Histological Assessment of the Bile Duct before Liver Transplantation: Does the Bile Duct Injury Score Predict Biliary Strictures?
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Graft Retrieval
2.3. Bile Duct Injury Scoring
2.4. Clinical Outcomes and Definitions
2.5. Statistics
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes
3.3. Bile Duct Injury Scores
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karimian, N.; Westerkamp, A.C.; Porte, R.J. Biliary complications after orthotopic liver transplantation. Curr. Opin. Organ. Transpl. Transplant. 2014, 19, 209–216. [Google Scholar] [CrossRef]
- op den Dries, S.; Westerkamp, A.C.; Karimian, N.; Gouw, A.S.; Bruinsma, B.G.; Markmann, J.F.; Lisman, T.; Yeh, H.; Uygun, K.; Martins, P.N.; et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 2014, 60, 1172–1179. [Google Scholar] [CrossRef]
- Karimian, N.; Op den Dries, S.; Porte, R.J. The origin of biliary strictures after liver transplantation: Is it the amount of epithelial injury or insufficient regeneration that counts? J. Hepatol. 2013, 58, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.M.; Junger, H.; Ruemmele, P.; Schnitzbauer, A.A.; Doenecke, A.; Kirchner, G.I.; Farkas, S.A.; Loss, M.; Scherer, M.N.; Schlitt, H.J.; et al. Bile duct damage after cold storage of deceased donor livers predicts biliary complications after liver transplantation. J. Hepatol. 2013, 58, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Kalloo, A.N. Biliary Complications After Liver Transplantation. Curr. Treat. Options Gastroenterol. 2002, 5, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Seehofer, D.; Eurich, D.; Veltzke-Schlieker, W.; Neuhaus, P. Biliary complications after liver transplantation: Old problems and new challenges. Am. J. Transpl. Transplant. 2013, 13, 253–265. [Google Scholar] [CrossRef]
- Howell, J.A.; Gow, P.J.; Angus, P.W.; Jones, R.M.; Wang, B.Z.; Bailey, M.; Fink, M.A. Early-onset versus late-onset nonanastomotic biliary strictures post liver transplantation: Risk factors reflect different pathogenesis. Transpl. Int. 2012, 25, 765–775. [Google Scholar] [CrossRef]
- Hansen, T.; Hollemann, D.; Pitton, M.B.; Heise, M.; Hoppe-Lotichius, M.; Schuchmann, M.; Kirkpatrick, C.J.; Otto, G. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation--a morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012, 461, 41–48. [Google Scholar] [CrossRef]
- Geuken, E.; Visser, D.; Kuipers, F.; Blokzijl, H.; Leuvenink, H.G.; de Jong, K.P.; Peeters, P.M.; Jansen, P.L.; Slooff, M.J.; Gouw, A.S.; et al. Rapid increase of bile salt secretion is associated with bile duct injury after human liver transplantation. J. Hepatol. 2004, 41, 1017–1025. [Google Scholar] [CrossRef]
- van Rijn, R.; van Leeuwen, O.B.; Matton, A.P.M.; Burlage, L.C.; Wiersema-Buist, J.; van den Heuvel, M.C.; de Kleine, R.H.J.; de Boer, M.T.; Gouw, A.S.H.; Porte, R.J. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018, 24, 655–664. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transpl. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Ciria, R.; Navarro, E.; Sanchez-Frias, M.; Gallardo, A.-B.; Medina, J.; Ayllon, M.-D.; Gomez-Luque, I.; Ruiz-Rabelo, J.; Luque, A.; de la Mata, M.; et al. Preliminary results from the use of intraoperative real-time biliary oxygen monitoring in liver transplantation. Clin. Transpl. Transplant. 2018, 32, e13433. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Randle, L.V.; Gimson, A.E.; Brais, R.; Klinck, J.R.; Hamed, M.; Tsyben, A.; Butler, A.J. Normothermic Perfusion in the Assessment and Preservation of Declined Livers Before Transplantation: Hyperoxia and Vasoplegia-Important Lessons From the First 12 Cases. Transplantation 2017, 101, 1084–1098. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Perera, M.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.; Cilliers, H.; Neil, D.; Hubscher, S.G.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transpl. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- TSANZ. Surgical Technique for Deceased Donor Abdominal Organ Procurement; Transplantation Society of Australia and New Zealand: Sydney, Australia, 2015; Volume ATCA-TSANZ Guidelines G003/2015. [Google Scholar]
- Chui, A.K.; Thompson, J.F.; Lam, D.; Koutalistras, N.; Wang, L.; Verran, D.J.; Sheil, A.G. Cadaveric liver procurement using aortic perfusion only. Aust. N. Z. J. Surg. 1998, 68, 275–277. [Google Scholar] [CrossRef]
- Greif, F.; Bronsther, O.L.; Van Thiel, D.H.; Casavilla, A.; Iwatsuki, S.; Tzakis, A.; Todo, S.; Fung, J.J.; Starzl, T.E. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann. Surg. 1994, 219, 40–45. [Google Scholar] [CrossRef]
- Buis, C.I.; Verdonk, R.C.; Van der Jagt, E.J.; van der Hilst, C.S.; Slooff, M.J.; Haagsma, E.B.; Porte, R.J. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007, 13, 708–718. [Google Scholar] [CrossRef]
- Verdonk, R.C.; Buis, C.I.; Porte, R.J.; van der Jagt, E.J.; Limburg, A.J.; van den Berg, A.P.; Slooff, M.J.; Peeters, P.M.; de Jong, K.P.; Kleibeuker, J.H.; et al. Anastomotic biliary strictures after liver transplantation: Causes and consequences. Liver Transpl. 2006, 12, 726–735. [Google Scholar] [CrossRef]
- Ly, M.; Crawford, M.; Verran, D. Biliary complications in donation after circulatory death liver transplantation: The Australian National Liver Transplantation Unit’s experience. Aust. N. Z. J. Surg. 2020, 91, 445–450. [Google Scholar] [CrossRef]
- Gilbo, N.; Fieuws, S.; Meurisse, N.; Nevens, F.; van der Merwe, S.; Laleman, W.; Verslype, C.; Cassiman, D.; van Malenstein, H.; Roskams, T.; et al. Donor Hepatectomy and Implantation Time Are Associated With Early Complications After Liver Transplantation: A Single-center Retrospective Study. Transplantation 2021, 105, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Zanierato, M.; Avolio, A.W.; Romagnoli, R. Different Timing of Cholangiocyte and Hepatocyte Damage in Liver Preservation: Time to Implement Donor Interventions and New Preservation Techniques to Prevent Ischemic-type Biliary Lesions. Transplantation 2021, 105, e77–e78. [Google Scholar] [CrossRef] [PubMed]
- Op den Dries, S.; Sutton, M.E.; Lisman, T.; Porte, R.J. Protection of bile ducts in liver transplantation: Looking beyond ischemia. Transplantation 2011, 92, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Matton, A.P.M.; de Vries, Y.; Burlage, L.C.; van Rijn, R.; Fujiyoshi, M.; de Meijer, V.E.; de Boer, M.T.; de Kleine, R.H.J.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, pH, and Glucose Are Suitable Biomarkers of Biliary Viability During Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019, 103, 1405–1413. [Google Scholar] [CrossRef]
- op den Dries, S.; Karimian, N.; Weeder, P.D.; Porte, R.J. Normothermic acellular machine perfusion and bile duct injury in pig livers retrieved after cardiac death. Am. J. Transpl. Transplant. 2013, 13, 3289. [Google Scholar] [CrossRef]
- Op den Dries, S.; Sutton, M.E.; Karimian, N.; de Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.; Leuvenink, H.G.; Lisman, T.; Porte, R.J. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS ONE 2014, 9, e88521. [Google Scholar] [CrossRef]
- Op den Dries, S.; Karimian, N.; Westerkamp, A.C.; Sutton, M.E.; Kuipers, M.; Wiersema-Buist, J.; Ottens, P.J.; Kuipers, J.; Giepmans, B.N.; Leuvenink, H.G.D.; et al. Normothermic machine perfusion reduces bile duct injury and improves biliary epithelial function in rat donor livers. Liver Transpl. 2016, 22, 994–1005. [Google Scholar] [CrossRef]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Best, J.; Manka, P.; Syn, W.K.; Dollé, L.; van Grunsven, L.A.; Canbay, A. Role of liver progenitors in liver regeneration. Hepatobiliary Surg. Nutr. 2015, 4, 48–58. [Google Scholar] [CrossRef]
- Buisson, E.M.; Jeong, J.; Kim, H.J.; Choi, D. Regenerative Medicine of the Bile Duct: Beyond the Myth. Int. J. Stem Cells 2019, 12, 183–194. [Google Scholar] [CrossRef]
- Abt, P.; Crawford, M.; Desai, N.; Markmann, J.; Olthoff, K.; Shaked, A. Liver transplantation from controlled non-heart-beating donors: An increased incidence of biliary complications. Transplantation 2003, 75, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Weeder, P.D.; Bomfati, F.; Gouw, A.S.; Porte, R.J. Preservation injury of the distal extrahepatic bile duct of donor livers is representative for injury of the intrahepatic bile ducts. J. Hepatol. 2015, 63, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Liu, Y.; Wang, J.L.; Lu, L.; Zhang, Y.F.; Lu, H.W.; Li, Y.M. Sequential vs simultaneous revascularization in patients undergoing liver transplantation: A meta-analysis. World J. Gastroenterol. 2015, 21, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
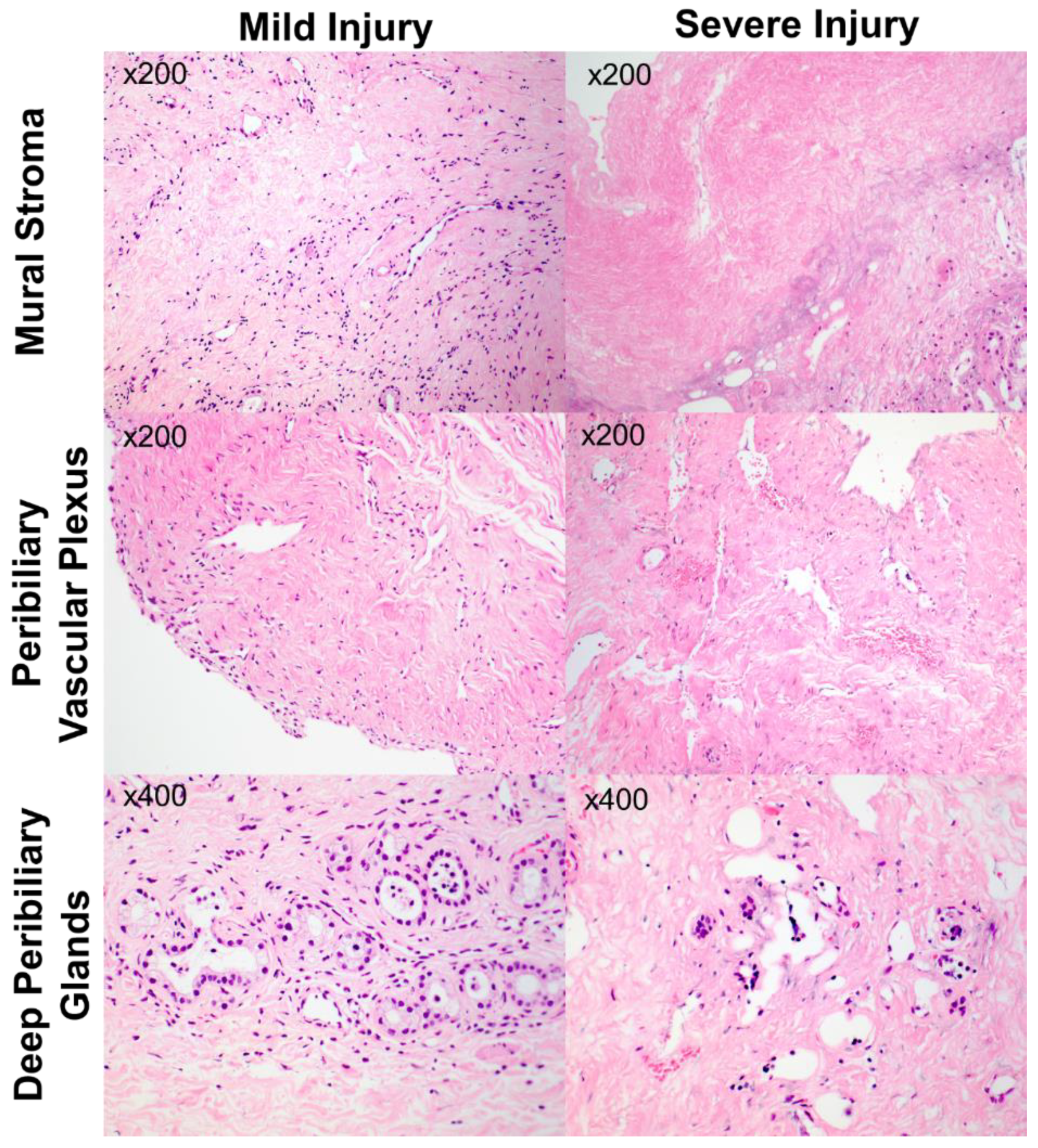
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Biliary Epithelial Injury | No epithelial loss | ≤50% loss | >50% loss | - |
| Mural Stromal Injury | No necrosis | ≤25% necrotic | 25–50% necrotic | >50% necrotic |
| Peribiliary Vascular Plexus Injury | No changes in vessels | ≤50% of vessels with changes | >50% vessels with changes | >50% vessels with changes and arteriolonecrosis |
| Thrombosis | Absent | Present | - | - |
| Intramural bleeding | None | ≤50% of duct wall | >50% of duct wall | |
| Periluminal Peribiliary Gland Loss | No cellular loss | ≤50% loss of cells | >50% loss of cells | |
| Deep Peribiliary Gland Loss | No cellular loss | ≤50% loss of cells | >50% loss of cells | |
| Inflammation | No inflammation | ≥10 leucocytes/HPF | ≥50 leucocytes/HPF |
| No-Stricture Group (n = 41) | Biliary Stricture Group (n = 16) | p-Value | ||||
|---|---|---|---|---|---|---|
| Median/Cases | IQR/% | Median/Cases | IQR/% | |||
| Donor Age | 54 | 43–61 | 61 | 55.5–68.3 | 0.018 | |
| Donor Risk Index * | 1.581 | 1.413–1.1766 | 1.746 | 1.349–1.960 | 0.304 | |
| Donor BMI | 26.9 | 23.7–29.1 | 23.9 | 22.7–26.9 | 0.051 | |
| Donor Gender (M) | 19 | 46% | 5 | 31% | 0.378 | |
| Cause of Donor Death | 0.122 | |||||
| Trauma | 5 | 12% | 2 | 13% | ||
| CVA | 20 | 49% | 12 | 75% | ||
| Hypoxia | 16 | 39% | 2 | 13% | ||
| Recipient Age | 60.5 | 55.6–64.01 | 52.3 | 47.7–60 | 0.026 | |
| Recipient BMI | 29.1 | 24.3–34.1 | 27.7 | 25.7–37.9 | 0.534 | |
| MELD Score * | 21 | 16–27 | 20 | 17–24 | 0.663 | |
| Primary Transplant | 35 | 85% | 13 | 81% | 0.402 | |
| Recipient Gender (M) | 27 | 66% | 12 | 75% | 0.752 | |
| Recipient Liver Disease | ||||||
| ETOH | 10 | 24% | 7 | 44% | 0.201 | |
| HCV | 12 | 29% | 5 | 31% | 1.00 | |
| NASH | 10 | 24% | 3 | 19% | 0.740 | |
| Acute Liver Failure | 3 | 7% | 1 | 6% | 1.00 | |
| PSC | 4 | 10% | 1 | 6% | 1.00 | |
| HCC | 11 | 27% | 5 | 31% | 0.752 | |
| CIT (min) | 325 | 261–389 | 209 | 273–386 | 0.894 | |
| WIT (min) | 40 | 33–54 | 50 | 41–64 | 0.041 | |
| Double bile duct flush | 17 | 41% | 8 | 50% | 0.570 | |
| Duct-to-Duct Biliary Anastomosis | 32 | 78% | 12 | 75% | 1.00 | |
| Interval between portal vein and hepatic artery revascularisation | 26 | 18–35 | 31 | 22–50 | 0.439 | |
| Hepatic Artery Complication | 4 | 10% | 5 | 31% | 0.099 | |
| Peak GGT levels within 7 days post-transplant | 247 | 149–345 | 388 | 283–527 | 0.083 | |
| No-Stricture Group (n = 41) | Biliary Stricture Group(n = 16) | p-Value | ||||
|---|---|---|---|---|---|---|
| Grade | Cases | % | Cases | % | ||
| Biliary Epithelial Injury | 0 | 0 | 0% | 0 | 0% | 0.317 |
| 1 | 9 | 22% | 6 | 38% | ||
| 2 | 32 | 78% | 10 | 63% | ||
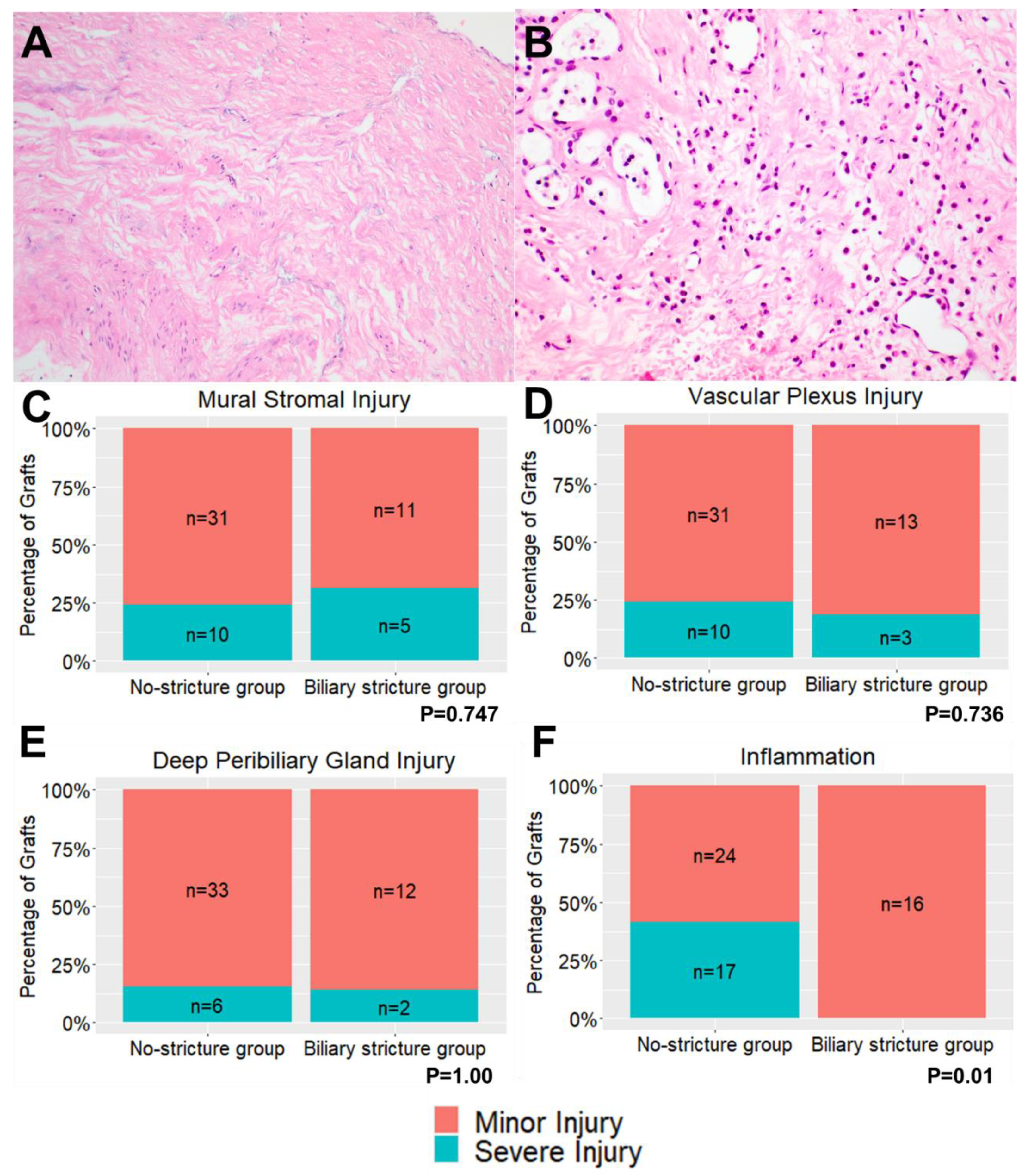
| Mural Stromal Injury | 0 | 11 | 27% | 6 | 38% | 0.700 |
| 1 | 20 | 49% | 5 | 31% | ||
| 2 | 6 | 15% | 3 | 19% | ||
| 3 | 4 | 10% | 2 | 13% | ||
| Peribiliary Vascular Plexus Injury | 0 | 0 | 0% | 2 | 13% | 0.158 |
| 1 | 31 | 76% | 11 | 69% | ||
| 2 | 9 | 22% | 3 | 19% | ||
| 3 | 1 | 2% | 0 | 0% | ||
| Thrombus | 0 | 38 | 93% | 16 | 100% | 0.552 |
| 1 | 3 | 7% | 0 | 0% | ||
| Intramural Bleeding | 0 | 34 | 83% | 15 | 94% | 0.420 |
| 1 | 7 | 17% | 1 | 6% | ||
| 2 | 0% | 0% | ||||
| Peribiliary Glands * | 0 | 6 | 15% | 5 | 33% | 0.261 |
| 1 | 24 | 60% | 6 | 40% | ||
| 2 | 10 | 25% | 4 | 27% | ||
| Deep Peribiliary Gland * | 0 | 10 | 26% | 5 | 36% | 0.831 |
| 1 | 23 | 59% | 7 | 50% | ||
| 2 | 6 | 15% | 2 | 14% | ||
| Inflammation | 0 | 12 | 29% | 8 | 50% | 0.003 |
| 1 | 12 | 29% | 8 | 50% | ||
| 2 | 17 | 41% | 0 | 0% | ||
| No-NAS Group (n = 53) | Non-Anastomotic Stricture Group (n = 4) | p-Value | ||||
|---|---|---|---|---|---|---|
| Grade | Cases | % | Cases | % | ||
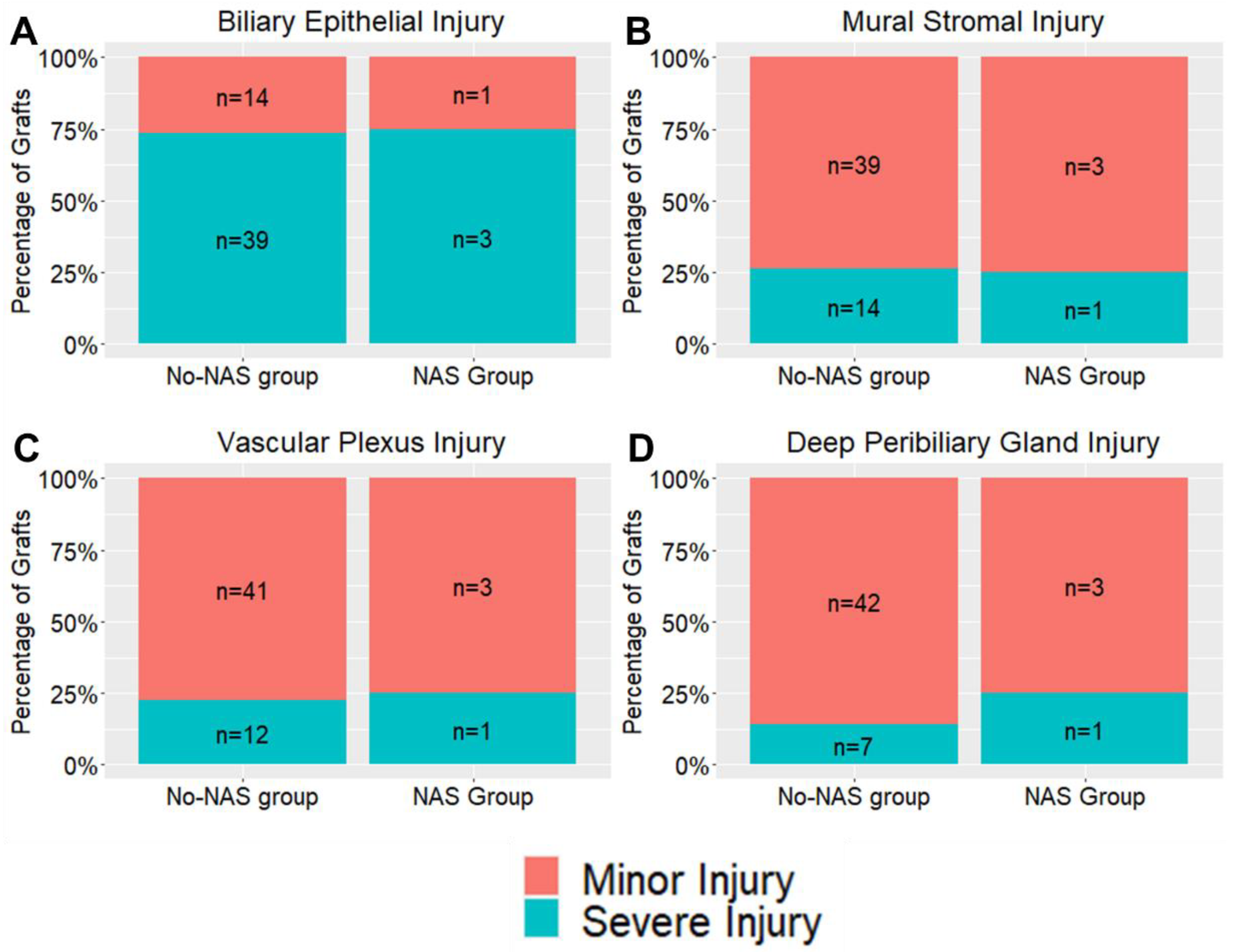
| Biliary Epithelial Injury | 0 | 0 | 0% | 0 | 0% | 1.00 |
| 1 | 14 | 26% | 1 | 17% | ||
| 2 | 39 | 74% | 3 | 50% | ||
| Mural Stromal Injury | 0 | 16 | 30% | 1 | 17% | 0.682 |
| 1 | 23 | 43% | 2 | 33% | ||
| 2 | 9 | 17% | 0 | 0% | ||
| 3 | 5 | 9% | 1 | 17% | ||
| Peribiliary Vascular Plexus Injury | 0 | 1 | 2% | 1 | 17% | 0.166 |
| 1 | 40 | 75% | 2 | 33% | ||
| 2 | 11 | 21% | 1 | 17% | ||
| 3 | 1 | 2% | 0 | 0% | ||
| Thrombus | 0 | 50 | 94% | 4 | 67% | 1.00 |
| 1 | 3 | 6% | 0 | 0% | ||
| Intramural Bleeding | 0 | 46 | 87% | 3 | 50% | 0.464 |
| 1 | 7 | 13% | 1 | 17% | ||
| 2 | 0% | |||||
| Peribiliary Glands * | 0 | 10 | 20% | 1 | 17% | 0.390 |
| 1 | 29 | 57% | 1 | 17% | ||
| 2 | 12 | 24% | 2 | 33% | ||
| Deep Peribiliary Gland * | 0 | 14 | 29% | 1 | 17% | 0.792 |
| 1 | 28 | 57% | 2 | 33% | ||
| 2 | 7 | 14% | 1 | 17% | ||
| Inflammation | 0 | 17 | 32% | 3 | 50% | 0.313 |
| 1 | 19 | 36% | 1 | 17% | ||
| 2 | 17 | 32% | 0 | 0% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, M.; Lau, N.-S.; McKenzie, C.; Kench, J.G.; Seyfi, D.; Majumdar, A.; Liu, K.; McCaughan, G.; Crawford, M.; Pulitano, C. Histological Assessment of the Bile Duct before Liver Transplantation: Does the Bile Duct Injury Score Predict Biliary Strictures? J. Clin. Med. 2023, 12, 6793. https://doi.org/10.3390/jcm12216793
Ly M, Lau N-S, McKenzie C, Kench JG, Seyfi D, Majumdar A, Liu K, McCaughan G, Crawford M, Pulitano C. Histological Assessment of the Bile Duct before Liver Transplantation: Does the Bile Duct Injury Score Predict Biliary Strictures? Journal of Clinical Medicine. 2023; 12(21):6793. https://doi.org/10.3390/jcm12216793
Chicago/Turabian StyleLy, Mark, Ngee-Soon Lau, Catriona McKenzie, James G. Kench, Doruk Seyfi, Avik Majumdar, Ken Liu, Geoffrey McCaughan, Michael Crawford, and Carlo Pulitano. 2023. "Histological Assessment of the Bile Duct before Liver Transplantation: Does the Bile Duct Injury Score Predict Biliary Strictures?" Journal of Clinical Medicine 12, no. 21: 6793. https://doi.org/10.3390/jcm12216793
APA StyleLy, M., Lau, N.-S., McKenzie, C., Kench, J. G., Seyfi, D., Majumdar, A., Liu, K., McCaughan, G., Crawford, M., & Pulitano, C. (2023). Histological Assessment of the Bile Duct before Liver Transplantation: Does the Bile Duct Injury Score Predict Biliary Strictures? Journal of Clinical Medicine, 12(21), 6793. https://doi.org/10.3390/jcm12216793







