Memory Impairments and Wellbeing in Breast Cancer Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Elegibility Criteria
2.2. Search Strategy
2.3. Selection Process and Data Extraction
2.4. Quality Appraisal
3. Results
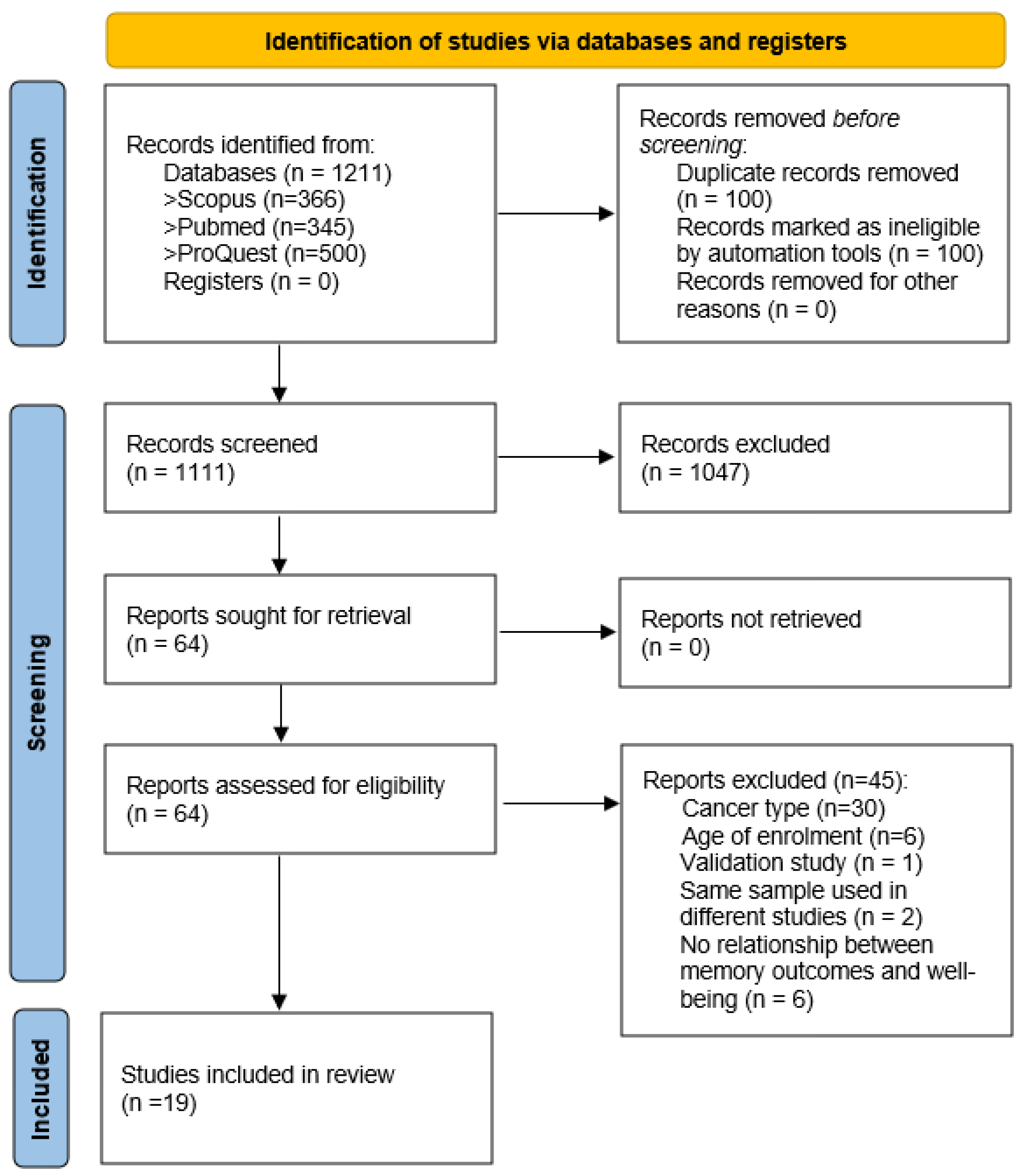
3.1. Study Selection
3.2. Study Characteristics
3.3. Memory Assessment: Objective and Subjective Measures
3.4. Effect of Breast Cancer and Associated Treatments on Memory
3.5. Relationship between Memory Outcomes and Wellbeing Indicators
3.6. Study Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://gco.iarc.fr/today (accessed on 3 July 2023).
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef]
- McDougall, G.J.; Oliver, J.S.; Scogin, F. Memory and cancer: A review of the literature. Arch. Psychiatr. Nurs. 2014, 28, 180–186. [Google Scholar] [CrossRef]
- Horowitz, T.S.; Suls, J.; Treviño, M. A Call for a Neuroscience Approach to Cancer-Related Cognitive Impairment. Trends Neurosci. 2018, 41, 493–496. [Google Scholar] [CrossRef]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-Term Cognitive Dysfunction in Cancer Survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Lange, M.; Dos Santos, M.; Vaz-Luis, I.; Di Meglio, A. Long-Term Fatigue and Cognitive Disorders in Breast Cancer Survivors. Cancers 2019, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hendrix, C.C. Cancer-Related Cognitive Impairment in Breast Cancer Patients: Influences of Psychological Variables. Asia-Pac. J. Oncol. Nurs. 2018, 5, 296–306. [Google Scholar] [CrossRef]
- Bray, V.J.; Dhillon, H.M.; Vardy, J.L. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J. Cancer Surviv. 2018, 12, 537–559. [Google Scholar] [CrossRef]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef]
- Jean-Pierre, P. Management of Cancer-related Cognitive Dysfunction-Conceptualization Challenges and Implications for Clinical Research and Practice. US Oncol. 2010, 6, 9–12. [Google Scholar] [CrossRef]
- Hodgson, K.D.; Hutchinson, A.D.; Wilson, C.J.; Nettelbeck, T.A. Meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat. Rev. 2013, 39, 297–304. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- JBI. Checklist for Analytical Cross Sectional and Cohort Studies: Critical Appraisal Tools for Use in JBI Systematic Reviews. 2020. Available online: https://jbi.global/critical-appraisal-tools (accessed on 14 June 2023).
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis. JBI, 2020. Available online: https://synthesismanual.jbi.global (accessed on 14 June 2023).
- Crouch, A.; Champion, V.L.; Von Ah, D. Comorbidity, cognitive dysfunction, physical functioning, and quality of life in older breast cancer survivors. Support. Care Cancer 2022, 30, 359–366. [Google Scholar] [CrossRef]
- Paquet, L.; Collins, B.; Song, X.; Chinneck, A.; Bedard, M.; Verma, S. A pilot study of prospective memory functioning in early breast cancer survivors. Breast 2013, 22, 455–461. [Google Scholar] [CrossRef]
- Ando-Tanabe, N.; Iwamitsu, Y.; Kuranami, M.; Okazaki, S.; Yasuda, H.; Nakatani, Y.; Yamamoto, K.; Watanabe, M.; Miyaoka, H. Cognitive function in women with breast cancer receiving adjuvant chemotherapy and healthy controls. Breast Cancer 2014, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Lloyd, G.R.; Awick, E.A.; McAuley, E. Relationship between self-reported and objectively measured physical activity and subjective memory impairment in breast cancer survivors: Role of self-efficacy, fatigue and distress. Psychooncology 2017, 26, 1390–1399. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, V.C.; Hsieh, C.C.; Wang, W.K.; Chen, H.M.; Weng, J.C.; Wu, S.I.; Gossop, M. Subjective and objective cognitive functioning among patients with breast cancer: Effects of chemotherapy and mood symptoms. Breast Cancer 2021, 28, 236–245. [Google Scholar] [CrossRef]
- Boele, F.W.; Schilder, C.M.; de Roode, M.L.; Deijen, J.B.; Schagen, S.B. Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause 2015, 22, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Crouch, A.; Champion, V.L.; Unverzagt, F.W.; Pressler, S.J.; Huber, L.; Moser, L.R.; Cella, D.; Von Ah, D. Cognitive dysfunction prevalence and associated factors in older breast cancer survivors. J. Geriatr. Oncol. 2022, 13, 33–39. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, J.; Ding, K.; Lv, Y.; Zhang, C.; Chao, H.H.; Li, C.S.; Cheng, H. Depression involved in self-reported prospective memory problems in survivors of breast cancer who have received chemotherapy. Medicine 2019, 98, e15301. [Google Scholar] [CrossRef]
- Merriman, J.D.; Sereika, S.M.; Brufsky, A.M.; McAuliffe, P.F.; McGuire, K.P.; Myers, J.S.; Phillips, M.L.; Ryan, C.M.; Gentry, A.L.; Jones, L.D.; et al. Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psychooncology 2017, 26, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Paquet, L.; Verma, S.; Collins, B.; Chinneck, A.; Bedard, M.; Song, X. Testing a novel account of the dissociation between self-reported memory problems and memory performance in chemotherapy-treated breast cancer survivors. Psychooncology 2018, 27, 171–177. [Google Scholar] [CrossRef]
- Shilling, V.; Jenkins, V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur. J. Oncol. Nurs. 2007, 11, 6–15. [Google Scholar] [CrossRef]
- Small, B.J.; Jim, H.S.L.; Eisel, S.L.; Jacobsen, P.B.; Scott, S.B. Cognitive performance of breast cancer survivors in daily life: Role of fatigue and depressed mood. Psychooncology 2019, 28, 2174–2180. [Google Scholar] [CrossRef]
- Vardy, J.L.; Stouten-Kemperman, M.M.; Pond, G.; Booth, C.M.; Rourke, S.B.; Dhillon, H.M.; Dodd, A.; Crawley, A.; Tannock, I.F. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2019, 13, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Vearncombe, K.J.; Rolfe, M.; Wright, M.; Pachana, N.A.; Andrew, B.; Beadle, G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J. Int. Neuropsychol. Soc. 2009, 15, 951–962. [Google Scholar] [CrossRef]
- Von Ah, D.; Tallman, E.F. Perceived cognitive function in breast cancer survivors: Evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy-cognitive function instrument. J. Pain Symptom Manag. 2015, 49, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.S.; Zhang, M.; Askren, M.K.; Berman, M.G.; Peltier, S.; Hayes, D.F.; Therrien, B.; Reuter-Lorenz, P.A.; Cimprich, B. Cognitive dysfunction and symptom burden in women treated for breast cancer: A prospective behavioral and fMRI analysis. Brain Imaging Behav. 2017, 11, 86–97. [Google Scholar] [CrossRef]
- Jung, M.S.; Visovatti, M.A.; Sohn, E.H.; Yoo, H.S.; Kim, M.; Kim, J.R.; Lee, J.S. Impact of changes in perceived attentional function on postsurgical health-related quality of life in breast cancer patients awaiting adjuvant treatment. Health Qual. Life Outcomes 2020, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Delbeuck, X.; Lefeuvre-Plesse, C.; Kramar, A.; Skrobala, E.; Pasquier, F.; Bonneterre, J. A phase III randomized multicenter trial evaluating cognition in post-menopausal breast cancer patients receiving adjuvant hormonotherapy. Breast Cancer Res. Treat. 2015, 152, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; Dayan, J.; Piolino, P.; Viard, A.; Allouache, D.; Noal, S.; Levy, C.; Joly, F.; Eustache, F.; Giffard, B. Emotional specificities of autobiographical memory after breast cancer diagnosis. Conscious. Cogn. 2015, 35, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, K.; Bower, J.E.; Crespi, C.M.; Petersen, L.; Ganz, P.A. Cognitive function following breast cancer treatment and associations with concurrent symptoms. NPJ Breast Cancer 2018, 4, 25. [Google Scholar] [CrossRef]
- Wirkner, J.; Weymar, M.; Löw, A.; Hamm, C.; Struck, A.M.; Kirschbaum, C.; Hamm, A.O. Cognitive functioning and emotion processing in breast cancer survivors and controls: An ERP pilot study. Psychophysiology 2017, 54, 1209–1222. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional MRI study. J. Clin. Oncol. 2012, 30, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, X.; Tao, L.; Li, J.; Wu, J.; Zhu, C.; Yu, F.; Zhang, L.; Zhang, J.; Qiu, B.; et al. The Working Memory and Dorsolateral Prefrontal-Hippocampal Functional Connectivity Changes in Long-Term Survival Breast Cancer Patients Treated with Tamoxifen. Int. J. Neuropsychopharmacol. 2017, 20, 374–382. [Google Scholar] [CrossRef]
- van der Willik, K.D.; Koppelmans, V.; Hauptmann, M.; Compter, A.; Ikram, M.A.; Schagen, S.B. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res. 2018, 20, 135. [Google Scholar] [CrossRef]
- Jim, H.S.; Phillips, K.M.; Chait, S.; Faul, L.A.; Popa, M.A.; Lee, Y.H.; Hussin, M.G.; Jacobsen, P.B.; Small, B.J. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. 2012, 30, 3578–3587. [Google Scholar] [CrossRef]
- Stewart, A.; Bielajew, C.; Collins, B.; Parkinson, M.; Tomiak, E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin. Neuropsychol. 2006, 20, 76–89. [Google Scholar] [CrossRef]
- Apple, A.C.; Schroeder, M.P.; Ryals, A.J.; Wagner, L.I.; Cella, D.; Shih, P.A.; Reilly, J.; Penedo, F.J.; Voss, J.L.; Wang, L. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin. 2018, 20, 110–118. [Google Scholar] [CrossRef]
- Van Dyk, K.; Hunter, A.M.; Ercoli, L.; Petersen, L.; Leuchter, A.F.; Ganz, P.A. Evaluating cognitive complaints in breast cancer survivors with the FACT-Cog and quantitative electroencephalography. Breast Cancer Res. Treat. 2017, 166, 157–166. [Google Scholar] [CrossRef]
- Veal, B.M.; Scott, S.B.; Jim, H.S.L.; Small, B.J. Subjective cognition and memory lapses in the daily lives of breast cancer survivors: Examining associations with objective cognitive performance, fatigue, and depressed mood. Psychooncology 2023, 32, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.L.; Reid, E.; Whitfield, P.; Moustafa, A.A. Subjective memory complaints as a predictor of mild cognitive impairment and Alzheimer’s disease. Discov. Psychol. 2022, 12, 13. [Google Scholar] [CrossRef]
- Rusin, A.; Seymour, C.; Cocchetto, A.; Mothersill, C. Commonalities in the Features of Cancer and Chronic Fatigue Syndrome (CFS): Evidence for Stress-Induced Phenotype Instability? Int. J. Mol. Sci. 2022, 23, 691. [Google Scholar] [CrossRef]
- García-Sánchez, J.; Torregrosa, M.D.; Cauli, O. Cognitive Functions under Anti-HER2 Targeted Therapy in Cancer Patients: A Scoping Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.E.E.; Jung, S.O.; Lee, D.; Abraham, I. Neuropsychological Effects of Chemotherapy: Systematic Review of Longitudinal Studies on Objective Cognitive Impairment in Breast Cancer Patients. Cancer Nurs. 2023, 46, E159–E168. [Google Scholar] [CrossRef] [PubMed]

| First Author Name, Year | Country | Study Design | Sample Size (N) | Sample Characteristics | Mean Age [M(SD)] | Memory Measures | Wellbeing Indicators | Data Analysis | Main Results |
|---|---|---|---|---|---|---|---|---|---|
| Ando-Tanabe et al., 2014 [20] | Japan | Longitudinal study (T1: before; and T2: 1 month after chemotherapy). | 18 breast cancer vs. 20 controls. | Chemotherapy-treated breast cancer survivors. | Cancer group: 51.2 (10.7); Controls: 60.2 (11.4). | Logical memory I and II from the Wechsler Memory Scale-Revised (WMS-R); Verbal paired associates I and II from the WMS-R; Visual reproduction I and II from the WMS-R. | Hospital Anxiety and Depression Scale (HADS). | Independent t-test; Pearson correlations. | No group differences were found for the memory performance tasks. In the chemotherapy group, ↓ logical memory I and II (r = −0.77, r = −0.50, p < 0.05, respectively) and ↓verbal memory (containing logical memory I; r = −0.76, p < 0.001) were significantly correlated with ↑ depressive symptoms; and ↓ verbal paired associates II were associated with anxiety (r = 0.57; p < 0.05). |
| Boele et al., 2015 [23] | Netherlands | Cross-sectional study | 107 (20 adjuvant tamoxifen vs. 43 surgical operation/radiotherapy vs. 44 healthy control). | Women who had undergone breast surgery with or without radiotherapy and adjuvant tamoxifen (AT group), and women who had experienced only breast surgery with or without radiotherapy (SR group). | AT: 61.60 (6.14) SR: 62.16 (7.99) Controls: 62.02 (6.32). | Rey auditory verbal learning test. Visual association test. Visual memory subtest (WMS; immediate recall, delayed recall). Letter-number sequencing (WAIS-III). | The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (QLQ-C30): physical functioning and fatigue subscales. The 25-item Hopkins. Symptom Checklist: depression and anxiety subscales. | Pearson’s Correlations and Multivariate analysis of variance. | ANOVAs revealed statistical differences among three groups in fatigue, specifically, AT presented the highest fatigue, the SR intermediate fatigue and HC the lowest fatigue. A negative correlation was found between depression and visual memory (r = −0.249, p = 0.010) for the three groups (combined). No other significant correlations were found between memory domains and the self-reported measures (p > 0.005). |
| Crouch et al., 2022 [24] | USA | Cross-sectional study | 335 Breast Cancer Survivors | Breast Cancer Survivors, 3–8 years postdiagnosis for stage I–IIIA breast cancer without recurrence and treated with adjuvant chemotherapy. | 63.9 | Rey Auditory-Verbal Learning Test (AVLT) Digit Span Backward. | Center for Epidemiologic Studies Depression Scale (CES-D) Spielberger State-Trait Anxiety Inventory (STAI)-State Functional Assessment of Cancer Therapy-Fatigue (FACT-F). | Linear regression models | Depressive symptoms were negatively related to delayed recall; the model accounted for 3% of the variance (β = −0.23, p < 0.01). More depressive symptoms were negatively related to delayed recall. |
| Hsu et al., 2021 [22] | China | Cross-sectional study | 45 prechemotherapy vs. 30 postchemotherapy [3–9 months]) vs. 30 controls. | Chemotherapy-treated breast cancer survivors. | Prechemotherapy: 51.29 (11.25); Postchemotherapy: 48.60 (9.98); Controls: 47.50 (10.88). | Word List subtest of the Taiwanese version of the Wechsler Memory Scale–Third Edition (WMS-III). | Hospital Anxiety and Depression Scale (HADS-A); Patient Health Questionnaire (PHQ-9); Brief Fatigue Inventory (BFI). | Analysis of covariance (ANCOVA); Pearson correlations. | There were no differences between the groups regarding performance on the memory task. A positive association was found between long-delay recall and anxiety levels only in the postchemotherapy group (r = 0.40, p < 0.05). |
| Huang et al., 2019 [25] | China [Anhui] | Case-control study (T1:before; and T2: after chemotherapy). | 63 (29 patients with depression; 34 patients without depressive symptoms). | Breast cancer survivors receiving chemotherapy with paclitaxel and doxorubicin. | Depression group: 50.66 (7.76) Nondepression group: 47.35 (8.56) | Neuropsychological background tests (e.g., mini-mental state examination [MMSE]; digit span test (DS); verbal fluency test [VFT]—short-term memory; Prospective memory questionnaire [PMQ]. | Depression [Self-rating depression scale—SDS]. | Paired-sample t-tests; 2 independent samples t-tests. | Breast cancer survivors had lower MMSE scores (short-term memory) at time T2 compared to T1 (p < 0.05); Depressed cancer patients after chemotherapy showed ↓ MMSE scores (short-term memory) and ↓ verbal fluency (p < 0.05). Data suggested ↑ prospective memory impairment after chemotherapy (p < 0.001); ↑ prospective memory impairment in the depression group after chemotherapy compared to the nondepression group (p < 0.001). |
| Jung et al., 2017 [33] | United States | Longitudinal study (T1: before adjuvant therapy; T2: 1 month after chemotherapy; and T3: 7 months after chemotherapy). | 36 breast cancer patients awaiting adjuvant chemotherapy vs. 41 awaiting radiotherapy without chemotherapy vs. 39 controls. | Surgically-treated breast cancer survivors. | Chemotherapy: 49.68 (9.74); Nonchemotherapy: 53.94 (8.42) and Controls: 51.13 (8.47). | Attentional Function Index (AFI); Verbal Working Memory Task (VWMT) during fMRI scanning. | Breast Cancer Prevention Trial Symptoms Scale; Three-Item Worry Index. | t-tests, Analysis of variance (ANOVA) and multivariable regression models. | There were no changes in verbal working memory over time for the cancer group in chemotherapy. The chemotherapy-treated cancer group showed ↓ performance on the verbal working memory task compared to controls (p < 0.05) even 7 months after treatment. In the model predicting memory deficits, being in the ‘chemotherapy group’ was significantly associated with worse performance at T3 (B = 0.79, p = 0.007). No group effects were found for self-reported cognitive complaints (including working memory). However, ↑ worry (B = −0.32, p = 0.013) and ↑ distress symptoms (B = −1.36, p < 0.001) at T3 were significant predictors of complaints. |
| Jung et al., 2020 [34] | South Korea | Pre-postdesign (T1: week before any planned surgery; T2: 1 month following baseline assessment). | 132 | Breast cancer patients before any treatment. | 50.80 (9.96). | Attentional Function Index (AFI). | >Functional Assessment of Cancer Therapy-General (FACT-G); >Patient Health Questionnaire (PHQ); >Pittsburgh Sleep Quality Index (PSQI). | Pearson correlations | Memory function ↓ from presurgery to 1-month postsurgery (p < 0.05); ↓ lower postsurgery health-related quality of life associated with ↓ memory function (r = 0.28, p = 0.001) |
| Le Rhun et al., 2015 [35] | France | Longitudinal study (M0: Baseline before any hormonal treatment; M1: 6 months; M2: 1 year after treatment). | 74 (Tamoxifen—37; Aromatase inhibitor—37) | Breast cancer survivors in tamoxifen treatment vs aromatase. inhibitor treatment. | Mdn = 62.0 (tamoxifen group) vs. Mdn = 61.0 (Aromatase group). | Rey auditory verbal learning test (RAVLT). Rey auditory Benton Visual Retention Test (BVRT). Forward and backward digit span. Forward spatial span. | The Hospital Anxiety and Depression Scale (HADS). | Analysis of covariance (ANCOVA). Mixed model. analyses of variance (Mixed ANOVAs). | Considering memory measures, there were no differences between the groups during the 6-month and 1-year follow up. The pattern of results remained similar after controlling HADS scores (p > 0.12). |
| Merriman et al., 2017 [26] | USA | Longitudinal study (Before systemic therapy vs. 6 months vs. 12 months vs. 18 months of follow up). | 368 (158: Aromatase inhibitor alone; 104: chemotherapy followed by aromatase; 106: controls). | Women newly diagnosed with stage I-IIIA breast cancer; who completed surgery; and were scheduled to receive anastrozole alone or chemotherapy followed by anastrozole. | 61.7 (6.42): aromatase inhibitor alone vs. 59.4 (5.49): chemotherapy followed by aromatase vs. 58.7 (5.91): control group. | Patient Assessment of Own Functioning Inventory (PAOFI): subscale memory. | Beck Depression Inventory-II; POMS-fatigue/inertia subscale. | Analysis of variance. Multilevel regressions. | Patients who received chemotherapy reported poorer memory (p < 0.001, d = 0.15), from before to after chemotherapy. These changes persisted after one year of anastrozole for memory (p = 0.005, d = 0.18). Patients who received chemotherapy reported poorer memory than the women who received anastrozole alone (p = 0.006, d = 0.13). |
| Morel et al., 2015 [36] | France | Cross-sectional study | 31 breast cancer patients vs. 49 controls. | Breast cancer patients who had not yet undergone chemotherapy. | Cancer group: 53.6 (5.2); Controls: 54.2(6.6). | Two tests of verbal and visual episodic memory processes, based on the Encoding, Storage, Retrieval (ESR) paradigm; Digit Span Backward, Letter–Number Sequencing, and Arithmetic subtests of the Wechsler Adult Intelligence Scale (WAIS), Trail Making Test (TMT) Parts A and B, formal and semantic verbal fluency, and d2 Test of Attention. | State-Trait Anxiety Inventory (STAI). | Factorial ANOVA. | Most anxious breast patients retrieved significantly fewer emotional details than the controls (p = 0.01). |
| Paquet et al., 2018 [27] | Canada | Cross-sectional study | 80 breast cancer survivors vs. 80 controls. | Chemotherapy-treated breast cancer survivors. | Breast cancer survivors: 54.1 (9.5); Controls: 54 (9.4): (30–75 years). | Prospective and Retrospective Memory Questionnaire (PRMQ); Memory for Intention Screening Test (MIST)—prospective memory; Standardized Logical Memory Test from the Wechsler Memory Scale-IV—retrospective memory. | >Functional Assessment of Cancer Therapy: Fatigue subscale; >20-item Center for Epidemiologic Studies Depression Scale. | Analysis of covariance (ANCOVA); Pearson correlations. | There were no group differences related to memory complaints; Cancer patients reported more prospective than retrospective memory complaints (p < 0.001; d = 1.12). Cancer group: ↑ fatigue was associated with ↑ prospective memory complaints (r = −0.547; p < 0.01) and ↑ retrospective memory complaints (r = −0.545; p < 0.01); ↑ depression symptoms were also related to increased memory complaints (r = 0.467, p < 0.01 and r = 0.475, p < 0.01, respectively prospective and retrospective);: Breast cancer survivors presented ↓ prospective memory functioning than controls (p < 0.001; d = 0.8) and ↓ retrospective memory (e.g., ↓ immediate recall [p < 0.001; d = 0.72] and ↓ delayed recall [p < 0.001; d = 0.77). Cancer group: No significant associations were found between performance on memory tasks and depression and fatigue. |
| Phillips et al., 2017 [21] | United States | Longitudinal (baseline and 6-month follow up). | 1477 | Post-treatment breast cancer survivors. | 56.3 (9.3) | Frequency of Forgetting Questionnaire. | Hospital Anxiety and Depression Scale; Perceived Stress Scale; Concerns About Recurrence Scale. | Panel analyses | ↑ levels of distress (β = −0.31) and fatigue (β = −0.18) were associated ↑ subjective memory impairment. This result was maintained at 6 months of follow up. |
| Shilling et al., 2007 [28] | United Kingdom | Longitudinal study (T1: baseline; T2: 4 weeks after the final chemotherapy session; and T3: 12 months after the final chemotherapy session). | 142 (126 completed cognitive assessment at T3). | Women receiving adjuvant therapy for breast cancer. | Chemotherapy: 51.71 (9.41); Nonchemotherapy: 59.43 (7.03). | Cognitive test battery (measures covering the functional areas of verbal and visual memory with both immediate and delayed recall, working memory, processing speed, vigilance and executive function). | General Health Questionnaire (GHQ12); Functional Assessment of Cancer Therapy questionnaire (Breast) (FACT B); and the fatigue (F); and endocrine symptoms (ES). | Independent t-tests; Odds ratios (Ors). | Women in the chemotherapy group were significantly more likely to report memory problems (OR 5.01, 95% CI 2.31–10.90, p < 0.001). At T2, a strong positive association between reporting feeling down and/or worried and reporting memory problems was found (OR 5.41, 95% CI 2.44–11.99, p < 0.001). |
| Small et al., 2019 [29] | USA | Cross-sectional study | 47 Breast Cancer Survivors. | Breast cancer survivors who had been treated for Stage I-II breast cancer. with a minimum of four cycles of chemotherapy. | 53.3 (6.5) | Dot Memory Hopkins Verbal Learning Test Digit Span. | Centers for Epidemiologic-Depression Scale (CEDS). Fatigue Symptom Inventory (FSI; 16). | Multilevel models. | Between-person differences in average levels of fatigue in daily life, as well as depressed mood, were unrelated to memory performance (p > 0.05). |
| Van Dyk et al., 2018 [37] | USA | Cross-sectional study | 189 (28—no adjuvant, 64—Radiotherapy only, 20 -Chemotherapy only, 77—Chemo + Rad). | Patients who had a recent early-stage breast cancer diagnosis had completed primary treatment within the last 3 months. | No adjuvant: 51.75 (6.08) vs. Rad only: 53.88 (7.95) vs. 4 chemo only: 6.95 (8.06) vs. Chemo + rad 50.31 (8.88). | CVLT-II List A Long Delay Free Recall; WMS-III LM II; BVMT-R Delayed Recall; ROCFT 3 min Delayed Recall. | Multidimensional Fatigue Symptom Inventory–Short Form (MFSI) Physical and the Breast Cancer Prevention Trial Symptom Checklist (BCPT); Beck Depression Inventory (BDI-II). | Correlations | There are minimal treatment-related neuropsychological differences in neuropsychological measures in early breast cancer survivorship. Specifically, the memory domain correlated negatively with BCPT (r = −0.16, p = 0.004), and with physical symptoms (r = −0.14, p = 0.006). BDI-II was not a predictor of memory performance. |
| Vardy et al., 2017 [30] | Canada | Cross-sectional study | CTh + CS + N = 44; CTh + CS − N = 52; CTh − N = 30 (CTh—Chemotherapy; CS—Cognitive symptoms). | Women receiving adjuvant or neoadjuvant chemotherapy for breast cancer vs. women not receiving chemotherapy. | Mdn = 48.39 (CTh + CS +); Mdn = 48.39 (CTh + CS −); Mdn = 54.10 (CTh −). | Cambridge Neuropsychological Test Automated Battery (CANTAB). Clinical neuropsychological tests 34-item Patient’s Assessment of Own Functioning Inventory (PAFI). | FACT-F fatigue subscale. The 12-item General Health Questionnaire (anxiety and depression). | Spearman correlations. ANOVAs. | There was a weak association between CANTAB GDS and the PAFI cognitive domain (rho = −0.27, p = 0.005). Patients who did not receive chemotherapy (CTh-) scored lower on verbal learning and memory (p = 0.054). A worse memory was reported by CTh + CS +N (27.2), followed by CTh + CS − N (38.0), and followed by CTh − N (38.4), p < 0.001 (lower values correspond to more symptoms). |
| Vearncombe et al., 2009 [31] | Australia | Longitudinal study (T1: after surgery but before the commencement of chemotherapy; T2—approximately 4 weeks after administration of the last course of chemotherapy). | 159 (138 Breast cancer survivors scheduled to receive standard-dose adjuvant chemotherapy + 21 Breast cancer survivors scheduled to receive no chemotherapy). | Breast cancer survivors scheduled to receive standard-dose adjuvant chemotherapy vs. breast cancer survivors scheduled to receive no chemotherapy). | 49.38 (7.92): with chemotherapy vs. 53.98 (8.24): without chemotherapy. | Auditory Verbal Learning Test (AVLT). WMS-III-Visual Reproduction Immediate. WMS-III Visual Reproduction Delayed. WMS-III Visual Reproduction Recognition. WAIS-III—Backward Digit Span. | Functional Assessment of Chronic Illness Therapy—fatigue scale Hospital Anxiety and Depression Scale (HADS). | Multiple binary logistic regressions correlations. | Decline in working memory (digit span) performance was associated with poorer initial emotional functioning (r = 0.21; p < 0.02).Pearson correlations between change in cognitive measures (T2-T1) and health and psychological measures did not reveal significant associations with memory performance. In the group with chemotherapy, results revealed (T2 – T1) a decrease in verbal memory (p < 0.001) and an increase in visual memory (p < 0.001) |
| Von Ah et al., 2015 [32] | USA | Cross-sectional study | 88 | Breast cancer survivors, postmenopausal, underwent at least 12 months of postcancer treatment, including chemotherapy. | 56.7 (8.54) | Rey Auditory Verbal Learning Test (AVLT), Rivermead Behavioral Memory Test (RBMT). | Center for Epidemiologic Studies Depression Scale (CES-D). The Functional Assessment of Cancer Therapy-Fatigue (FACT-F). State-Trait Anxiety Inventory-State Subscale (STAI-S). | Pearson’s correlation coefficient. | There was a positive correlation between fatigue and immediate memory (AVLT), r = 0.25, p < 0.05). |
| Wirkner et al., 2017 [38] | German | Cross-sectional study | 51 (20 Breast Cancer Survivors Group and 31 Healthy Control Group). | Breast cancer survivors have undergone medical treatment including surgery, chemotherapy, and endocrine therapy, no longer than 7 years ago. | 52.75 (BCS) and 51.74 (Control) | Subtests digit span forward and backward (Wechsler Memory Scale revised, WMS-R). Logical memory I and II of the WMS-R). Verbal memory (Verbaler Lern- und Merkfähigkeitstest, VLMT) A recognition memory test in which 90 old pictures Memory performance on an analogical scale from 0 (very bad) to 100 (very good). | Trait version of the State-Trait Anxiety Inventory. Beck Depression Inventory (BDI-II. Multidimensional Fatigue Inventory. Fragebogen erlebter Defizite der Aufmerksamkeit, FEDA (fatigue). | Correlations | Depression and trait anxiety were not related to neuropsychological test performance in BCS. BCS showed poorer performance in verbal memory tasks (VLMT and the WMS-R logical memory) compared to the control group. Poorer performance was also found in the digit span (forward) subscale. |
| Longitudinal studies | |||||||||||
| First author name, year | Were the criteria for inclusion in the sample clearly defined? | Were the study subjects and the setting described in detail? | Was the exposure measured in a valid and reliable way? | Were objective, standard criteria used for measurement of the condition? | Were confounding factors identified? | Were strategies to deal with confounding factors stated? | Were the outcomes measured in a valid and reliable way? | Was appropriate statistical analysis used? | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | Was the follow up complete, and if not, were the reasons for loss to follow up described and explored? | Were strategies to address incomplete follow up utilized? |
| Ando-Tanabe et al., 2014 [20] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | NA |
| Boele et al., 2014 [23] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | NA | NA | NA |
| Crouch et al., 2022 [24] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | NA | NA | NA |
| Hsu et al., 2021 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | NA |
| Huang et al., 2019 [25] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Unclear | Yes | NA |
| Jung et al., 2017 [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Jung et al., 2020 [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Le Rhun et al., 2015 [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Merriman et al., 2017 [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear |
| Morel et al., 2015 [36] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | NA | NA | NA |
| Paquet et al., 2018 [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | NA |
| Phillips et al., 2017 [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Shilling et al., 2007 [28] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | No |
| Small et al., 2019 [29] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | NA | NA | NA |
| Van Dyk et al., 2018 [37] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | NA |
| Vardy et al., 2017 [30] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | NA | NA | NA |
| Vearn-combe et al., 2009 [31] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | NA |
| Von Ah et al., 2015 [32] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | NA |
| Wirkner et al., 2017 [38] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.F.S.; Bártolo, A.; Albuquerque, P.B. Memory Impairments and Wellbeing in Breast Cancer Patients: A Systematic Review. J. Clin. Med. 2023, 12, 6968. https://doi.org/10.3390/jcm12226968
Rodrigues PFS, Bártolo A, Albuquerque PB. Memory Impairments and Wellbeing in Breast Cancer Patients: A Systematic Review. Journal of Clinical Medicine. 2023; 12(22):6968. https://doi.org/10.3390/jcm12226968
Chicago/Turabian StyleRodrigues, Pedro F. S., Ana Bártolo, and Pedro B. Albuquerque. 2023. "Memory Impairments and Wellbeing in Breast Cancer Patients: A Systematic Review" Journal of Clinical Medicine 12, no. 22: 6968. https://doi.org/10.3390/jcm12226968
APA StyleRodrigues, P. F. S., Bártolo, A., & Albuquerque, P. B. (2023). Memory Impairments and Wellbeing in Breast Cancer Patients: A Systematic Review. Journal of Clinical Medicine, 12(22), 6968. https://doi.org/10.3390/jcm12226968








