Semin. Arthritis Rheum. 2019, 48, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Heras-Recuero, E.; Landaeta-Kancev, L.C.; Martínez de Bourio-Allona, M.; Torres-Rosello, A.; Blázquez-Sánchez, T.; Ferraz-Amaro, I.; Castañeda, S.; Martínez-López, J.A.; Martínez-Dhier, L.; Largo, R.; et al. Positron Emission Computed Tomography Spectrum of Large Vessel Vasculitis in a Tertiary Center: Differences in 18F-Fluorodeoxyglucose uptake between large vessel vasculitis with predominant cranial and extracranial giant cell arteritis phenotypes. J. Clin. Med. 2023, 12, 6164. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Writing group; Reviewer group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees, SNMMI Cardiovascular; Members of Council, PET Interest Group; Members of ASNC; EANM Committee Coordinator. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [CrossRef]
- Casadepax-Soulet, C.; Benali, K.; Crestani, B.; Piekarski, E.; Mahida, B.; Ebstein, E.; Juge, P.-A.; Forien, M.; Dieudé, P.; Ottaviani, S. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in polymyalgia rheumatica: An observational study. Clin. Exp. Rheumatol. 2022, 41, 1456–1462. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Rodríiguez-Valverde, V.; Blanco, R.; Fernández-Sueiro, J.L.; Armona, J.; Figueroa, M.; Martínez-Taboada, V.M. Polymyalgia rheumatica without significantly increased erythrocyte sedimentation rate. A more benign syndrome. Arch. Intern. Med. 1997, 157, 317–320. [Google Scholar] [CrossRef]
- Cantini, F.; Salvarani, C.; Olivieri, I.; Niccoli, L.; Macchioni, P.; Boiardi, L.; Mastrorosato, M.; Ciancio, G.; Padula, A.; Bozza, A.; et al. Inflamed shoulder structures in polymyalgia rheumatica with normal erythrocyte sedimentation rate. Arthritis Rheum. 2001, 44, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Garcia-Porrua, C.; Salvarani, C.; Olivieri, I.; Hunder, G.G. The spectrum of conditions mimicking polymyalgia rheumatica in Northwestern Spain. J. Rheumatol. 2000, 27, 2179–2184. [Google Scholar]
- Paltta, J.; Suuronen, S.; Pirilä, L.; Palomäki, A. Differential diagnostics of polymyalgia rheumatica in a university hospital in Finland. Scand. J. Rheumatol. 2023, 19, 1–7. [Google Scholar] [CrossRef]
- Yamashita, H.; Kubota, K.; Takahashi, Y.; Minaminoto, R.; Morooka, M.; Ito, K.; Kano, T.; Kaneko, H.; Takashima, H.; Mimoiri, A. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: Evidence for distinctive bursitis and large-vessel vasculitis. Mod. Rheumatol. 2012, 22, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Barozzi, L.; Boiardi, L.; Pipitone, N.; Bajocchi, G.L.; Macchioni, P.L.; Catanoso, M.; Pazzola, G.; Valentino, M.; De Luca, C.; et al. Lumbar interspinous bursitis inactive poly-myalgia rheumatica. Clin. Exp. Rheumatol. 2013, 31, 526–531. [Google Scholar]
- Owen, C.E.; Poon, A.M.T.; Yang, V.; McMaster, C.; Lee, S.T.; Liew, D.F.L.; Leung, J.L.; Scott, A.M.; Buchanan, R.R.C. Abnormalities at three musculoskeletal sites on whole-body positron emission tomography/computed tomography can diagnose polymyalgia rheumatica with high sensitivity and specificity. Eur. J. Nucl. Med. 2020, 47, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Flaus, A.; Amat, J.; Prevot, N.; Olagne, L.; Descamps, L.; Bouvet, C.; Barres, B.; Valla, C.; Mathieu, S.; Andre, M.; et al. Decision Tree With Only Two Musculoskeletal Sites to Diagnose Polymyalgia Rheumatica Using [18F]FDG PET-CT. Front. Med. 2021, 8, 646974. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A. Giant cell arteritis and polymyalgia rheumatica: Two different but often overlapping conditions. Semin. Arthritis Rheum. 2004, 33, 289–293. [Google Scholar] [CrossRef]
- Salvarani, C.; Padoan, R.; Iorio, L.; Tomelleri, A.; Terrier, B.; Muratore, F.; Dasgupta, B. Subclinical giant cell arteritis in polymyalgia rheumatica: Concurrent conditions or a common spectrum of inflammatory diseases? Autoimmun. Rev. 2023, 23, 103415. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; García-Porrúa, C.; Vázquez-Caruncho, M. Polymyalgia rheumatica in biopsy proven giant cell arteritis does not constitute a different subset but differs from isolated polymyalgia rheumatica. J. Rheumatol. 1998, 25, 1750–1755. [Google Scholar]
- Muratore, F.; Kermani, T.A.; Crowson, C.S.; Green, A.B.; Salvarani, C.; Matteson, E.L.; Warrington, K.J. Large-vessel giant cell arteritis: A cohort study. Rheumatology 2015, 54, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Tomelleri, A.; Campochiaro, C.; Dagna, L. Giant cell arteritis: Update on clinical manifestations, diagnosis, and management. Eur. J. Intern. Med. 2023, 107, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, P.; de Thurah, A.; Nielsen, B.D.; Hansen, I.T.; Eldrup, N.; Nørgaard, M.; Hauge, E.-M. Increased risk of thoracic aortic complications among patients with giant cell arteritis: A nationwide, population-based cohort study. Rheumatology 2022, 61, 2931–2941. [Google Scholar] [CrossRef]
- Moreel, L.; Coudyzer, W.; Boeckxstaens, L.; Betrains, A.; Molenberghs, G.; Vanderschueren, S.; Claus, E.; Van Laere, K.; Blockmans, D. Association Between Vascular 18F-Fluorodeoxyglucose Uptake at Diagnosis and Change in Aortic Dimensions in Giant Cell Arteritis: A Cohort Study. Ann. Intern. Med. 2023, 176, 1321–1329. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Vicente-Rabaneda, E.F.; Heras-Recuero, E.; Castañeda, S. Polymyalgia rheumatica: When should we suspect an underlying large vessel vasculitis? Clin. Exp. Rheumatol. 2023, 41, 774–776. [Google Scholar] [CrossRef]
- Desvages, A.; Hives, F.; Deprez, X.; Pierache, A.; Béhal, H.; Flipo, R.-M.; Paccou, J. Usefulness of 18F-Fluorodeoxyglucose Positron Emission Tomography in Diagnosing Polymyalgia Rheumatica and Large-Vessel Vasculitis: A Case-Control Study. J. Clin. Med. 2023, 12, 2844. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, K.S.M.; van Sleen, Y.; Nienhuis, P.; Sandovici, M.; Westerdijk, N.; Glaudemans, A.W.J.M.; Brouwer, E.; Slart, R.H.J.A. Comparison and validation of FDG-PET/CT scores for polymyalgia rheumatica. Rheumatology 2022, 61, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.D.; Gormsen, L.C.; Hansen, I.T.; Keller, K.K.; Therkildsen, P.; Hauge, E.-M. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
| Extravascular Area | Site | No Clinically Evident PMR (N = 33) | Clinically Evident PMR (N = 21) | p |
|---|---|---|---|---|
| Acromioclavicular | Total | 2 (6.1) | 3 (14.3) | 0.37 |
| Left | 2 (6.1) | 2 (9.5) | 0.64 | |
| Right | 1 (3.0) | 3 (14.3) | 0.29 | |
| Sternoclavicular | Total | 7 (21.2) | 6 (28.6) | 0.75 |
| Left | 7 (21.2) | 6 (28.6) | 0.75 | |
| Right | 7 (21.2) | 6 (28.6) | 0.75 | |
| Shoulder | Total | 8 (24.2) | 16 (76.2) | <0.001 |
| Left | 8 (24.2) | 16 (76.2) | <0.001 | |
| Right | 7 (21.2) | 14 (66.7) | 0.001 | |
| Cervical interspinous | 1 (3.0) | 5 (23.8) | 0.03 | |
| Lumbar interspinous | 4 (12.1) | 9 (42.9) | 0.02 | |
| Hip | Total | 6 (18.2) | 10 (47.6) | 0.03 |
| Left | 6 (18.2) | 10 (47.6) | 0.03 | |
| Right | 5 (15.2) | 10 (47.6) | 0.01 | |
| Ischial tuberosity | Total | 2 (6.1) | 5 (23.8) | 0.10 |
| Left | 1 (3.0) | 5 (23.8) | 0.03 | |
| Right | 2 (6.1) | 5 (23.8) | 0.10 | |
| Greater trochanter | Total | 3 (9.1) | 7 (33.3) | 0.04 |
| Left | 3 (9.1) | 6 (28.6) | 0.13 | |
| Right | 3 (9.1) | 7 (33.3) | 0.04 | |
| Symphysis pubis | Total | 0 (0.0) | 1 (4.8) | 0.39 |
| Left | 0 (0.0) | 1 (4.8) | 0.39 | |
| Right | 0 (0.0) | 1 (4.8) | 0.39 | |
| Number of sites involved (SCORE016) | (mean ± SD) | 1.73 ± 2.31 | 5.10 ± 4.05 | <0.001 |
| Area under ROC curve = 0.753 (0.615, 0.890) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heras-Recuero, E.; Martínez de Bourio-Allona, M.; Landaeta-Kancev, L.C.; Blázquez-Sánchez, T.; Torres-Roselló, A.; Álvarez-Rubio, M.; Belhaj-Gandar, M.; Martínez-López, J.A.; Martínez-Dhier, L.; Llorca, J.; et al. 18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography Findings of Polymyalgia Rheumatica in Patients with Giant Cell Arteritis. J. Clin. Med. 2023, 12, 6983. https://doi.org/10.3390/jcm12226983
Heras-Recuero E, Martínez de Bourio-Allona M, Landaeta-Kancev LC, Blázquez-Sánchez T, Torres-Roselló A, Álvarez-Rubio M, Belhaj-Gandar M, Martínez-López JA, Martínez-Dhier L, Llorca J, et al. 18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography Findings of Polymyalgia Rheumatica in Patients with Giant Cell Arteritis. Journal of Clinical Medicine. 2023; 12(22):6983. https://doi.org/10.3390/jcm12226983
Chicago/Turabian StyleHeras-Recuero, Elena, Marta Martínez de Bourio-Allona, Laura Cristina Landaeta-Kancev, Teresa Blázquez-Sánchez, Arantxa Torres-Roselló, Miguel Álvarez-Rubio, Mariam Belhaj-Gandar, Juan Antonio Martínez-López, Luis Martínez-Dhier, Javier Llorca, and et al. 2023. "18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography Findings of Polymyalgia Rheumatica in Patients with Giant Cell Arteritis" Journal of Clinical Medicine 12, no. 22: 6983. https://doi.org/10.3390/jcm12226983
APA StyleHeras-Recuero, E., Martínez de Bourio-Allona, M., Landaeta-Kancev, L. C., Blázquez-Sánchez, T., Torres-Roselló, A., Álvarez-Rubio, M., Belhaj-Gandar, M., Martínez-López, J. A., Martínez-Dhier, L., Llorca, J., Largo, R., & González-Gay, M. Á. (2023). 18F-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography Findings of Polymyalgia Rheumatica in Patients with Giant Cell Arteritis. Journal of Clinical Medicine, 12(22), 6983. https://doi.org/10.3390/jcm12226983









